Abstract
Background: Onychomycosis is a common fungal infection of the nail.
Objective: The study aimed to provide an update on the evaluation, diagnosis, and treatment of onychomycosis.
Methods: A PubMed search was completed in Clinical Queries using the key term “onychomycosis”. The search was conducted in May 2019. The search strategy included meta-analyses, randomized controlled trials, clinical trials, observational studies, and reviews published within the past 20 years. The search was restricted to English literature. Patents were searched using the key term “onychomycosis” in www.freepatentsonline.com.
Results: Onychomycosis is a fungal infection of the nail unit. Approximately 90% of toenail and 75% of fingernail onychomycosis are caused by dermatophytes, notably Trichophyton mentagrophytes and Trichophyton rubrum. Clinical manifestations include discoloration of the nail, subungual hyperkeratosis, onycholysis, and onychauxis. The diagnosis can be confirmed by direct microscopic examination with a potassium hydroxide wet-mount preparation, histopathologic examination of the trimmed affected nail plate with a periodic-acid-Schiff stain, fungal culture, or polymerase chain reaction assays. Laboratory confirmation of onychomycosis before beginning a treatment regimen should be considered. Currently, oral terbinafine is the treatment of choice, followed by oral itraconazole. In general, topical monotherapy can be considered for mild to moderate onychomycosis and is a therapeutic option when oral antifungal agents are contraindicated or cannot be tolerated. Recent patents related to the management of onychomycosis are also discussed.
Conclusion: Oral antifungal therapies are effective, but significant adverse effects limit their use.Although topical antifungal therapies have minimal adverse events, they are less effective than oral antifungal therapies, due to poor nail penetration. Therefore, there is a need for exploring more effective and/or alternative treatment modalities for the treatment of onychomycosis which are safer and more effective.
Keywords: Dermatophytes, itraconazole, nail discoloration, onychauxis, onycholysis, subungual hyperkeratosis, terbinafine
1. INTRODUCTION
Onychomycosis is an infection of the nail unit caused by fungi (dermatophytes, non-dermatophyte molds, and yeasts), presenting with discoloration of the nail, onycholysis, and nail plate thickening [1, 2]. Any component of the nail unit, including the nail plate, nail matrix, and nail bed can be affected [3]. The term “onychomycosis” is derived from the Greek words “onyx” meaning nail and “mykes” meaning fungus [4]. Onychomycosis is the most common disorder affecting the nail unit and accounts for at least 50% of all nail diseases [2, 5, 6]. Laboratory confirmation of the clinical diagnosis of onychomycosis prior to initiating treatment is cost effective and is recommended [5]. In recent years, newer techniques enabling accurate and sensitive diagnosis of onychomycosis and novel treatments of this condition have emerged. The purpose of this communication is to provide readers with an update on current approaches to diagnosis and treatment of onychomycosis.
2. ETIOLOGY
Onychomycosis can be caused by dermatophytes (tinea unguium), non-dermatophyte molds and yeasts [1, 6, 7]. Approximately 90% of toenail and 75% of fingernail onychomycosis are caused by dermatophytes notably Trichophyton mentagrophytes and Trichophyton rubrum [8-12]. The remaining dermatophyte infections are caused by Epidermophyton floccosum, Microsporum species, Trichophyton verrucosum, Trichophyton tonsurans, Trichophyton violaceum, Trichophyton soundanense, Trichophyton krajdenii, Trichophyton equinum, and Arthroderma species [13-17]. Non-dermatophyte molds that can cause onychomycosis include Aspergillus species, Scopulariopsis species, Fusarium species, Acremonium species, Syncephalastrum species, Scytalidium species, Paecilomyces species, Neoscytalidium species, Chaetomium species, Onychocola species, and Alternaria species [11, 17, 18-31]. Non-dermatophyte molds account for approximately 10% of onychomycosis cases globally [6, 32]. Onychomycosis caused by yeasts is uncommon [33]. Candida albicans accounts for approximately 70% of onychomycosis caused by yeasts [1]. Other Candida species include Candida tropicalis and Candida parapsilosis [12, 17, 28, 33-35]. Patients with chronic mucocutaneous candidiasis and immunodeficiency are more likely infected with the yeast organism, especially in the fingernails [11, 28, 33, 36, 37].
3. EPIDEMIOLOGY
The overall worldwide prevalence of onychomycosis in the general population is approximately 5.5%, based on recently published epidemiological studies [6, 11, 14]. A 2013 systemic review of 11 population-based and 21 hospital-based studies showed that the mean prevalence of onychomycosis in North America and Europe was 4.3% (95% confidence interval: 1.9 to 6.8) in the population-based studies and 8.9% (95% confidence interval: 4.3 to 13.6) in the hospital-based studies [38]. There is evidence that the prevalence is rising, possibly because of longer life expectancy, use of occlusive modern footwear, increased prevalence of obesity, and increased urbanisation [17, 39, 40]. The condition is much more common in adults than in children and the prevalence increases with age [8, 14, 36, 41]. The prevalence in children in North America is approximately 0.4% [17], whereas the prevalence may be as high as 35% in the elderly (> 65 years of age) [42]. Toenail onychomycosis is more common in males whereas Candida fingernail onychomycosis is more common in females [43-45]. Other predisposing factors include fungal infection elsewhere on the body (in particular, tinea pedis), chronic paronychia, previous onychomycosis, wearing of occlusive and tight shoes, hyperhidrosis, participation in sports or fitness activities, nail trauma, poor nail grooming, use of commercial swimming pools, communal bathing, living with family members with fungal infection, poor health, genetic factors, immunodeficiency (in particular, acquired immune deficiency syndrome and transplant patients), diabetes mellitus, obesity, Down syndrome, psoriasis, smoking, peripheral vascular disease, venous insufficiency, hallux valgus, and asymmetric gait nail unit syndrome [44-56].
4. PATHOGENESIS
Onychomycosis is acquired through direct contact of the nail with dermatophytes, non-dermatophyte molds, or yeasts. Because the nail unit does not have effective cell-mediated immunity, it is susceptible to fungal infection [14]. Fungal production of enzymes that have proteolytic, keratinolytic, and lipolytic activities help to degrade the keratin in the nail plate and facilitate fungal invasion of the nail [36, 57]. Factors that compromise barriers to fungal infection may increase the risk for fungal infection [36]. The site and pattern of fungal invasion account for the production of different clinical subtypes of onychomycosis [57]. The formation of fungal biofilms allows the fungi to evade current antifungal therapies and contribute to antifungal resistance [58].
5. CLINICAL MANIFESTATIONS
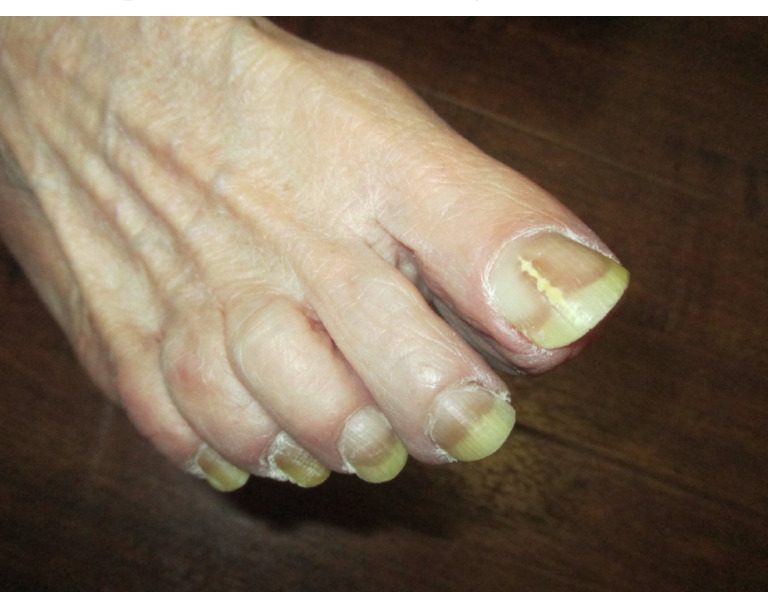
Typically, onychomycosis presents as a white or yellow-brown discoloration of the nail [11, 46]. Violaceous, green, and black discoloration of the nail plate have also been observed [11, 14, 59]. Other clinical manifestations include subungual hyperkeratosis, detachment of the nail from the nail bed (onycholysis) and thickening of the nail plate (onychauxis) [14, 46, 60, 61]. Dermatophytoma presenting as linear, single or multiple white, yellow, orange or brown bands on the nail plate is specific for onychomycosis (Fig. 1) [14]. In general, toenails are affected seven to ten times more frequently than fingernails [6, 9]. The big toenails are most often affected [11]. Generally, several toenails are affected and tinea pedis is often present (Fig. 2) [14, 28]. Also, it is unusual to have more than one fingernail involved without concomitant toenail involvement unless the patient is immunocompromised or there is a history of trauma [14].
Fig. (1).
Dermatophytoma presenting as a linear, yellow, band on the nail plate of the right big toe in a patient with distal lateral subungual onychomycosis.
Fig. (2).
Onychomycosis in a patient with coexisting tinea pedis.
Based on the pattern of invasion, onychomycosis can be divided into the five clinical subtypes described below. It should be noted that patients may have a combination of these subtypes.
5.1. Distal Lateral Subungual Onychomycosis
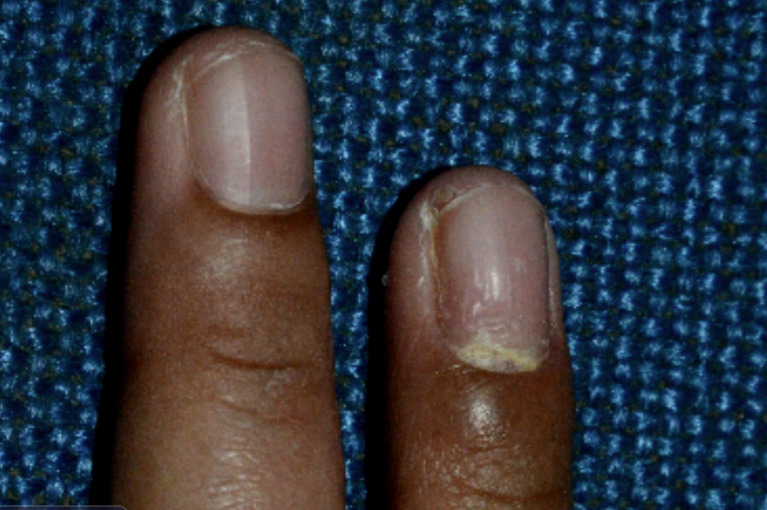
Distal lateral subungual onychomycosis is the most common clinical subtype [35, 36, 62, 63]. In distal lateral subungual onychomycosis, the fungal invasion begins at the hyponychium and then progresses to involve the distal nail bed and subsequently the nail plate [57, 63, 64]. The fungus then migrates proximally through the nail plate, causing linear channels or “spikes” [35, 57]. This clinical subtype is usually caused by Trichophyton rubrum and less commonly by Trichophyton mentagrophytes [36, 57, 63]. Clinically, distal lateral subungual onychomycosis presents as yellowish, whitish, or brownish discoloration of a distal corner of a nail (Fig. 3) [36, 63]. Distal subungual hyperkeratosis, onycholysis, and/or onychauxis of the lateral and distal aspects of the nail plate are common [17, 28, 36].
Fig. (3).
Distal lateral subungual onychomycosis: yellowish discoloration and onycholysis.
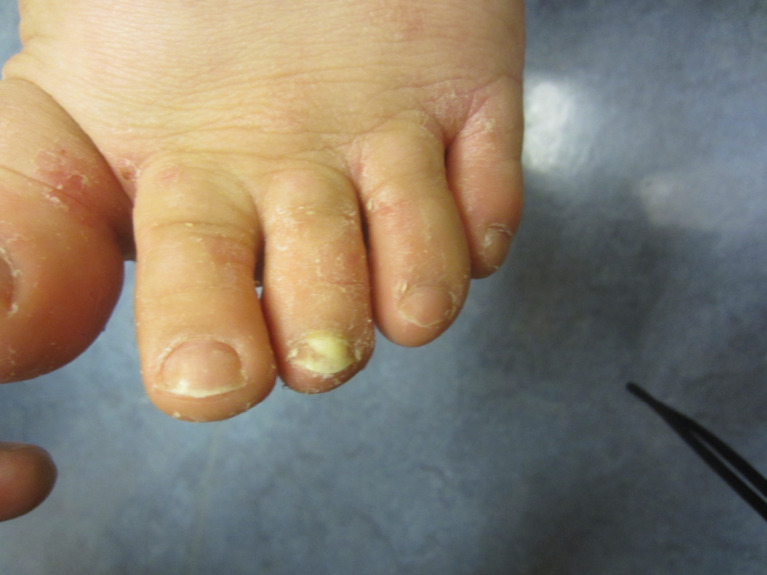
5.2. White Superficial Onychomycosis
In white superficial onychomycosis, the upper surface of the nail plate is affected by the fungus, notably Trichophyton mentagrophytes [36, 57, 63]. Typically, white superficial onychomycosis presents as white dots or patches on the surface of the nail plate (Fig. 4) [36, 57, 63]. The white dots and patches can be easily scraped off [8, 14, 65].
Fig. (4).
White superficial onychomycosis.
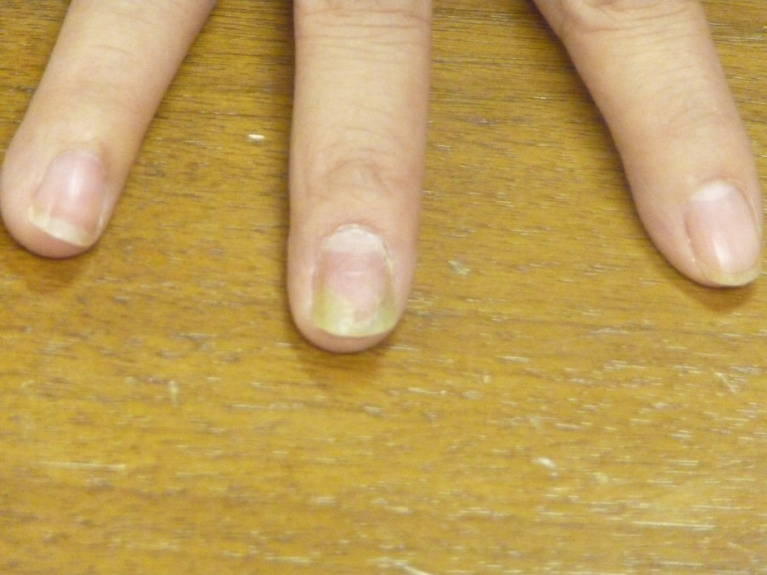
5.3. Proximal Subungual Onychomycosis
Proximal subungual onychomycosis develops when the fungus invades the undersurface of the proximal nail fold in the vicinity of the cuticle and then extends distally (Fig. 5) [14, 36, 53]. This clinical subtype is usually caused by Trichophyton rubrum and Fusarium spp. [63]. Clinically, proximal subungual onychomycosis presents as an area of leukonychia in the proximal nail plate and moves distally with nail growth [57]. Proximal subungual onychomycosis usually occurs in patients with immunodeficiency, especially Acquired Immunodeficiency Syndrome (AIDS) [8, 14].
Fig. (5).
Proximal subungual onychomycosis.
5.4. Endonyx Onychomycosis
Endonyx onychomycosis is caused by fungal infection of the nail plate without infection of the nail bed [14, 28, 53, 63]. This clinical subtype is usually caused by Trichophyton soundanense and Trichophyton violaceum [14, 28, 63]. Clinically, endonyx onychomycosis is characterized by milky patches of the nail plate, indentations, and lamellar splitting [14, 28, 64]. The nail plate is firmly attached to the nail bed and subungual hyperkeratosis is absent [28, 36].
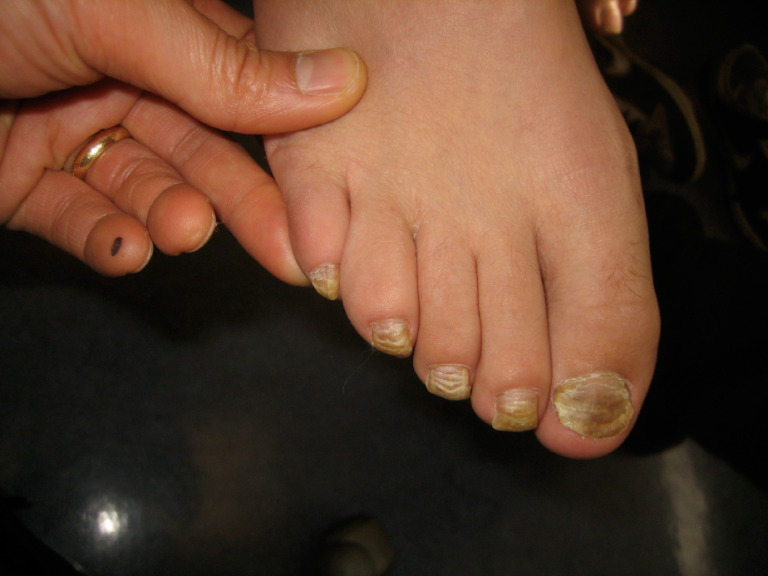
5.5. Total Dystrophic Onychomycosis
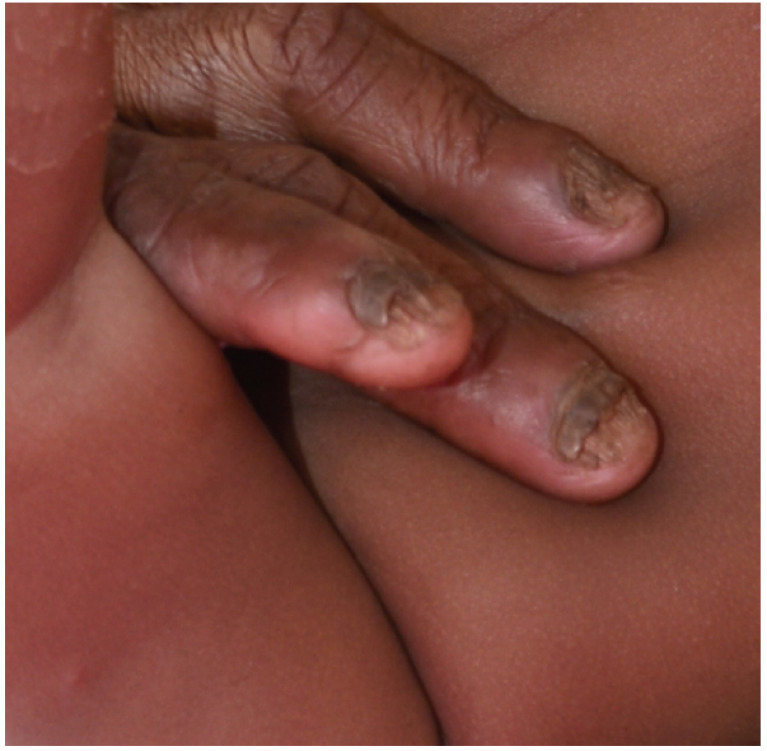
Total dystrophic onychomycosis is characterized by the total destruction of the entire nail apparatus and is often the end-stage of onychomycosis that may follow any of the other subtypes [14, 53, 57, 63]. Clinically, total dystrophic onychomycosis presents with a severely dystrophic and crumbed nail plate which is yellowish, diffusely thickened, and friable (Fig. 6) [28, 57].
Fig. (6).
Total dystrophic onychomycosis.
6. DIAGNOSIS AND DIAGNOSTIC STUDIES
A diagnosis of onychomycosis can be strongly suspected based on the typical clinical features such as nail discoloration, subungual hyperkeratosis/debris, onycholysis, and onychauxis [46, 66, 67]. In one study, the clinical diagnostic accuracy of onychomycosis among non-dermatologists and dermatologists was approximately 66% and 75%, respectively [68]. Dermoscopy of the nails is a useful, quick, non-invasive, and highly effective tool that may help to differentiate onychomycosis from other nail disorders [36, 69]. The most common dermoscopic pattern is a jagged proximal edge with spikes in the onycholytic area [8, 14, 70, 71]. Other dermoscopic findings include “ruined” appearance of the subungual hyperkeratosis, white to yellow longitudinal streaks/striae, leukonychia, chromonychia, parallel bands of different colors (“aurora borealis”), and dermatophytoma [36, 69-75]. On the other hand, transverse onycholysis is suggestive of microtraumatic nail dystrophy [8].
The diagnosis can be confirmed by direct microscopic examination with a Potassium Hydroxide (KOH) wet-mount preparation, histopathologic examination of the trimmed affected nail plate with a Periodic-Acid-Schiff (PAS) stain, fungal culture, or Polymerase Chain Reaction (PCR) assays. The ideal test would identify the fungus and the species, determine its viability, be easy to perform with rapid result and low cost, and be highly specific and sensitive [14, 76].
Depending on the clinical presentation, nail clippings, nail plate scrapings, nail bed scrapings, and subungual scrapings may be necessary for sample collection [76, 77]. A sterile nail clipper should be used to clip the full thickness nail plate and a sterile curette or blade should be used to obtain subungual debris [57, 64, 78]. Nail plate dermoscopy can be used to identify the best location for localized abrasion to obtain adequate samples for mycological examination [79]. The clinical presentation also determines the site of sample collection [14]. For distal and lateral subungual onychomycosis, the sample should be obtained from the most proximal area of involvement (the most active area of infection where the highest concentration of hyphae is located) after clipping the distal onycholytic nail plate [17, 78]. In this regard, the hyphae at the distal end of the nail are less likely to be viable and to grow on culture media [57]. In white superficial onychomycosis, the specimen can be obtained by scraping the affected superficial aspect of the nail plate with a number 15 blade [3, 14, 36, 64, 78]. In proximal subungual onychomycosis, the upper nail plate of the proximal nail should be debrided or pared and the underlying nail debris collected with a curette [3, 14, 36, 64, 78]. Prior to sample collection, the nail plate and the surrounding soft tissue should be cleaned with 70% isopropyl or ethyl alcohol to prevent contamination [32, 64, 78]. Adequate samples should be collected, stored in sterile paper for transport, and delivered to the laboratory in a sterile container without delay [76, 78].
A potassium hydroxide preparation is a useful screening test to rule out the presence of fungi which provides almost immediate results at low cost [36, 68, 80]. The test is performed by adding a drop of 10 to 20% potassium hydroxide, to the nail specimen which is placed on a glass slide for examination by light microscopy [64, 78]. The potassium hydroxide dissolves the keratin, leaving behind easily visualized septate hyphae [64]. The specimen can be gently heated if no dimethyl sulfoxide is added to accelerate keratin dissolution [57, 64]. A positive test demonstrates fungal hyphae, spores, and yeasts cells [36, 64]. The test, however, does not provide information on the species of the fungus or fungus viability [6, 14, 36, 57]. A potassium hydroxide preparation is expertise dependent. The sensitivity has been reported as 48 to 60% [6, 57, 81]. The specificity ranges from 38 to 78% [36].
Histopathologic examination of the clipped affected nail plate with a PAS stain allows hyphae, pseudohyphae, spores, and yeasts to be visualized [32, 78]. The sensitivity ranges from 82 to 88% [57]. The sensitivity can be increased to 96% when PAS stain is combined with fungal culture [64]. PAS stain can produce results within 24 hours [6, 64]. However, the species of the offending fungus and its viability cannot be determined in a PAS stain [32, 36, 78]. The cost associated with PAS stain is higher than potassium hydroxide preparation [36, 68, 78]. PAS stain is also more labor intensive than potassium hydroxide preparation [6].
Fungal cultures are specific (specificity 83 to 100%) [36] but are not very sensitive (sensitivity 60 to 65%) [6, 8]. Cultures are expensive and can take 2 to 4 weeks for results [6, 17]. Nevertheless, fungal cultures are useful for identifying the fungal species, provide information on fungal viability, and guide therapy [6, 14, 17]. For fungal cultures, the clinical sample is plated onto a properly selected culture medium such as Sabouraud dextrose agar [6]. Cycloheximide can be added to the Sabouraud dextrose agar to inhibit growth of non-dermatophyte molds [6, 76]. Gentamycin and chloramphenicol can be added to inhibit bacterial growth [6, 76]. To avoid false negative results, an adequate amount of scrapings is needed [14]. False negative results can also occur due to partial treatment or lingering antifungal drugs from previous treatment [6].
PCR testing allows for rapid and highly specific amplification of fungal DNA fragments [36, 82]. The PCR technique can accurately identify the causal dermatophyte [8, 40, 44, 45, 82]. Also, the results are available quickly (days instead of weeks) [8, 40, 44, 45, 82]. However, PCR assays are costly and not widely available which limit their use in general practice [78].
7. DIFFERENTIAL DIAGNOSIS
Differential diagnosis includes nail changes in psoriasis, lichen planus, alopecia areata, chronic dermatitis, onychogryphosis, chronic paronychia, pityriasis rubra pilaris, pachyonychia congenita, trachyonychia, onychogryphosis, median nail dystrophy, melanonychia striata, subungual melanoma, pemphigus vulgaris, pemphigoid, epidermolysis bullosa acquisita, bullous epidermolysis, subungual wart, subungual exostosis, subungual keratoacanthoma, rheumatoid arthritis, scleroderma, lupus erythematosus, scabies, tungiasis, twenty nail dystrophy, yellow nail syndrome, traumatic onychodystrophy, onychomatricoma, idiopathic onycholysis, porphyria, amyloidosis, myxoid cyst, fibroma, glomus tumor, Bowen disease, and squamous cell carcinoma [2, 3, 21, 24, 36, 44-46, 64, 83-87].
8. COMPLICATIONS
Onychomycosis may serve as a reservoir for cutaneous fungal infections such as tinea pedis, tinea corporis, and tinea cruris [11, 36]. The fungus may also disseminate to other nails [3]. There is an increased risk for bacterial infections such as cellulitis and paronychia, especially in immunocompromised individuals including diabetics [36, 88]. Severe onychomycosis may interfere with standing, walking, nail function, and daily activities [11, 53]. The condition, if left untreated, may cause discomfort, pain, paresthesia, nail deformities such as transverse over-curvature, difficulties in trimming thick nail plates, difficulties in fitting shoes, and low self-esteem [7, 9, 14, 37, 53, 78, 89]. In addition, onychomycosis can be unsightly and socially embarrassing (especially for females) and may have an adverse affect on quality of life [4, 6, 7, 90-92].
9. TREATMENT
Laboratory confirmation of onychomycosis before beginning a treatment regimen is cost-effective and should be considered to avoid misdiagnosis [14, 53, 66,, 88, 93-95]. A misdiagnosis might result in unnecessary treatment and expose the patient to inherent risks of the side effects of the medications, potential negative drug-to-drug interactions associated with systemic antifungal medications, and therapeutic failure. It might also impose a financial burden to the patient [5]. However, empiric treatment of onychomycosis is still performed by many physicians [96]. Onychomycosis is notoriously difficult to treat because of the deep-seated nature of the fungus within the nail plate, the prolonged treatment required for resolution, poor patient compliance, and frequent recurrences [39]. Treatment options include oral antifungal therapy, topical antifungal therapy, laser therapy, photodynamic therapy, and surgical avulsion (e.g. very thick and chronic fungal nail).
9.1. Oral Antifungal Agents
Oral antifungal therapy is considered the gold standard for onychomycosis both in children and adults because of shorter courses of treatment and higher cure rates when compared with topical antifungal therapy [88, 95, 97, 98]. The incidence of adverse events associated with oral antifungal agents is lower in children [6]. Oral antifungal agents used for the treatment of onychomycosis include terbinafine (Lamisil), itraconazole (Sporanox, Sporaz, Orungal), and fluconazole (Diflucan, Celozole) [14, 53, 95]. Terbinafine, an antifungal agent of the allylamine group, is fungicidal. On the other hand, itraconazole and fluconazole are fungistatic and have more potential side effects and drug interactions than terbinafine [1, 88]. A 2017 Cochrane meta-analysis of 48 randomized controlled trials (n = 10, 200) assessing the effects of oral antifungal medications for the treatment of toenail onychomycosis found that terbinafine leads to better clinical and mycological cure rates than other treatments [99]. Currently, oral terbinafine (< 25kg, 125mg once daily; 25 to 35kg, 187.5mg once daily; > 35mg, 250mg once daily) is the drug of choice for the treatment of onychomycosis [39, 53, 62, 88, 100, 101]. Adverse events include headaches, taste disturbance, dermatitis, anorexia, vomiting, epigastric pain, diarrhea, drug-to-drug interactions and, rarely, depression, neutropenia, hepatic dysfunction, and Steven-Johnson syndrome [4, 17, 53, 81]. Generally, continuous terbinafine treatment has similar efficacy as pulse terbinafine treatment although some studies have shown superiority of continuous versus pulsed terbinafine treatment for toenail onychomycosis [102]. Oral itraconazole (children: < 20kg, 5mg/kg daily; 20 to 40kg, 100mg daily; > 40kg, 200mg daily for one week per month; adults: 200mg daily for one week per month for 3 to 6 months) should be considered for patients who cannot tolerate or fail to respond to oral terbinafine or whose onychomycosis is caused by non-dermatophyte molds or yeasts [17, 40, 61, 88]. Adverse events include headaches, gastrointestinal upset, upper respiratory infection, hypertriglyceridemia, hepatic dysfunction, and ventricular dysfunction [14, 44, 45, 53, 88]. Although oral fluconazole is approved for the treatment of onychomycosis in Europe, it is not approved by the United States Food and Drug Administration (FDA) for onychomycosis [14, 53]. Oral fluconazole (children: 3 to 6mg/kg once per week; adults: 150mg once per week) is used off-label for the treatment of onychomycosis in the United States, Canada, and Australia [14, 53]. The medication may be considered in patients who are unable to tolerate terbinafine or itraconazole [61]. Oral griseofulvin (Gris-peg, Grifulvin V) (not available in many countries, like Canada) is less effective, has more adverse events, and requires longer courses of treatment [1, 103]. For these reasons, oral griseofulvin is not the medication of choice in the treatment of onychomycosis [1, 61]. Likewise, oral ketoconazole (Nizoral) should not be used for the treatment of onychomycosis because of severe adverse events such as hepatotoxicity [61, 88].
Oral antifungal agents are recommended for all types of onychomycosis, especially when ≥ 50% of the nail is affected, multiple nails are infected, the nail matrix is involved, or dermatophytoma is present [1, 61, 63, 88, 104]. Oral antifungals, when used in combination with topical antifungals, increase the cure rate [8, 28, 63, 88]. Combination therapy can be used sequentially or in parallel. The treatment regimen should be tailored to the individual patient. Repeated treatment courses may be required, especially for chronic onychomycosis.
9.2. Topical Antifungal Agents
Topical antifungal therapy involves the use of nail lacquers and solutions in addition to topical antifungal agents [105, 106]. Generally, the nail is more permeable to topical antifungal agents formulated in aqueous vehicles [2]. The route of drug delivery is transungual, with the topical antifungal agent applied to the dorsal aspect of the nail [3, 60]. Although the efficacy of topical antifungal agents (e.g., efinaconazole) is not diminished with concurrent nail polish use, concurrent nail polish use may produce undesirable cosmetic changes to the quality of nail polish over time [3, 107, 108]. As such, concurrent nail polish use should be avoided. Commonly used topical antifungal agents include efinaconazole (Jublia, Clenafin) (10% nail solution), tavaborole (Kerydin) (5% nail solution), ciclopirox (Ciclodan, Penlac, Loprox) (8% nail lacquer or hydrolacquer), amorolfine (Curanail, Loceryl, Locetar, Odenil) (5% nail lacquer), and terbinafine (Lamisil) (10% nail solution) [4, 6, 43, 53, 106, 109, 110]. Generally, topical antifungal agents are well-tolerated; adverse events are minimal and include periungual erythema and burning at the application site [6, 111, 112]. However, topical therapy requires longer treatment courses (often 48 weeks or longer) and may be less effective than oral treatment possibly because of insufficient nail plate penetration [4, 14, 88]. The impermeability of the nail can be attributed to the highly stable disulfide linkages and hydrogen bonds in the keratin network [105]. A 2019 meta-analysis of 26 randomized controlled trials (n = 8, 136) assessing the efficacy of monotherapy for toenail onychomycosis found that the odds of mycological cure with continuous oral terbinafine or itraconazole are significantly greater than topical antifungal treatments [104]. In general, topical monotherapy may be considered for mild to moderate onychomycosis when less than 50% of the nail is affected without matrix involvement and only a few (< 3) nails are infected [1, 2, 88]. In this regard, topical antifungal treatment is often sufficient for white superficial onychomycosis because of the superficial nature of the infection [2, 17, 61, 88]. Topical antifungal therapy is a therapeutic option when oral antifungal agents are contraindicated or cannot be tolerated [1, 2, 44, 45, 88]. Topical antifungals may also be used in combination with oral antifungal therapy as an adjunctive therapy to increase the cure rate due to synergistic antifungal action of the drugs [32, 88, 105, 113]. Because the rate of nail growth is faster and the nail plate is thinner in children, children are more likely to respond to topical antifungal therapy better than adults [17, 53, 88, 114, 115].
9.3. Lasers
Lasers have emerged as promising treatment options for the treatment of onychomycosis, although data are still lacking [116]. Most lasers use the principle of selective photothermolysis, whereby laser energy is preferentially absorbed by the fungal mycelia resulting in a rapid elevation of temperature within the fungal mycelia with resultant fungal cell death [39, 117]. As the treatment is targeted, the surrounding tissue is not affected, thus eliminating the potential of systemic adverse events [6, 7]. To be effective, lasers should have a wavelength between 750 and 1300 nm to penetrate the nail, a pulse duration that is shorter than the “thermal relaxation time” of the fungus, and a spatially uniform beam that does not cause “hot spots” [6, 107]. Several types of lasers have been used for the treatment of onychomycosis including long pulsed neodymium-doped yttrium aluminium garnet (Nd:YAG) laser, diode laser, and fractional carbon dioxide (CO2) laser [118-129]. Studies have shown that laser therapies are somewhat effective in achieving cosmetic endpoints in onychomycosis, but do not exceed or equal the efficacy of current topical and oral antifungal therapies in terms of achieving medical endpoints [39, 130]. Laser therapies are safe but expensive and may be considered for patients in whom systemic antifungal agents are contraindicated or as part of combination therapy to presumably increase the chances of successful fungal clearance [131].
9.4. Photodynamic Therapy
Photodynamic therapy involves photoactivation of a photosensitizer with light in specific wavelengths [132]. The photoactivation increases the energetic level of the photosensitizer [132]. The energy consequently generated reacts with the dissolved oxygen in the treated tissue, resulting in the formation of reactive oxygen species and free radicals which are cytotoxic [53, 132, 133]. The fungus absorbs the photosensitizer, making it more susceptible to destruction by apoptosis or necrosis than surrounding healthy tissue [3, 61]. Photosensitizers that have been used include 5-Aminolevulinic Acid (5-ALA), Methyl Aminolevulinate (MAL), porphyrins, aluminium-phthalocyanine chloride, methylene blue, toluidine blue, and rose bengal [28, 39, 132, 134]. Data on the use of photodynamic therapy are scarce and mainly limited to case reports and non randomized trials. A 2016 systematic review of five in vitro and 12 in vivo studies found that photodynamic therapy may be of benefit in the treatment of onychomycosis [134]. Adverse events included mild pain, erythema, burning, edema, and blistering and were well tolerated [7, 134]. Two recent studies, each involving 20 patients with onychomycosis also showed the effectiveness of photodynamic therapy in the treatment of onychomycosis [132, 133]. Well-designed, large-scale, randomized studies are necessary to confirm the efficacy of photodynamic therapy in order to make formal recommendations regarding its use in the treatment of onychomycosis.
9.5. Miscellaneous
Nail abrasion, trimming, avulsion, and debridement can be performed, if necessary, to enhance topical penetration of antifungal agents and reduce fungal load [2, 7, 60, 105]. White superficial onychomycosis can be treated by mechanical removal (e.g., scraping) of the involved area followed by topical antifungal therapy (vide supra) [88]. Surgical avulsion of the nail is painful and may cause disfigurement. Bristow et al. reported the use of a novel nail drill system that permits controlled micro-penetration of the nail without penetrating the nail bed beneath [135]. The authors reported the successful use of the device to deliver a topical antifungal agent directly and rapidly to the site of fungal infection with minimal adverse events, while maintaining the integrity of the nail. Chiu et al. used a dermaroller (Infinitive Beauty, Birmingham, UK) to produce micropores on the nail surface [136]. PathFormer (Path Scientific, Carlisle, USA) is a FDA-approved device for this microporation technique [7].
Keratolytic agents such as urea, salicylic acid, lactic acid, and papain may enhance the delivery of topical antifungal agents into the nails [60]. Nam disclosed an invention for treating onychomycosis [137]. The invention comprises of urea, fumaric acid, 1, 3-buthylene glycol, a gel-forming polymer, a cross-linking agent, and 45 to 60% by weight of water. The preparation has keratolytic and moisture-retention abilities and can be used in the treatment of onychomycosis. For patients with thick, dystrophic nails that are difficult to trim, the use of topical urea (40% ointment or cream) may be considered [88]. The topical application of urea to the treated area prior to therapy may soften the nail and increase the efficacy of the therapy [17, 132, 134]. A systematic review of six randomized, controlled trials (n = 407) showed that topical urea, as an adjunct to oral and topical antifungal treatment regimens, improves the efficacy of topical antifungal treatment [138]. K101 nail solution (Emtrix, Nalox, Naloc) is a combination of urea, propylene glycol, and lactic acid in a topical formulation. A retrospective study (n = 91) showed that combination therapy with topical K101 nail solution and oral terbinafine or itraconazole results in clearance of onychomycosis earlier than that of oral terbinafine or itraconazole alone [139]. Repka et al. showed that the topical application of phosphoric acid gel to the nail plate was effective to improve the permeability of the nail to topical ketoconazole; the treated nail showed sixfold higher ketoconazole permeation than the nonetched nail [140].
Preliminary studies have shown that iontophoresis could enhance the delivery of topical antifungal agents into the nail plate and other parts of the nail apparatus [7, 66, 88, 141, 142]. A tingling sensation may be experienced with the current application [7]. Additional studies are necessary to determine the efficacy and safety of iontophoresis in the treatment of onychomycosis.
10. PREVENTION
Because fungi thrive best in moist warm environments, patients should be advised to wear non-occlusive shoes, keep feet dry and cold, use absorbent socks, and clip nails short [66]. Tinea pedis, if present, should be treated [2, 143]. Also, family members with tinea pedis and onychomycosis should be appropriately treated [144]. To prevent recurrence, some authors suggest the use of topical antifungal therapy once weekly or twice monthly in high-risk patients for up to two years after completion of treatment [6, 40, 145, 146]. An ultraviolet C-based treatment device for footwear can be considered, as well as washing of running shoes (non-leather) in hot water.
11. PROGNOSIS
Generally, the prognosis is good with appropriate treatment. Yellow streaks along the lateral margin of the nail, the presence of dermatophytoma, and onychomycosis caused by non-dermatophyte molds (in particular, Fusarium species) are associated with a poor response to therapy. Other factors associated with a poor response include noncompliance, old age, advanced disease, nail matrix involvement, subungual hyperkeratosis greater than 2 mm, two feet-one hand syndrome, and immunodeficiency [14, 53, 64]. Poor response to therapy may also result from poor permeation of topical antifungal drugs across the nail plate and the deep-seated and recalcitrant nature of fungal infection [4]. Recurrences are not uncommon, with reported rates ranging from 10 to 53% [40, 67, 147]. Typically, recurrences occur within 3 years of completing therapy [14, 40]. Recurrence may be caused by relapse or reinfection [40].
CONCLUSION
Onychomycosis is the most common nail disorder with a significant burden. The condition is most commonly caused by dermatophytes followed by non-dermatophyte molds and yeasts. Although the diagnosis can be strongly suspected based on clinical grounds, laboratory confirmation is necessary prior to treatment. Current treatment modalities of onychomycosis include oral antifungal therapies, topical antifungal therapies, and device-based therapies, alone or in combination. Oral antifungal therapies are recommended for onychomycosis especially for moderate to severe disease or when multiple nails are affected. On the other hand, topical antifungal therapies may be considered for mild to moderate disease. Topical antifungal therapy may also be used in combination with oral antifungal therapy to increase the cure rate of recalcitrant or severe disease. Laser therapies have shown promising results when used alone or in combination with oral and/or topical antifungal therapy. Very thick nails or nails stubborn to treatment can also be considered for surgical or chemical avulsion (e.g. high concentration urea).
Oral antifungal therapies are effective, but significant adverse effects and potential negative drug-to-drug interactions limit their use. Although topical antifungal therapies have minimal adverse events, they are less effective than oral antifungal therapies, due to poor nail penetration. As such, there is a need in exploring more effective and/or alternative treatment modalities for onychomycosis which are safer and more effective. Combination therapies hold promise for improving patient outcomes.
CURRENT & FUTURE DEVELOPMENTS
Novel broad spectrum oral antifungal agents that have shown promising results in the treatment of onychomycosis include posaconazole (Posanol, Noxafil), albaconazole (code name: UR-9825), ravuconazole (code names: BMS-207147, ER-30346), fosravuconazole, VT-1161, and P-3051 [53, 81, 88, 148-153]. Novel broad spectrum topical antifungal agents that have shown promising results in the treatment of onychomycosis include tazarotene (Tazorac), lanoconazole (Astat), luliconazole (Luzu, Luzam, Lulicon), NCV-422, and ME1111 [53, 66]. Very recently, Mercer et al. disclosed a synthetic antifungal peptide in a water-based solution, NP213 (Novexatin), which is in the late-stage of development [154]. According to the authors, NP213 can penetrate the nail more effectively than other topical antifungal agents and shows promise as a drug of choice for topical treatment of onychomycosis. Thus far, these medications have not been approved by the FDA or Health Canada for the treatment of onychomycosis [81].
Viant disclosed antifungal preparations for topical treatment of onychomycosis [155]. The preparations comprise gamma-butyrolactone as the solvent vehicle, 1 to 6% by weight of salicylic acid, and a topical antifungal agent such as ciclopirox, amorolfine, and and terbinafine. The therapeutic effects of these preparations are reinforced by the keratolytic action of the salicylic acid and the preparations may advantageously be made viscous, gelled or filmogenic, with or without a colorant. The author claimed that these antifungal preparations are very effective in the treatment of onychomycosis.
Zderic et al. disclosed an ultrasound-enhanced drug delivery method for the treatment of onychomycosis [156]. The method includes submerging a nail infected with onychomycosis in a solution containing at least one pharmaceutical agent and applying ultrasound to the infected nail for 1 to 10 minutes. Another method includes applying ultrasound to a subject's nail infected with onychomycosis first, and then applying a solution containing at least one pharmaceutical agent to the infected nail. The ultrasound device for applying ultrasound has a frequency of between 200kHz and 3,000kHz.
Mizutani et al. patented a film-forming preparation which contains efinaconazole or a salt thereof; 1 to 30% by weight of ethyl cellulose; 20% by weight or more of a volatile solvent (defined here as water or a liquid component having a boiling point lower than that of water); and 0.01 to 30% by weight of a surfactant [157]. The preparation does not leave a sticky surface but has sufficient cohesiveness after the preparation hardens and can be easily peeled off without washing. According to the authors, the preparation is more effective in the treatment of onychomycosis than the plain topical antifungal agent alone.
Lundahl disclosed a novel method of photodynamic therapy for the treatment of onychomycosis by applying a solution to the affected nail, wherein the solution comprises Aminolevulinic Acid (ALA) or its pharmaceutically acceptable salts at a concentration of 5 to 20% [158]. After a waiting period of at least 10 days, the nail is exposed to a light source that emits red light in the 600-650nm wavelength range. The author claimed that the invention is safe and the long interval between the application of the photosensitizer and the photoactivating light increases the efficacy of the photodynamic therapy.
Nonthermal plasma is being investigated for the treatment of onychomycosis [53, 159]. Nonthermal plasma is created in the air by short pulses (about 10ns) of strong (about 20kV/mm peak) electric field which ionises air molecules, generating electrons, ions, hydroxy radicals, ozone, and nitric acid [53, 159]. Unlike thermal plasma which may cause extensive tissue heating and damage, nonthermal plasma with its small current and duration, does not cause substantial tissue damage [53, 159]. It has been shown that nonthermal plasma, generated by surface microdischarge technology, inhibited the growth of Trichophyton rubrum in vitro [160]. In a pilot study, Lipner et al. used nonthermal plasma to treat 19 patients with toenail onychomycosis [159]. The overall clinical cure was 53.8% and the mycological cure was 15.4% [159]. The authors concluded that nonthermal plasma may have a beneficial effect on toenail onychomycosis and that the procedure is safe. Well designed, large-scale clinical trials are needed to determine the true efficacy and safety of nonthermal plasma in treating onychomycosis.
Roe et al. disclosed a method that includes delivery of a redox gas solution to the affected nail for the treatment of onychomycosis, wherein the redox gas solution comprises a reactive species dissolved in a perfluorocarbon liquid [161]. The reactive species may include, alone or in combination, one or more of reactive oxygen, reactive nitrogen, reactive chlorine, or reactive bromine species [161]. The perfluorocarbon liquid may include perfluorodecalin. The treatment system includes a chamber assembly housing including a chamber formed therein; a plasma head assembly disposed within the chamber; and perfluorodecalin for forming a coating on a nail inserted within the chamber for treatment.
Topical non-prescription agents that have been used with varying success in the treatment of onychomycosis include tea tree (Melaleuca alternifolia) oil, cedar leaf oil (Vicks, VapoRub), snakeroot (Ageratina pichinchensis) extract, and a combination of cyanoacrylate, hydroquinone, and undecylenic acid (Renewed Nail) [64]. These agents have been evaluated in only a small number of studies involving a few patients. Larger, well-designed studies are necessary to evaluate the efficacy of these agents.
Fernandez and Leon disclosed a procedure for the preparation of a product based on the leaves of the plant Sedum telephium for the treatment of nails infected with onychomycosis [162]. The procedure consists of stripping away one side of the leaf of the Sedum telephium plant, after which the stripped surface is impregnated with sulfanilamide powder and then applied as a dressing on nails infected with onychomycosis.
Recently, Sonthalia et al. reported the successful use of Black peel (a combination of 0.5% salicylic acid, 50% black acetic acid, 6% tetrahydrojasmonic acid, 0.1% potassium iodide, 10% bio sulphur) in two recalcitrant cases of onychomycosis [163]. Further studies have to be done to confirm or refute their findings.
Veiga and coworkers showed that propolis, an extract derived from an apiary of hives of honey bees (Apis mellifera L.) has shown promise in the treatment of onychomycosis [164]. This natural product is able to penetrate the nail without cytotoxicity and has good antifungal activity [165]. The authors used propolis with success in the treatment of three elderly patients with onychomycosis. This new finding warrants further investigations to further elucidate its clinical efficacy. Allergic contact dermatitis would be of some concern with this agent.
Mailland et al. disclosed an invention to treat onychomycosis by topically administering to the infected nail a composition which contains hydroxypropyl chitosan, water, and a lower alkanol as solvent [166]. The composition does not need to contain an antifungal agent or any nail penetration enhancer. After applying the composition, washing the nails should be avoided for at least six hours. The application should be repeated once daily for a minimum period of six months to one year. The authors claimed that the preparation is effective in the treatment of onychomycosis.
Most fungi produce biofilms [40]. Biofilms are sessile microbial communities that attach irreversibly to the epithelial surfaces such as the nail plate by means of an extracellular matrix [95, 167]. The extracellular matrix acts as a protective barrier to the organism such as a fungus within the biofilm [167]. As such, biofilms increase fungal resistance to antifungal agents by reducing penetration of these agents, along with protection from host defenses [81, 168]. Therefore, fungal biofilms may account for the persistence of onychomycosis as well as high rate of recurrence and relapse of onychomycosis [167]. Diagnostic tests to detect biofilms are needed. Device-based therapies that can destroy biofilms, enzymes such as with DNAase that can break down biofilms, dispersal agents and chitosan that can prevent biofilm adhesion, and antibodies that can inhibit matrix polysaccharides production, if available, may be used to improve treatment outcomes [6, 53, 167-171].
The inclusion of nanoparticles in topical antifungal agents enhances the drug profile and permeation, as small molecules can pass through nail pores easier than large molecules [7, 105]. The nanoparticles can be in the form of nanocapsules, nanoemulsion, nanovesicles, and polymeric nanoparticles [105, 172]. In a recent study, topical efinaconazole was combined with nitric oxide releasing nanoparticles [173]. The authors showed that the compound offered sustained nitric oxide release over time and enhanced efinaconazole penetration of the nail while exerting broad spectrum antifungal and immunomodulating properties [173]. Krainbring disclosed a novel pharmaceutically active composition which is able to permeate into the keratin sheets of the nails [174]. The composition is a microemulsion comprising ethoxy sulfate, ethoxylated glycerol fatty acid ester, and ethoxylated sorbitol or sorbitol anhydride fatty acid ester. The composition is particularly suitable for treating onychomycosis.
Definitions of treatment outcome measures in onychomycosis vary widely. As such, it is very difficult, if not impossible to compare data across studies. It is hoped that definitions of clinical cure, almost complete cure, clinical success, and clinical improvement need to be unified and consistently adhered to in all future studies.
ACKNOWLEDGEMENTS
Professor Alexander K.C. Leung is the principal author. Dr. Joseph M. Lam, Dr. Kin F. Leong, Professor Kam L. Hon, Dr. Benjamin Barankin, Dr. Amy A.M. Leung, and Dr. Alex H.C. Wong are the coauthors who contributed and helped with the drafting of this manuscript.
LIST OF ABBREVIATIONS
- AIDS
Acquired Immunodeficiency Syndrome
- ALA
Aminolevulinic Acid
- FDA
Food and Drug Administration
- MAL
Methyl Aminolevulinate
- PAS
Periodic-Acid-Schiff
- PCR
Polymerase Chain Reaction
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Professor Alexander K.C. Leung, Dr. Joseph M. Lam, Dr. Kin F. Leong, Professor Kam L. Hon, Dr. Benjamin Barankin, Dr. Amy A.M. Leung, and Dr. Alex H.C. Wong disclose no relevant financial relationship.
REFERENCES
- 1.Hoy N.Y., Leung A.K., Metelitsa A.I., Adams S. New concepts in median nail dystrophy, onychomycosis, and hand, foot, and mouth disease nail pathology. ISRN Dermatol. 2012;2012: 680163. doi: 10.5402/2012/680163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlahovic T.C. Onychomycosis: Evaluation, treatment options, managing recurrence, and patient outcomes. Clin. Podiatr. Med. Surg. 2016;33(3):305–318. doi: 10.1016/j.cpm.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Queller J.N., Bhatia N. The dermatologist’s approach to onychomycosis. J. Fungi (Basel) 2015;1(2):173–184. doi: 10.3390/jof1020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas J., Peterson G.M., Christenson J.K., Kosari S., Baby K.E. Antifungal drug use for onychomycosis. Am. J. Ther. 2019;26(3):e388–e96. doi: 10.1097/MJT.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A.K., Versteeg S.G., Shear N.H. Confirmatory testing prior to initiating onychomycosis therapy is cost-effective. J. Cutan. Med. Surg. 2018;22(2):129–141. doi: 10.1177/1203475417733461. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A.K., Mays R.R., Versteeg S.G., Shear N.H., Piguet V. Update on current approaches to diagnosis and treatment of onychomycosis. Expert Rev. Anti Infect. Ther. 2018;16(12):929–938. doi: 10.1080/14787210.2018.1544891. [DOI] [PubMed] [Google Scholar]
- 7.Angelo T., Borgheti-Cardoso L.N., Gelfuso G.M., Taveira S.F., Gratieri T. Chemical and physical strategies in onychomycosis topical treatment: A review. Med. Mycol. 2017;55(5):461–475. doi: 10.1093/mmy/myw084. [DOI] [PubMed] [Google Scholar]
- 8.Bodman MA, Krishnamurthy K. Onychomycosis. StatPearls [Internet]. aTreasure Island (FL): StatPearls Publishing; 2019 Jan- 2019 Jan 5.
- 9.Gupta A.K., Sibbald R.G., Andriessen A., Belley R., Boroditsky A., Botros M., et al. Toenail onychomycosis - A Canadian approach with a new transungual treatment: Development of a clinical pathway. J. Cutan. Med. Surg. 2015;19(5):440–449. doi: 10.1177/1203475415581310. [DOI] [PubMed] [Google Scholar]
- 10.Joyce A., Gupta A.K., Koenig L., Wolcott R., Carviel J. Fungal diversity and onychomycosis: An analysis of 8,816 toenail samples using quantitative PCR and next-generation sequencing. J. Am. Podiatr. Med. Assoc. 2019;109(1):57–63. doi: 10.7547/17-070. [DOI] [PubMed] [Google Scholar]
- 11.Thomas J., Jacobson G.A., Narkowicz C.K., Peterson G.M., Burnet H., Sharpe C. Toenail onychomycosis: An important global disease burden. J. Clin. Pharm. Ther. 2010;35(5):497–519. doi: 10.1111/j.1365-2710.2009.01107.x. [DOI] [PubMed] [Google Scholar]
- 12.Youssef A.B., Kallel A., Azaiz Z., Jemel S., Bada N., Chouchen A., et al. Onychomycosis: Which fungal species are involved? Experience of the Laboratory of Parasitology-Mycology of the Rabta Hospital of Tunis. J. Mycol. Med. 2018;28(4):651–654. doi: 10.1016/j.mycmed.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Fike J.M., Kollipara R., Alkul S., Stetson C.L. Case report of onychomycosis and tinea corporis due to Microsporum gypseum. J. Cutan. Med. Surg. 2018;22(1):94–96. doi: 10.1177/1203475417724439. [DOI] [PubMed] [Google Scholar]
- 14.Lipner S.R., Scher R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatol. 2019;80(4):835–851. doi: 10.1016/j.jaad.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 15.Pang S.M., Pang J.Y.Y., Fook-Chong S., Tan A.L. Tinea unguium onychomycosis caused by dermatophytes: A ten-year (2005-2014) retrospective study in a tertiary hospital in Singapore. Singapore Med. J. 2018;59(10):524–527. doi: 10.11622/smedj.2018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato T., Kitahara H., Honda H., Katsukawa F., Hiruma M., Yaguchi T. Onychomycosis of the middle finger of a Japanese judo athlete due to Trichophyton tonsurans. Med. Mycol. J. 2019;60(1):1–4. doi: 10.3314/mmj.18-00012. [DOI] [PubMed] [Google Scholar]
- 17.Solís-Arias M.P., García-Romero M.T. Onychomycosis in children. A review. Int. J. Dermatol. 2017;56(2):123–130. doi: 10.1111/ijd.13392. [DOI] [PubMed] [Google Scholar]
- 18.Bombace F., Iovene M.R., Galdiero M., Martora F., Nicoletti G.F., D’Andrea M., et al. Non-dermatophytic onychomycosis diagnostic criteria: an unresolved question. Mycoses. 2016;59(9):558–565. doi: 10.1111/myc.12504. [DOI] [PubMed] [Google Scholar]
- 19.Bongomin F., Batac C.R., Richardson M.D., Denning D.W. A review of onychomycosis due to Aspergillus species. Mycopathologia. 2018;183(3):485–493. doi: 10.1007/s11046-017-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirose M., Noguchi H., Yaguchi T., Matsumoto T., Hiruma M., Fukushima S., et al. Onychomycosis caused by Aspergillus subramanianii. J. Dermatol. 2018;45(11):1362–1366. doi: 10.1111/1346-8138.14616. [DOI] [PubMed] [Google Scholar]
- 21.Hon K.L., Leung A.K. Alopecia areata. Recent Pat. Inflamm. Allergy Drug Discov. 2011;5(2):98–107. doi: 10.2174/187221311795399291. [DOI] [PubMed] [Google Scholar]
- 22.Kimura U., Hiruma M., Kano R., Matsumoto T., Takamori K., Suga Y. Onychomycosis caused by Scopulariopsis brevicaulis: The third documented case in Japan. J. Dermatol. 2019;46(5):e167–e168. doi: 10.1111/1346-8138.14677. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Herrera E.O., Arroyo-Camarena S., Tejada-García D.L., Porras-López C.F., Arenas R. Onychomycosis due to opportunistic molds. An. Bras. Dermatol. 2015;90(3):334–337. doi: 10.1590/abd1806-4841.20153521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuyama Y., Nakamura T., Hagi T., Asanuma K., Sudo A. Subungual onychomycosis due to Aspergillus niger mimicking a glomus tumor: A case report. Biomed. Rep. 2017;7(6):532–534. doi: 10.3892/br.2017.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohanty P., Dash S., Mohapatra L., Jain M. Total dystrophic onychomycosis due to Syncephalastrum racemosum - A rare cause and its novel treatment option. Indian Dermatol. Online J. 2019;10(2):171–173. doi: 10.4103/idoj.IDOJ_155_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno G., Arenas R. Other fungi causing onychomycosis. Clin. Dermatol. 2010;28(2):160–163. doi: 10.1016/j.clindermatol.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Noguchi H., Hiruma M., Miyashita A., Makino K., Miyata K., Ihn H. A case of fingernail onychomycosis due to Aspergillus flavus. Med. Mycol. J. 2016;57(2):E21–E25. doi: 10.3314/mmj.57.E21. [DOI] [PubMed] [Google Scholar]
- 28.Piraccini B.M., Alessandrini A. Onychomycosis: A review. J. Fungi (Basel) 2015;1(1):30–43. doi: 10.3390/jof1010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pontini P., Gorani A., Veraldi S. Onychomycosis by Paecilomyces lilacinus. G. Ital. Dermatol. Venereol. 2016;151(6):706–709. [PubMed] [Google Scholar]
- 30.Pote S.T., Khan U., Lahiri K.K., Patole M.S., Thakar M.R., Shah S.R. Onychomycosis due to Achaetomium strumarium. J. Mycol. Med. 2018;28(3):510–513. doi: 10.1016/j.mycmed.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Veiga F.F., de Castro-Hoshino L.V., Sato F., Bombassaro A., Vicente V.A., Mendes V., et al. Fusarium oxysporum is an onychomycosis etiopathogenic agent. Future Microbiol. 2018;13:1745–1756. doi: 10.2217/fmb-2018-0245. [DOI] [PubMed] [Google Scholar]
- 32.Welsh O., Vera-Cabrera L., Welsh E. Onychomycosis. Clin. Dermatol. 2010;28(2):151–159. doi: 10.1016/j.clindermatol.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Subramanya S.H., Subedi S., Metok Y., Kumar A., Prakash P.Y., Nayak N. Distal and lateral subungual onychomycosis of the finger nail in a neonate: A rare case. BMC Pediatr. 2019;19(1):168. doi: 10.1186/s12887-019-1549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ge G., Yang Z., Li D., Sybren de Hoog G., Shi D. Onychomycosis with greenish-black discolorations and recurrent onycholysis caused by Candida parapsilosis. Med. Mycol. Case Rep. 2019;24:48–50. doi: 10.1016/j.mmcr.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seebacher C., Brasch J., Abeck D., Cornely O., Effendy I., Ginter-Hanselmayer G., et al. Onychomycosis. J. Dtsch. Dermatol. Ges. 2007;5(1):61–66. doi: 10.1111/j.1610-0387.2007.06134.x. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein A.O., Bhatia N. Post TW, ed.Up To Date. Onychomycosis: Epidemiology, clinical features, and diagnosis. Waltham, MA. (Accessed on June 30, 2019). [Google Scholar]
- 37.Zane L.T., Chanda S., Coronado D., Del Rosso J. Antifungal agents for onychomycosis: New treatment strategies to improve safety. Dermatol. Online J. 2016;22(3):1. [PubMed] [Google Scholar]
- 38.Sigurgeirsson B., Baran R. The prevalence of onychomycosis in the global population: A literature study. J. Eur. Acad. Dermatol. Venereol. 2014;28(11):1480–1491. doi: 10.1111/jdv.12323. [DOI] [PubMed] [Google Scholar]
- 39.Christenson J.K., Peterson G.M., Naunton M., Bushell M., Kosari S., Baby K.E., et al. Challenges and opportunities in the management of onychomycosis. J. Fungi (Basel) 2018;4(3):pii: E87. doi: 10.3390/jof4030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta A.K., Cernea M., Foley K.A. Improving cure rates in onychomycosis. J. Cutan. Med. Surg. 2016;20(6):517–531. doi: 10.1177/1203475416653734. [DOI] [PubMed] [Google Scholar]
- 41.Ghannoum M.A., Hajjeh R.A., Scher R., Konnikov N., Gupta A.K., Summerbell R., et al. A large-scale North American study of fungal isolates from nails: The frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 2000;43(4):641–648. doi: 10.1067/mjd.2000.107754. [DOI] [PubMed] [Google Scholar]
- 42.Cozzani E., Agnoletti A.F., Speziari S., Schiavetti I., Zotti M., Persi A., et al. Epidemiological study of onychomycosis in older adults with onychodystrophy. Geriatr. Gerontol. Int. 2016;16(4):486–491. doi: 10.1111/ggi.12496. [DOI] [PubMed] [Google Scholar]
- 43.Gupta A.K., Studholme C. Update on efinaconazole 10% topical solution for the treatment of onychomycosis. Skin Therapy Lett. 2016;21(6):7–11. [PubMed] [Google Scholar]
- 44.Rosen T., Friedlander S.F., Kircik L., Zirwas M.J., Stein Gold L., Bhatia N., et al. Onychomycosis: Epidemiology, diagnosis, and treatment in a changing landscape. J. Drugs Dermatol. 2015;14(3):223–233. [PubMed] [Google Scholar]
- 45.Rosen T. Tinea and onychomycosis. Semin. Cutan. Med. Surg. 2016;35(Suppl. 6):S110–S113. doi: 10.12788/j.sder.2016.035. [DOI] [PubMed] [Google Scholar]
- 46.Allevato M.A. Diseases mimicking onychomycosis. Clin. Dermatol. 2010;28(2):164–177. doi: 10.1016/j.clindermatol.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Assadamongkol R., Lertwattanarak R., Wannachalee T., Bunyaratavej S., Leeyaphan C., Matthapan L. Prevalence, risk factors, and type of organism in fungal foot infection and toenail onychomycosis in Thai diabetic patients. J. Med. Assoc. Thai. 2016;99(6):659–664. [PubMed] [Google Scholar]
- 48.Cathcart S., Cantrell W., Elewski Be. Onychomycosis and diabetes. J. Eur. Acad. Dermatol. Venereol. 2009;23(10):1119–1122. doi: 10.1111/j.1468-3083.2009.03225.x. [DOI] [PubMed] [Google Scholar]
- 49.Daggett C., Brodell R.T., Daniel C.R., Jackson J. Onychomycosis in athletes. Am. J. Clin. Dermatol. 2019;20(5):691–698. doi: 10.1007/s40257-019-00448-4. [DOI] [PubMed] [Google Scholar]
- 50.Gallo L., Cinelli E., Fabbrocini G., Vastarella M. A 15-year retrospective study on the prevalence of onychomycosis in psoriatic vs non-psoriatic patients: A new European shift from dermatophytes towards yeast. Mycoses. 2019;62(8):659–664. doi: 10.1111/myc.12925. [DOI] [PubMed] [Google Scholar]
- 51.Gómez-Moyano E., Crespo-Erchiga V. HIV infection manifesting as proximal white onychomycosis. N. Engl. J. Med. 2017;377(18): e26. doi: 10.1056/NEJMicm1703082. [DOI] [PubMed] [Google Scholar]
- 52.Jimenez-Gonzalez C., Mata-Marin J.A., Arroyo-Anduiza C.I., Ascencio-Montiel Ide J., Fuentes-Allen J.L., Gaytan-Martinez J. Prevalence and etiology of onychomycosis in the HIV-infected Mexican population. Eur. J. Dermatol. 2013;23(3):378–381. doi: 10.1684/ejd.2013.2015. [DOI] [PubMed] [Google Scholar]
- 53.Lipner S.R., Scher R.K. Onychomycosis: Treatment and prevention of recurrence. J. Am. Acad. Dermatol. 2019;80(4):853–867. doi: 10.1016/j.jaad.2018.05.1260. [DOI] [PubMed] [Google Scholar]
- 54.Papini M., Piraccini B.M., Difonzo E., Brunoro A. Epidemiology of onychomycosis in Italy: Prevalence data and risk factor identification. Mycoses. 2015;58(11):659–664. doi: 10.1111/myc.12396. [DOI] [PubMed] [Google Scholar]
- 55.Shemer A., Gupta A.K., Amichai B., Baum S., Barzilai A., Farhi R., et al. Increased risk of tinea pedis and onychomycosis among swimming pool employees in Netanya area, Israel. Mycopathologia. 2016;181(11-12):851–856. doi: 10.1007/s11046-016-0040-5. [DOI] [PubMed] [Google Scholar]
- 56.Tabassum S., Rahman A., Awan S., Jabeen K., Farooqi J., Ahmed B., et al. Factors associated with onychomycosis in nail psoriasis: A multicenter study in Pakistan. Int. J. Dermatol. 2019;58(6):672–678. doi: 10.1111/ijd.14364. [DOI] [PubMed] [Google Scholar]
- 57.Grover C., Khurana A. Onychomycosis: Newer insights in pathogenesis and diagnosis. Indian J. Dermatol. Venereol. Leprol. 2012;78(3):263–270. doi: 10.4103/0378-6323.95440. [DOI] [PubMed] [Google Scholar]
- 58.Gupta A.K., Foley K.A. Evidence for biofilms in onychomycosis. G. Ital. Dermatol. Venereol. 2019;154(1):50–55. doi: 10.23736/S0392-0488.18.06001-7. [DOI] [PubMed] [Google Scholar]
- 59.Monteagudo B., Figueroa O., Suárez-Magdalena O., Méndez-Lage S. Green nail caused by onychomycosis coinfected with Pseudomonas aeruginosa. Actas Dermosifiliogr. 2019;110(9):783–785. doi: 10.1016/j.adengl.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 60.Akhtar N., Sharma H., Pathak K. Onychomycosis: Potential of nail lacquers in transungual delivery of antifungals. Scientifica (Cairo) 2016;2016: 1387936. doi: 10.1155/2016/1387936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta A.K., Mays R.R., Versteeg S.G., Piraccini B.M., Takwale A., Shemer A., et al. Global perspectives for the management of onychomycosis. Int. J. Dermatol. 2019;58(10):1118–1129. doi: 10.1111/ijd.14346. [DOI] [PubMed] [Google Scholar]
- 62.Loo D.S. Onychomycosis in the elderly: Drug treatment options. Drugs Aging. 2007;24(4):293–302. doi: 10.2165/00002512-200724040-00003. [DOI] [PubMed] [Google Scholar]
- 63.Shemer A. Update: Medical treatment of onychomycosis. Dermatol. Ther. . 2012;25(6):582–593. doi: 10.1111/j.1529-8019.2012.01551.x. [DOI] [PubMed] [Google Scholar]
- 64.Westerberg D.P., Voyack M.J. Onychomycosis: Current trends in diagnosis and treatment. Am. Fam. Physician. 2013;88(11):762–770. [PubMed] [Google Scholar]
- 65.Baran R., Hay R., Perrin C. Superficial white onychomycosis revisited. J. Eur. Acad. Dermatol. Venereol. 2004;18(5):569–571. doi: 10.1111/j.1468-3083.2004.01015.x. [DOI] [PubMed] [Google Scholar]
- 66.Gupta A.K., Versteeg S.G., Shear N.H. Confirmatory testing prior to treating toenail onychomycosis is recommended in Canada. J. Cutan. Med. Surg. 2018;22(2):244–245. doi: 10.1177/1203475417746126. [DOI] [PubMed] [Google Scholar]
- 67.Scher R.K., Tavakkol A., Sigurgeirsson B., Hay R.J., Joseph W.S., Tosti A., et al. Onychomycosis: Diagnosis and definition of cure. J. Am. Acad. Dermatol. 2007;56(6):939–944. doi: 10.1016/j.jaad.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 68.Li D.G., Cohen J.M., Mikailov A., Williams R.F., Laga A.C., Mostaghimi A. Clinical diagnostic accuracy of onychomycosis: A multispecialty comparison study. Dermatol. Res. Pract. 2018;2018: 2630176. doi: 10.1155/2018/2630176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yorulmaz A., Yalcin B. Dermoscopy as a first step in the diagnosis of onychomycosis. Postepy Dermatol. Alergol. 2018;35(3):251–258. doi: 10.5114/ada.2018.76220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nirmal B. Utility of a multispectral dermatoscope in onychomycosis. Indian J. Dermatol. 2018;63(1):87–88. doi: 10.4103/ijd.IJD_37_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piraccini B.M., Balestri R., Starace M., Rech G. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2013;27(4):509–513. doi: 10.1111/j.1468-3083.2011.04323.x. [DOI] [PubMed] [Google Scholar]
- 72.Bodman M.A. Point-of-care diagnosis of onychomycosis by dermoscopy. J. Am. Podiatr. Med. Assoc. 2017;107(5):413–418. doi: 10.7547/16-183. [DOI] [PubMed] [Google Scholar]
- 73.De Crignis G., Valgas N., Rezende P., Leverone A., Nakamura R. Dermatoscopy of onychomycosis. Int. J. Dermatol. 2014;53(2):e97–e99. doi: 10.1111/ijd.12104. [DOI] [PubMed] [Google Scholar]
- 74.Kaynak E., Göktay F., Güneş P., Sayman E., Turan D., Baygül A., et al. The role of dermoscopy in the diagnosis of distal lateral subungual onychomycosis. Arch. Dermatol. Res. 2018;310(1):57–69. doi: 10.1007/s00403-017-1796-2. [DOI] [PubMed] [Google Scholar]
- 75.Nargis T., Pinto M., Shenoy M.M., Hegde S. Dermoscopic features of distal lateral subungual onychomycosis. Indian Dermatol. Online J. 2018;9(1):16–19. doi: 10.4103/idoj.IDOJ_40_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta A.K., Simpson F.C. Diagnosing onychomycosis. Clin. Dermatol. 2013;31(5):540–543. doi: 10.1016/j.clindermatol.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 77.Bertanha L., Chiacchio N.D. Nail clipping in onychomycosis. An. Bras. Dermatol. 2016;91(5):688–690. doi: 10.1590/abd1806-4841.20164968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghannoum M., Mukherjee P., Isham N., Markinson B., Rosso J.D., Leal L. Examining the importance of laboratory and diagnostic testing when treating and diagnosing onychomycosis. Int. J. Dermatol. 2018;57(2):131–138. doi: 10.1111/ijd.13690. [DOI] [PubMed] [Google Scholar]
- 79.Bet D.L., Reis A.L., Di Chiacchio N., Belda W., Junior Dermoscopy and onychomycosis: Guided nail abrasion for mycological samples. An. Bras. Dermatol. 2015;90(6):904–906. doi: 10.1590/abd1806-4841.20154615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karaman B.F.O., Açıkalın A., Ünal İ., Aksungur V.L. Diagnostic values of KOH examination, histological examination, and culture for onychomycosis: A latent class analysis. Int. J. Dermatol. 2019;58(3):319–324. doi: 10.1111/ijd.14255. [DOI] [PubMed] [Google Scholar]
- 81.Gupta A.K., Versteeg S.G., Shear N.H. Onychomycosis in the 21st century: An update on diagnosis, epidemiology, and treatment. J. Cutan. Med. Surg. 2017;21(6):525–539. doi: 10.1177/1203475417716362. [DOI] [PubMed] [Google Scholar]
- 82.Watanabe S., Ishida K. Molecular diagnostic techniques for onychomycosis: Validity and potential application. Am. J. Clin. Dermatol. 2017;18(2):281–286. doi: 10.1007/s40257-016-0248-7. [DOI] [PubMed] [Google Scholar]
- 83.Kandi V. Tungiasis presenting as onychomycosis: Probably the first report of flea infestation of the nail observed using modified potassium hydroxide mount technique. Cureus. 2018;10(3): e2278. doi: 10.7759/cureus.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leung A.K., Robson W.L. Hair loss in children. J. R. Soc. Health. 1993;113(5):252–256. doi: 10.1177/146642409311300509. [DOI] [PubMed] [Google Scholar]
- 85.Leung A.K., Lam J.M., Leong K.F., Sergi C.M. Melanonychia striata: Clarifying behind the black curtain. A review of clinical evaluation and management of the 21st century. Int. J. Dermatol. 2019;58(11):1239–1245. doi: 10.1111/ijd.14464. [DOI] [PubMed] [Google Scholar]
- 86.Oztürk Durmaz E., Sezer E., Dikicioğlu Çetin E., Sahin S. Onychomatricoma masquerading as candidal onychomycosis and paronychia. Acta Dermatovenerol. Croat. 2013;21(3):198–201. [PubMed] [Google Scholar]
- 87.Riahi R.R., Cohen P.R., Goldberg L.H. Subungual amelanotic melanoma masquerading as onychomycosis. Cureus. 2018;10(3): e2307. doi: 10.7759/cureus.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldstein A.O., Bhatia N. Post TW, ed UpToDate. Onychomycosis: Management. Waltham, MA. (Accessed on June 30, 2019). [Google Scholar]
- 89.Ocampo-Garza J., Di Chiacchio N.G., Di Chiacchio N., Machado-Filho C.D. Acute transverse overcurvature of the nail due to onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2017;31(4):e202–e203. doi: 10.1111/jdv.13955. [DOI] [PubMed] [Google Scholar]
- 90.Chacon A., Franca K., Fernandez A., Nouri K. Psychosocial impact of onychomycosis: A review. Int. J. Dermatol. 2013;52(11):1300–1307. doi: 10.1111/ijd.12122. [DOI] [PubMed] [Google Scholar]
- 91.Gupta A.K., Mays R.R. The impact of onychomycosis on quality of life: A systematic review of the available literature. Skin Appendage Disord. 2018;4(4):208–216. doi: 10.1159/000485632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milobratović D., Janković S., Vukičević J., Marinković J., Janković J., Railić Z. Quality of life in patients with toenail onychomycosis. Mycoses. 2013;56(5):543–551. doi: 10.1111/myc.12072. [DOI] [PubMed] [Google Scholar]
- 93.Gupta AK, Stec N. Recent advances in therapies for onychomycosis and its management. F1000Res. 2019;8 doi: 10.12688/f1000research.18646.1. pii: F1000 Faculty Rev-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hanna S., Andriessen A., Beecker J., Gilbert M., Goldstein E., Kalia S., et al. Clinical insights about onychomycosis and its treatment: A consensus. J. Drugs Dermatol. 2018;17(3):253–262. [PubMed] [Google Scholar]
- 95.Lipner S.R. Pharmacotherapy for onychomycosis: New and emerging treatments. Expert Opin. Pharmacother. 2019;20(6):725–735. doi: 10.1080/14656566.2019.1571039. [DOI] [PubMed] [Google Scholar]
- 96.Koshnick R.L., Lilly K.K., St Clair K., Finnegan M.T., Warshaw E.M. Use of diagnostic tests by dermatologists, podiatrists and family practitioners in the United States: Pilot data from a cross-sectional survey. Mycoses. 2007;50(6):463–469. doi: 10.1111/j.1439-0507.2007.01422.x. [DOI] [PubMed] [Google Scholar]
- 97.Gupta A.K., Paquet M. Systemic antifungals to treat onychomycosis in children: A systematic review. Pediatr. Dermatol. 2013;30(3):294–302. doi: 10.1111/pde.12048. [DOI] [PubMed] [Google Scholar]
- 98.Gupta A.K., Mays R.R., Versteeg S.G., Shear N.H., Friedlander S.F. Onychomycosis in children: Safety and efficacy of antifungal agents. Pediatr. Dermatol. 2018;35(5):552–559. doi: 10.1111/pde.13561. [DOI] [PubMed] [Google Scholar]
- 99.Kreijkamp-Kaspers S., Hawke K., Guo L., Kerin G., Bell-Syer S.E., Magin P., et al. Oral antifungal medication for toenail onychomycosis. Cochrane Database Syst. Rev. 2017;7: CD010031. doi: 10.1002/14651858.CD010031.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ameen M., Lear J.T., Madan V., Mohd Mustapa M.F., Richardson M. British Association of Dermatologists’ guidelines for the management of onychomycosis 2014. Br. J. Dermatol. 2014;171(5):937–958. doi: 10.1111/bjd.13358. [DOI] [PubMed] [Google Scholar]
- 101.de Sá D.C., Lamas A.P., Tosti A. Oral therapy for onychomycosis: An evidence-based review. Am. J. Clin. Dermatol. 2014;15(1):17–36. doi: 10.1007/s40257-013-0056-2. [DOI] [PubMed] [Google Scholar]
- 102.Yadav P., Singal A., Pandhi D., Das S. Comparative efficacy of continuous and pulse dose terbinafine regimes in toenail dermatophytosis: A randomized double-blind trial. Indian J. Dermatol. Venereol. Leprol. 2015;81(4):363–369. doi: 10.4103/0378-6323.158634. [DOI] [PubMed] [Google Scholar]
- 103.Faergemann J., Anderson C., Hersle K., Hradil E., Nordin P., Kaaman T., et al. Double-blind, parallel-group comparison of terbinafine and griseofulvin in the treatment of toenail onychomycosis. J. Am. Acad. Dermatol. 1995;32(5 Pt 1):750–753. doi: 10.1016/0190-9622(95)91454-4. [DOI] [PubMed] [Google Scholar]
- 104.Gupta A.K., Foley K.A., Mays R.R., Shear N.H., Piguet V. Monotherapy for toenail onychomycosis: A systematic review and network meta-analysis. Br. J. Dermatol. 2019 doi: 10.1111/bjd.18155. [DOI] [PubMed] [Google Scholar]
- 105.Dhamoon R.K., Popli H., Gupta M. Novel drug delivery strategies for the treatment of onychomycosis. Pharm. Nanotechnol. 2019;7(1):24–38. doi: 10.2174/2211738507666190228104031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piraccini B.M., Tosti A. Ciclopirox hydroxypropyl chitosan: Efficacy in mild-to-moderate onychomycosis. Skin Appendage Disord. 2018;5(1):13–19. doi: 10.1159/000488606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Canavan T.N., Bevans S.L., Cantrell W.C., Wang C., Elewski B.E. Single-center, prospective, blinded study comparing the efficacy and compatibility of efinaconazole 10% solution in treating onychomycosis with and without concurrent nail polish use. Skin Appendage Disord. 2018;5(1):9–12. doi: 10.1159/000488369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeichner J.A., Stein Gold L., Korotzer A. Penetration of ((14)C)-efinaconazole topical solution, 10%, does not appear to be influenced by nail polish. J. Clin. Aesthet. Dermatol. 2014;7(9):34–36. [PMC free article] [PubMed] [Google Scholar]
- 109.Aly R., Gupta A.K., Winter T., Zane L.T., Vlahovic T. Tavaborole in difficult-to-treat onychomycosis cases: A post-hoc assessment of Phase III subjects. J. Drugs Dermatol. 2017;16(10):1016–1021. [PubMed] [Google Scholar]
- 110.Gupta A.K., Hall S., Zane L.T., Lipner S.R., Rich P. Evaluation of the efficacy and safety of tavaborole topical solution, 5%, in the treatment of onychomycosis of the toenail in adults: A pooled analysis of an 8-week, post-study follow-up from two randomized Phase 3 studies. J. Dermatolog. Treat. 2018;29(1):44–48. doi: 10.1080/09546634.2017.1329510. [DOI] [PubMed] [Google Scholar]
- 111.Krasaeath R., Elizondo J. Topical antifungals for treatment of onychomycosis. Am. Fam. Physician. 2016;94(9):734. [PubMed] [Google Scholar]
- 112.Rich P., Spellman M., Purohit V., Zang C., Crook T.J. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: Results from a Phase 4 open-label study. J. Drugs Dermatol. 2019;18(2):190–195. [PubMed] [Google Scholar]
- 113.Feng X., Xiong X., Ran Y. Efficacy and tolerability of amorolfine 5% nail lacquer in combination with systemic antifungal agents for onychomycosis: A meta-analysis and systematic review. Dermatol. Ther. . 2017;30(3) doi: 10.1111/dth.12457. [DOI] [PubMed] [Google Scholar]
- 114.Eichenfield L.F., Friedlander S.F. Pediatric onychomycosis: The emerging role of topical therapy. J. Drugs Dermatol. 2017;16(2):105–109. [PubMed] [Google Scholar]
- 115.Feldstein S., Totri C., Friedlander S.F. Antifungal therapy for onychomycosis in children. Clin. Dermatol. 2015;33(3):333–339. doi: 10.1016/j.clindermatol.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 116.Ibrahim S.A., Albalat W., Ebrahim H.M. Evaluation of long pulsed Nd-YAG laser in the treatment of onychomycosis. J. Cosmet. Laser Ther. 2019;21(2):76–81. doi: 10.1080/14764172.2018.1469765. [DOI] [PubMed] [Google Scholar]
- 117.Gupta A.K., Foley K.A., Versteeg S.G. Lasers for onychomycosis. J. Cutan. Med. Surg. 2017;21(2):114–116. doi: 10.1177/1203475416677722. [DOI] [PubMed] [Google Scholar]
- 118.Abd El-Aal E.B., Abdo H.M., Ibrahim S.M., Eldestawy M.T. Fractional carbon dioxide laser assisted delivery of topical tazarotene versus topical tioconazole in the treatment of onychomycosis. J. Dermatolog. Treat. 2019;30(3):277–282. doi: 10.1080/09546634.2018.1509046. [DOI] [PubMed] [Google Scholar]
- 119.Bonhert K., Dorizas A., Sadick N.S. Efficacy of combination therapy with efinaconazole 10% solution and 1064 nm Nd: YAG laser for treatment of toenail onychomycosis. J. Cosmet. Laser Ther. 2019;21(3):179–183. doi: 10.1080/14764172.2018.1502451. [DOI] [PubMed] [Google Scholar]
- 120.do Espírito Santo R.B., Deps P.D. Case study of onychomycosis patients treated with 1,064-nm Nd: YAG laser. Case Rep. Dermatol. 2018;10(2):216–225. doi: 10.1159/000492526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Francuzik W., Fritz K., Salavastru C. Laser therapies for onychomycosis - Critical evaluation of methods and effectiveness. J. Eur. Acad. Dermatol. Venereol. 2016;30(6):936–942. doi: 10.1111/jdv.13593. [DOI] [PubMed] [Google Scholar]
- 122.Khater M.H., Khattab F.M. Combined long pulsed Nd-Yag laser and itraconazole versus itraconazole alone in the treatment of onychomycosis nails. J. Dermatolog. Treat. 2019;3:1–19. doi: 10.1080/09546634.2019.1623861. [DOI] [PubMed] [Google Scholar]
- 123.Okan G., Tarikci N., Gokdemir G. The effect of long-pulsed Nd: YAG laser for the treatment of onychomycosis. J. Am. Podiatr. Med. Assoc. 2017;107(1):54–59. doi: 10.7547/15-137. [DOI] [PubMed] [Google Scholar]
- 124.Piccolo D., Kostaki D., Del Duca E., Cannarozzo G., Sannino M., Nisticò S. Long-pulsed 1064-nm Nd: YAG laser for the treatment of onychomycosis. Photomed. Laser Surg. 2017;35(4):213–216. doi: 10.1089/pho.2016.4153. [DOI] [PubMed] [Google Scholar]
- 125.Rivers J.K., Vestvik B.J., Berkowitz J. Real-world efficacy of 1064-nm Nd: YAG laser for the treatment of onychomycosis. J. Cutan. Med. Surg. 2017;21(2):108–113. doi: 10.1177/1203475416676804. [DOI] [PubMed] [Google Scholar]
- 126.Weber G.C., Firouzi P., Baran A.M., Bölke E., Schrumpf H., Buhren B.A., et al. Treatment of onychomycosis using a 1064-nm diode laser with or without topical antifungal therapy: A single-center, retrospective analysis in 56 patients. Eur. J. Med. Res. 2018;23(1):53. doi: 10.1186/s40001-018-0340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wiznia L.E., Quatrano N.A., Mu E.W., Rieder E.A. A clinical review of laser and light therapy for nail psoriasis and onychomycosis. Dermatol. Surg. 2017;43(2):161–172. doi: 10.1097/DSS.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 128.Zawar V., Sarda A., De A. Clearance of recalcitrant onychomycosis following Q-switched Nd-Yag laser. J. Cutan. Aesthet. Surg. 2017;10(4):226–227. doi: 10.4103/JCAS.JCAS_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou B.R., Lu Y., Permatasari F., Huang H., Li J., Liu J., et al. The efficacy of fractional carbon dioxide (CO2) laser combined with luliconazole 1% cream for the treatment of onychomycosis: A randomized, controlled trial. Medicine (Baltimore) 2016;95(44): e5141. doi: 10.1097/MD.0000000000005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gupta A.K., Versteeg S.G. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2017;31(7):1111–1118. doi: 10.1111/jdv.14212. [DOI] [PubMed] [Google Scholar]
- 131.El-Tatawy R.A., Aliweh H.A., Hegab D.S., Talaat R.A.Z., Shams Eldeen M.A. Fractional carbon dioxide laser and topical tioconazole in the treatment of fingernail onychomycosis. Lasers Med. Sci. 2019;34(9):1873–1880. doi: 10.1007/s10103-019-02789-2. [DOI] [PubMed] [Google Scholar]
- 132.Morgado L.F., Trávolo A.R.F., Muehlmann L.A., Narcizo P.S., Nunes R.B., Pereira P.A.G., et al. Photodynamic therapy treatment of onychomycosis with aluminium-phthalocyanine chloride nanoemulsions: A proof of concept clinical trial. J. Photochem. Photobiol. B. 2017;173:266–270. doi: 10.1016/j.jphotobiol.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 133.Alberdi E., Gómez C. Efficiency of methylene blue-mediated photodynamic therapy vs. intense pulsed light in the treatment of onychomycosis in the toenails. Photodermatol. Photoimmunol. Photomed. 2019;35(2):69–77. doi: 10.1111/phpp.12420. [DOI] [PubMed] [Google Scholar]
- 134.Bhatta A.K., Keyal U., Wang X.L. Photodynamic therapy for onychomycosis: A systematic review. Photodiagn. Photodyn. Ther. 2016;15:228–235. doi: 10.1016/j.pdpdt.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 135.Bristow I., Baran R., Score M. Rapid treatment of subungual onychomycosis using controlled micro nail penetration and terbinafine solution. J. Drugs Dermatol. 2016;15(8):974–978. [PubMed] [Google Scholar]
- 136.Chiu WS, Belsey NA, Garrett NL, Moger J, Price GJ, Delgado-Charro MB, et al. Drug delivery into microneedle-porated nails from nanoparticle reservoirs. J Control Release. 2015. 220(Pt A):98-106. [DOI] [PubMed]
- 137.Nam T.S. Pharmaceutical composition for preventing or treating onychomycosis and preparation method therefor. WO2017018625 (2017).
- 138.Dars S., Banwell H.A., Matricciani L. The use of urea for the treatment of onychomycosis: A systematic review. J. Foot Ankle Res. 2019;12:22. doi: 10.1186/s13047-019-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shemer A., Gupta A.K., Babaev M., Barzilai A., Farhi R., Daniel Iii C.R. A retrospective study comparing K101 nail solution as a monotherapy and in combination with oral terbinafine or itraconazole for the treatment of toenail onychomycosis. Skin Appendage Disord. 2018;4(3):166–170. doi: 10.1159/000484211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Repka M.A., Mididoddi P.K., Stodghill S.P. Influence of human nail etching for the assessment of topical onychomycosis therapies. Int. J. Pharm. 2004;282(1-2):95–106. doi: 10.1016/j.ijpharm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 141.Amichai B., Nitzan B., Mosckovitz R., Shemer A. Iontophoretic delivery of terbinafine in onychomycosis: A preliminary study. Br. J. Dermatol. 2010;162(1):46–50. doi: 10.1111/j.1365-2133.2009.09414.x. [DOI] [PubMed] [Google Scholar]
- 142.Kushwaha A., Shivakumar H.N., Murthy S.N. Iontophoresis for drug delivery into the nail apparatus: Exploring hyponychium as the site of delivery. Drug Dev. Ind. Pharm. 2016;42(10):1678–1682. doi: 10.3109/03639045.2016.1165690. [DOI] [PubMed] [Google Scholar]
- 143.Lipner S.R., Scher R.K. Management of onychomycosis and co-existing tinea pedis. J. Drugs Dermatol. 2015;14(5):492–494. [PubMed] [Google Scholar]
- 144.Scher R.K., Tosti A., Joseph W.S., Vlahovic T.C., Plasencia J., Markinson B.C., et al. Onychomycosis diagnosis and management: Perspectives from a joint dermatology-podiatry roundtable. J. Drugs Dermatol. 2015;14(9):1016–1021. [PubMed] [Google Scholar]
- 145.Shemer A, Gupta AK, Kamshov A, Babaev M, Farhi R, Daniel CR, et al. Topical antifungal treatment prevents recurrence of toenail onychomycosis following cure. Dermatol Ther . 2017. 30(5). [DOI] [PubMed]
- 146.Tosti A., Elewski B.E. Onychomycosis: Practical approaches to minimize relapse and recurrence. Skin Appendage Disord. 2016;2(1-2):83–87. doi: 10.1159/000448056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kemna M.E., Elewski B.E. A U.S. epidemiologic survey of superficial fungal diseases. J. Am. Acad. Dermatol. 1996;35(4):539–542. doi: 10.1016/S0190-9622(96)90675-1. [DOI] [PubMed] [Google Scholar]
- 148.Al-Hatmi A.M., Bonifaz A., Calderón L., Curfs-Breuker I., Meis J.F., van Diepeningen A.D., et al. Proximal subungual onychomycosis caused by Fusarium falciforme successfully cured with posaconazole. Br. J. Dermatol. 2015;173(1):253–255. doi: 10.1111/bjd.13589. [DOI] [PubMed] [Google Scholar]
- 149.Elewski B., Pollak R., Ashton S., Rich P., Schlessinger J., Tavakkol A. A randomized, placebo- and active-controlled, parallel-group, multicentre, investigator-blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br. J. Dermatol. 2012;166(2):389–398. doi: 10.1111/j.1365-2133.2011.10660.x. [DOI] [PubMed] [Google Scholar]
- 150.Garvey E.P., Hoekstra W.J., Moore W.R., Schotzinger R.J., Long L., Ghannoum M.A. VT-1161 dosed once daily or once weekly exhibits potent efficacy in treatment of dermatophytosis in a guinea pig model. Antimicrob. Agents Chemother. 2015;59(4):1992–1997. doi: 10.1128/AAC.04902-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sigurgeirsson B., van Rossem K., Malahias S., Raterink K. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J. Am. Acad. Dermatol. 2013;69(3):416–425. doi: 10.1016/j.jaad.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 152.Watanabe S., Tsubouchi I., Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: A multicenter, double-blind, randomized Phase III study. J. Dermatol. 2018;45(10):1151–1159. doi: 10.1111/1346-8138.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yamaguchi H. Potential of ravuconazole and its prodrugs as the new oral therapeutics for onychomycosis. Med. Mycol. J. 2016;57(4):E93–E110. doi: 10.3314/mmj.16-00006. [DOI] [PubMed] [Google Scholar]
- 154.Mercer D.K., Stewart C.S., Miller L., Robertson J., Duncan V.M.S., O’Neil D.A. Improved methods for assessing therapeutic potential of antifungal agents against dermatophytes and their application in the development of NP213, a novel onychomycosis therapy candidate. Antimicrob. Agents Chemother. 2019;63(5):e2117–e2118. doi: 10.1128/AAC.02117-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Viant P. Antifungal preparations intended for topical treatment of onychomycosis. US20180185490 (2018).
- 156.Zderic V., Kline-Schoder A., Lee Z. Ultrasound-enhanced drug delivery for treatment of onychomycosis. US20180092840 (2018). [DOI] [PubMed]
- 157.Mizutani M., Tanaka T., Ogino H., Akazawa M. Onychomycosis therapeutic agent. WO2018110693 (2018).
- 158.Lundahl S. Method of treating onychomycosis. US20160220674 (2016).
- 159.Lipner S.R., Friedman G., Scher R.K. Pilot study to evaluate a plasma device for the treatment of onychomycosis. Clin. Exp. Dermatol. 2017;42(3):295–298. doi: 10.1111/ced.12973. [DOI] [PubMed] [Google Scholar]
- 160.Heinlin J., Maisch T., Zimmermann J.L., Shimizu T., Holzmann T., Simon M., Heider J., et al. Contact-free inactivation of Trichophyton rubrum and Microsporum canis by cold atmospheric plasma treatment. Future Microbiol. 2013;8(9):1097–1106. doi: 10.2217/fmb.13.86. [DOI] [PubMed] [Google Scholar]
- 161.Roe J.N., Grammer T.C., Barrera-Barraza R.I., Tridas E. Onychomycosis treatment system and method. US20170189349 (2017).& WO2018175327 (2018).
- 162.Fernandez A.G., Leon M.F. Method for preparing product based on leaves of the Sedum telephium plant for the treatment of nails infected with onychomycosis. US20190167744 (2019).
- 163.Sonthalia S., Jakhar D., Yadav P., Kaur I. Chemical peeling as an innovative treatment alternative to oral antifungals for onychomycosis in special circumstances. Skin Appendage Disord. 2019;5(3):181–185. doi: 10.1159/000495152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Veiga F.F., Costa M.I., Cótica É.S.K., Svidzinski T.I.E., Negri M. Propolis for the treatment of onychomycosis. Indian J. Dermatol. 2018;63(6):515–517. doi: 10.4103/ijd.IJD_365_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Veiga F.F., Gadelha M.C., da Silva M.R.T., Costa M.I., Kischkel B., de Castro-Hoshino L.V., et al. Propolis extract for onychomycosis topical treatment: From bench to clinic. Front. Microbiol. 2018;9:779. doi: 10.3389/fmicb.2018.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mailland F., Caserini M., Ceriani D. Method to treat onychomycosis by hydroxypropyl chitosan. US20160213705 (2016) & US20170056386 (2017).
- 167.Gupta A.K., Daigle D., Carviel J.L. The role of biofilms in onychomycosis. J. Am. Acad. Dermatol. 2016;74(6):1241–1246. doi: 10.1016/j.jaad.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 168.Gupta A.K., Carviel J., Shear N.H. Antibiofilm treatment for onychomycosis and chronic fungal infections. Skin Appendage Disord. 2018;4(3):136–140. doi: 10.1016/j.jaad.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carlson R.P., Taffs R., Davison W.M., Stewart P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008;19(8):1035–1046. doi: 10.1163/156856208784909372. [DOI] [PubMed] [Google Scholar]
- 170.Granger B.L., Flenniken M.L., Davis D.A., Mitchell A.P., Cutler J.E. Yeast wall protein 1 of Candida albicans. Microbiology. 2005;151(Pt 5):1631–1644. doi: 10.1099/mic.0.27663-0. [DOI] [PubMed] [Google Scholar]
- 171.Rajendran R., Williams C., Lappin D.F., Millington O., Martins M., Ramage G. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot. Cell. 2013;12(3):420–429. doi: 10.1128/EC.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Flores F.C., Rosso R.S., Cruz L., Beck R.C., Silva C.B. An innovative polysaccharide nanobased nail formulation for improvement of onychomycosis treatment. Eur. J. Pharm. Sci. 2017;100:56–63. doi: 10.1016/j.ejps.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 173.Costa-Orlandi C.B., Mordorski B., Baltazar L.M., Mendes-Giannini M.J.S., Friedman J.M., Nosanchuk J.D., et al. Nitric oxide releasing nanoparticles as a strategy to improve current onychomycosis treatments. J. Drugs Dermatol. 2018;17(7):717–720. [PubMed] [Google Scholar]
- 174.Krainbring V.G.A. Composition for treating onychomycosis.WO2019105793 (2019). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.