Abstract
Amniotic band syndrome is an unusual congenital condition characterized by manifestations of disfigurement and disablement. Patients with this condition may experience an array of clinical deformities, including constriction rings, digital defects, and even visceral defects. Although this disease has been identified for centuries, its etiology is still unknown. The present male patient was born by cesarean section at 34 weeks and 4 days of gestation. At birth, an amniotic band that encircled and constricted his right upper limb was observed. Four hours after the amniotic band was cut off, amputation was performed because the right limb remained insensate. The patient suffered from amniotic band syndrome and presented with a gangrenous limb leading to amputation at birth, which is extremely rare. Moreover, the patient’s mother suffered from a uterine septum, which has not been previously reported in this situation. Timely surgical treatment avoided further tissue necrosis threating the patient’s life. This rare case of amniotic band syndrome provides new clinical evidence for the “extrinsic theory”.
Keywords: Amniotic constriction band syndrome, septate uterus, amputation, misoprostol, extrinsic theory, neonate
Introduction
Amniotic band syndrome is an unusual congenital abnormality characterized by a series of clinical manifestations, which range from constriction rings to amputations and to spontaneous abortion. This syndrome is sporadic, with a morbidity of approximately 1:1200 to 1:15,000 live births.1–4 The present report describes a case of amniotic band syndrome with the upper third of the right upper extremity encircled and constricted by an amniotic band. This eventually led to amputation at 4 hours after birth. The patient’s mother had a history of uterine septum, which has never been reported in this situation. This rare case of amniotic band syndrome provides new clinical evidence for the “extrinsic theory”.
Case presentation
A 24-year-old pregnant woman (gravida 3, para 0) with a 6-year history of septate uterus presented to the emergency room with premature rupture of the membranes and the threat of premature labor for 9 hours. In her personal medical history, she had used misoprostol for medical abortion 6 and 3 years previously. There was no history of smoking, alcoholic beverage consumption, or drug abuse during the pregnancy, and no hereditary anomalies were identified in her family.
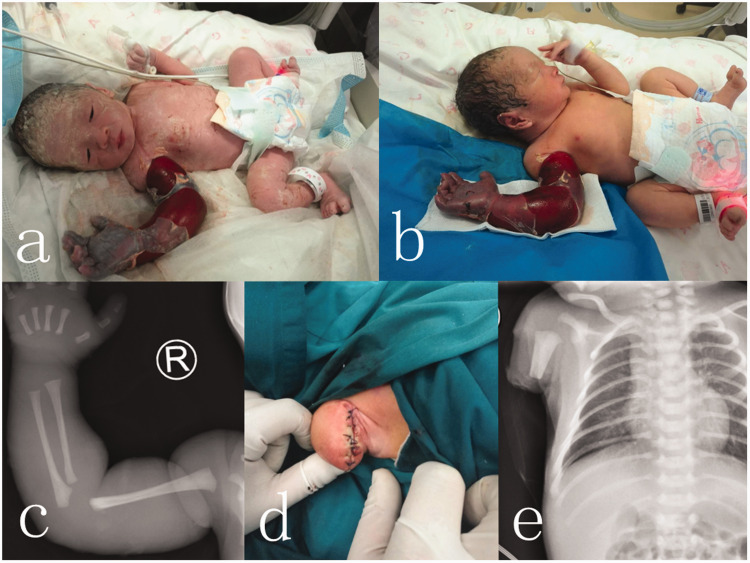
A male neonate who weighed 2400 g was born at 34 weeks and 4 days of gestation by cesarean section. His Apgar scores were 10 at 1 minute and 10 at 5 minutes. At birth, we noticed an amniotic band that consisted of a layer of annular amniotic tissue approximately 1 cm wide, which was tightened around the upper third of the right upper limb. The distal limb displayed cyanoderma, edema of the extremity, and focal peeling, with no autonomous activities or reactions of tenderness (Figure 1a). An X-ray showed a negative result for the humerus, but clear swelling of the soft tissues (Figure 1c). During the operation, the space between the amniotic band and upper limb skin was carefully separated and the band was cut off directly. The neonate was then transferred to the neonatal intensive care unit. Four hours later, swelling of the right limb was further aggravated, the radial artery could not be reached, and the local skin color did not improve. Hypoperfusion of the limb was continued and the limb remained insensate without motor compromise (Figure 1b). We considered the possibility that further necrosis of the limb and local toxin absorption could lead to death of the neonate. We decided to amputate the upper third of the right upper extremity. The patient was relatively stable during anesthesia and successfully underwent surgery (Figure 1d). No severe complications occurred after the operation. The incision was almost healed without infection, dehiscence, or other complications, as observed by postoperative X-ray images (Figure 1e). The neonate recovered rapidly and was discharged 9 days after surgery. The stitches were removed 14 days after surgery. The patient was followed up at 1, 3, and 6 months post-surgery, with no complaints of pain or clinical evidence of infection or further limb necrosis.
Figure 1.
At birth, the distal limb showed cyanoderma, edema of the extremity, and focal peeling (a). After 4 hours, the right limb continued to show signs of hypoperfusion and more edema without any compromise (b). X-ray result before surgery (c). The residual limb after surgery (d). Postoperative X-ray image (e).
Discussion
Since the term amniotic constriction band was originally mentioned by Montgomery in 1832,5 it has been extensively used to represent a wide variety of associated congenital abnormalities, including constriction rings of the extremities, disfigurements, and hemangiomas. Additionally, uncommon manifestations of this condition include complete absence of a limb, a short umbilical cord, craniofacial deformities, a defect of the neural tube, cranial defects, scoliosis, and body wall defects, such as gastroschisis and an extrathoracic heart.6,7
The extent of constrictions of the amniotic band was first defined in 1961 by Patterson as follows.8 (i) There is involvement of simple annular constriction of the extremities. Despite the presence of subcutaneous tissue defects at the level of the ring, the extremity distal to the ring is normal. (ii) A constriction ring with a distal deformity is present, including atrophy and lymphedema. These manifestations are representative of lymphatic or neurovascular disruption, which result from the ring. (iii) There is the presence of acrosyndactyly or fenestrated syndactyly, which is distal cutaneous fusion with separation of the proximal digits. (iv) Finally, there is involvement of amputation at any level of the extremity or digit.
The pathogenesis of constriction rings has been disputed by many researchers for centuries. However, there are three main theories of the etiology of an amniotic constriction band, including the intrinsic theory, extrinsic theory, and vascular theory. The intrinsic theory was presented by Streeter in 1930,9 who considered that both anomalies and fibrous bands originate from perturbation of a developing germinal disc in the early embryo. This theory is usually used to account for major craniofacial abnormalities, body wall defects, and internal organ abnormalities. The extrinsic theory was proposed by Torpin in 1965,10 who suggested that rupture of the amniotic sac is responsible for the formation of amniochorionic mesodermal bands leading to development of an amniotic constriction band.11–13 The vascular theory was described by Van Allen in 1981, who claimed that internal and external defects can result from vascular disruption. A number of traumatic events, including direct trauma, rupture of the amnion, amniocentesis, or teratogen exposure at 4 to 6 weeks of gestation, may restrain fetal development. This effect on development is achieved by disrupting the blood supply, thereby leading to hemorrhagic necrosis and embryonic circulatory collapse. The timing of the insult determines the presenting abnormality.14,15 Several reports have provided evidence that vascular compromise may be the underlying cause of craniofacial and abdominal wall defects.12,16–18
The pathogenesis of an amniotic constriction band has remained a topic in the literature for centuries without a firm conclusion. Each theory of this pathogenesis has limitations. The extrinsic theory appears to apply to our rare case. We clearly observed an amniotic band encircling and constricting the patient’s right upper extremity at birth. Furthermore, the pregnancy of the patient’s mother was complicated by a septate uterus. The uterine space may have been much smaller compared with the uterus of a healthy individual. Intrauterine pressure increases with growth of the fetus, restricting embryonic development and amniotic fluid production, and thereby promoting rupture of the amnion and amniotic sac. Amniotic bands may result from premature rupture of the amniotic sac, during which amniotic fluid may disappear and the fetus passes, at least in part, into the chorionic cavity, resulting in amputation.
Sentilhes et al.19 described a pregnant woman who took misoprostol at 9 weeks of pregnancy. This resulted in a nonviable fetus with amniotic band presentation of the upper limb requiring amputation and other malformations. Despite temporal differences in medical knowledge compared with our case, a history of misoprostol abuse may be another cause of amniotic constriction band syndrome.
Conclusion
Our patient suffered from amniotic band syndrome and presented with a gangrenous limb, which led to amputation at birth, which is extremely rare. Moreover, a maternal septate uterus in this situation has not yet been reported. Our timely surgical treatment avoided further tissue necrosis threating the patient’s life. This rare case of amniotic band syndrome provides new clinical evidence for the external theory.
Authors’ contributions
YJS and HX performed the operation and drafted the manuscript. PS participated in the design of the study and data collection. XW performed statistical analysis and a literature search. TH conceived the study, and participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Availability of data and supporting materials
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
This article does not contain any studies with animal subjects. All procedures followed were in accordance with the ethical standards of the Ethics Committee of Yantai Yuhuangding Hospital and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from the patient’s parents for being included in the study. Written informed consent for publication of the patient’s clinical details and clinical images was obtained from the parents of the patient.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Yujie Sun https://orcid.org/0000-0001-7767-7552
References
- 1.Taub PJ, Bradley JP, Setoguchi Y, et al. Typical facial clefting and constriction band anomalies: an unusual association in three unrelated patients. Am J Med Genet A 2003; 120: 256–260. [DOI] [PubMed] [Google Scholar]
- 2.Murasakas JK, Mcdannell JF, Chudik RJ, et al. Amniotic band syndrome with significant orofacial clefts and disruptions and distortions of craniofacial structures. J Pediatr Surg 2003; 38: 635–638. [DOI] [PubMed] [Google Scholar]
- 3.Morovic CG, Berwart F, Varas J. Claniofacial anomalies of the amniotic band syndrome in serial clinical cases. Plast Reconstr Surg 2004; 113: 1556–1562. [DOI] [PubMed] [Google Scholar]
- 4.Coady MS, Moore MH, Wallis K. Amniotic band syndrome: the association between rare facial clefts and limb ring constrictions. Plast Reconstr Surg 1998; 101: 640–649. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery WF. Observations on the spontaneous amputations of the limbs of the foetus in utero, with an attempt to explain the occasional cause of its production. Dublin J Med Chem Sci 1832; 1: 140–144. [Google Scholar]
- 6.Jones K. Smith’s recognizable patterns of human malformation. 6th ed Philadelphia: WB Saunders, 2005. pp. 1–976. [Google Scholar]
- 7.Robin NH, Franklin J, Prucka S, et al. Clefting, amniotic bands, and polydactyly: a distinct phenotype that supports an intrinsic mechanism for amniotic band sequence. Am J Med Genet A 2005; 137: 298–301. [DOI] [PubMed] [Google Scholar]
- 8.Patterson TJ. Congenital ring-constrictions. Br J Plast Surg 1961; 14: 1–31. [DOI] [PubMed] [Google Scholar]
- 9.Streeter GL. Focal deficiencies in fetal tissues and their relation to intrauterine amputations. Contrib Embryol Carnegie Inst 1930; 22: 1–44. [Google Scholar]
- 10.Torpin R. Amniochorionic mesoblastic fibrous strings and amnionic bands: associated constricting fetal malformations or fetal death. Am J Obstet Gynecol 1965; 91: 65–75. [DOI] [PubMed] [Google Scholar]
- 11.Levy PA. Amniotic bands. Pediatr Rev 1998; 19: 249. [DOI] [PubMed] [Google Scholar]
- 12.Lockwood C, Ghidini A, Romero R, et al. Amniotic band syndrome: re-evaluation of its pathogenesis. Am J Obstet Gynecol 1989; 160: 1030–1033. [DOI] [PubMed] [Google Scholar]
- 13.Kim JB, Berry MG, Watson JS. Abdominal constriction band: A rare location for amniotic band syndrome. J Plast Reconstr Aesthet Surg 2007; 60: 1241–1243. [DOI] [PubMed] [Google Scholar]
- 14.Higginbottom MC, Jones KL, Hall BD, et al. The amniotic band disruption complex: timing of amniotic rupture and variable spectra of consequent defects. J Pediatr 1979; 95: 544–549. [DOI] [PubMed] [Google Scholar]
- 15.Webster WS, Lipson AH, Brown-Woodman PD. Uterine trauma and limb defects. Teratology 1987; 35: 253–260. [DOI] [PubMed] [Google Scholar]
- 16.Herva R, Karkinen-Jääskeläinen M. Amniotic adhesion malformation syndrome: fetal and placental pathology. Teratology 1984; 29: 11–19. [DOI] [PubMed] [Google Scholar]
- 17.Hunter AGW, Carpenter BF. Implication of malformations not due to amniotic bands in the amniotic band sequence. Am J Med Genet 1986; 24: 691–700. [DOI] [PubMed] [Google Scholar]
- 18.Van Allen MI, Curry C, Gallager L. Limb body wall complex: I, pathogenesis. Am J Med Genet 1987. a; 28: 529–548. [DOI] [PubMed] [Google Scholar]
- 19.Sentilhes L, Patrier S, Chouchene S, et al. Amniotic band syndrome with limb amputation after exposure to mifepristone in early pregnancy. Fetal Diagn Ther 2007; 22: 51–54. [DOI] [PubMed] [Google Scholar]