Abstract
Background:
The anterior bundle (AB) of the ulnar collateral ligament is the most important structure for valgus stabilization of the elbow. However, anatomic relationships among the AB, posterior bundle (PB) of the ulnar collateral ligament, and common tendon (CT) of the flexor-pronator muscles have not been fully clarified.
Purpose:
To classify the AB, PB, and CT and to clarify their morphological features.
Study Design:
Descriptive laboratory study.
Methods:
This investigation examined 56 arms from 31 embalmed Japanese cadavers. The CT investigation examined 34 arms from 23 embalmed Japanese cadavers with CTs remaining. Type classification was performed by focusing on positional relationships with surrounding structures. Morphological features measured were length, width, thickness, and footprint for the AB and PB and attachment length, thickness, and footprint for the CT.
Results:
The AB was classified as type I (44 elbows; 78.6%), can be separated as a single bundle, or type II (12 elbows; 21.4%), cannot be separated from the PB and joint capsule. The PB was classified as type I (28 elbows; 50.0%), can be separated as a single bundle; type IIa (6 elbows; 10.7%), posterior edge cannot be separated; type IIb (7 elbows; 12.5%), anterior edge cannot be separated; or type III (15 elbows; 26.8%), cannot be separated from the joint capsule. The CT was classified as type I (18 elbows; 52.9%), can be separated from the AB, or type II (16 elbows; 47.1%), cannot be separated from the AB. Significant differences in frequencies of AB, PB, and CT types were identified between men and women. Morphological features were measured only for type I of each structure, and reliability was almost perfect.
Conclusion:
These results suggest that the AB, PB, and CT each can be classified into an independent form and an unclear form. Presence of the unclear form was suggested as one factor contributing to morphological variation.
Clinical Relevance:
This study may provide basic information for clarifying functional roles of the AB, PB, and CT.
Keywords: elbow, anatomy, baseball, ulnar collateral ligament injury
Injury to the ulnar collateral ligament (UCL) is common among throwing athletes.5 The mechanism of UCL injury is thought to involve repeated valgus stress on the elbow during throwing motions.25 The UCL is composed of the anterior bundle (AB), the posterior bundle (PB), and the transverse bundle. Of these, the AB is the most important for valgus stabilization of the elbow.18
The site of UCL injury is generally the AB, and a recent study showed damage to the anterior and posterior bands of the AB.27 This distribution is attributed to strain on the anterior and posterior bands of the AB through the full range of joint motion27 because strain during elbow flexion and extension differs for each band.27
The AB is functionally divisible into anterior, central, and posterior bands.29 In the anterior and central bands, strain reportedly increases with elbow flexion and extension movements6,7,36 and is constant through the full range of motion.6,7,24 Strain on the posterior band has been shown to increase with elbow flexion movements.6,7,24,36 PB strain reportedly increases with elbow flexion movements.36 This may be related to differences in anatomic features.
Anatomic studies have reported variations in the form of AB,8,20 and no consensus on the definition of the PB has been established.28 In recent years, some reports have stated that the AB and common tendon (CT) of the flexor-pronator muscles can be separated macroscopically,30 whereas other studies have stated that they cannot.23 Anatomic features of the medial elbow thus appear inconsistent. Other ligaments and tendons have been reported to function differently because of differences in anatomic features.13,14 A detailed examination of the anatomic characteristics of the AB, PB, and CT may thus provide useful information to clarify the functional roles of each structure. The purpose of this study was to classify the AB, PB, and CT and to clarify their morphological features.
Methods
Cadavers
This investigation examined 56 elbows from 31 Japanese cadavers (mean age at death, 82 ± 11 years; 36 sides from men, 20 sides from women; 28 right sides, 28 left sides) donated to the university anatomy program. No CT remained in 22 of these 56 elbows. The CT was therefore investigated in the remaining 34 elbows from 23 Japanese cadavers (mean age at death, 81 ± 11 years; 27 sides from men, 7 sides from women; 16 right sides, 18 left sides). All cadavers were placed in 10% formalin and then dehydrated in alcohol. No sides showed signs of previous major surgery around the upper extremity.
Procedures
Referring to previous studies23,30 for dissection, we sectioned the humerus and forearm at their midpoints to prepare an isolated elbow joint. The skin, subcutaneous tissue, and muscular parts (pronator teres, flexor carpi radialis, palmaris longus [PL], flexor digitorum superficialis [FDS], flexor carpi ulnaris [FCU], biceps brachii, brachialis, and triceps brachii muscles) were then removed, and the AB, PB, anterior common tendon (ACT), and posterior common tendon (PCT) were carefully dissected. Specimens were then moved through elbow flexion and extension, and the AB, PB, and CT were classified by positional relationships with surrounding structures. Regarding the positional relationships with surrounding structures, characterization of the AB focused on the positional relationships with the PB and joint capsule, characterization of the PB focused on the positional relationship with the joint capsule, and characterization of the CT focused on the positional relationship with the AB.
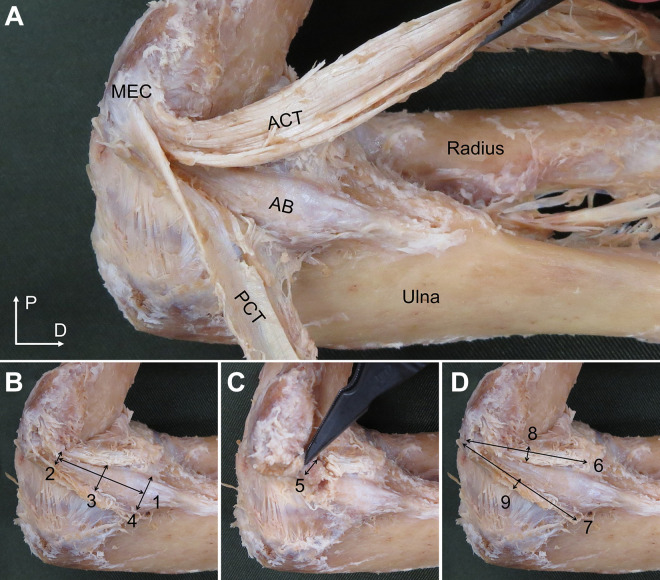
Morphological measurements were performed by 2 examiners, with 1 examiner (M.I.) taking the measurement and the other examiner (K.M. or S.S.) ensuring that the specimen did not move. All measurements were performed with the cadaveric elbow in 90° of flexion/forearm supination, in accordance with a previous study.9 Morphological features of the AB and PB that were measured included length, width, thickness, and footprint. Morphological features of the ACT and PCT that were measured included attachment length, thickness, and footprint (Figures 1 and 2).
Figure 1.
Sites of measurement of morphological features (left side, medial view). (A) Dissections of the anterior bundle (AB), anterior common tendon (ACT), and posterior common tendon (PCT). D, distal; MEC, medial epicondyle of the humerus; P, proximal. (B) Measurement site of AB length and width. (C) Measurement site of AB thickness. (D) Measurement site of attachment length and thickness of the ACT and PCT. AB length (1); AB width (proximal site) (2); AB width (intermediate site) (3); AB width (distal site) (4); AB thickness (intermediate site) (5); ACT attachment length (6); PCT attachment length (7); ACT thickness (intermediate site) (8); PCT thickness (intermediate site) (9).
Figure 2.
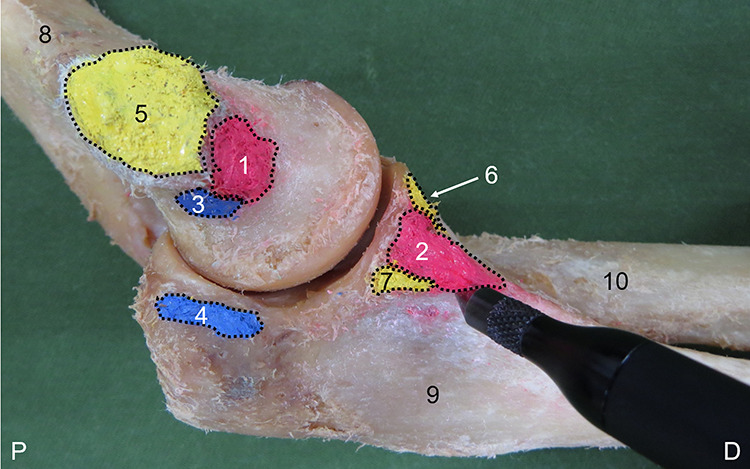
Measurement of the footprint using the MicroScribe system (left side, medial view). Anterior bundle (AB) origin (1); AB insertion (2); posterior bundle (PB) origin (3); PB insertion (4); anterior common tendon (ACT) and posterior common tendon (PCT) humeral attachment (5); ACT ulnar attachment (6); PCT ulnar attachment (7); humerus (8); ulna (9); radius (10). D, distal; P, proximal.
AB and PB length, width, and thickness and ACT and PCT attachment length and thickness were measured using digital calipers (model IP54; Shinwa Rules). AB and PB lengths were measured by connecting the midpoint of the origin and insertion. ACT and PCT humeral attachments were structures that could not be separated from each other. The mixed ACT and PCT attachment was therefore designated as the humeral attachment. ACT and PCT attachment lengths were measured by connecting the proximal end of the humeral attachment and the distal end of the ulnar attachment. AB and PB widths were measured at 3 sites: a proximal site (humeral attachment), intermediate site, and distal site (ulnar attachment). Thickness was measured at the intermediate site of each. The footprint was marked using a marking pen, and the circumference of the footprint was digitized at about 2-mm intervals through use of the MicroScribe system (G2XSYS; Revware) with reference to a previous study (Figure 2).9 A computational mesh was then created from the digitized points, and the footprint was calculated as the total area of the plane of the resulting computational mesh. Rhinoceros 3D software (McNeel) was used to analyze the footprint. All measurements were made by the same examiner (M.I.); each site was measured 3 times, and the mean value and SD was then calculated.
This study examined the intrarater reliability of morphological characteristics. Retesting was performed at an interval of 3 to 7 days and involved moving the specimen and then repositioning it before repeated measurement. The one exception was for retest of the footprint, which was performed the same day because ink bled over time and could be overestimated during digitization.
Statistical Analysis
Intersession measurement reliability was assessed using the intraclass correlation coefficient (ICC) (1,3). The criteria for ICC were as follows26: <0.00 = poor; 0.00-0.20 = slight; 0.21-0.40 = fair; 0.41-0.60 = moderate; 0.61-0.80 = substantial; and 0.81-1.00 = almost perfect. Minimal detectable difference at the 95% CI (MDD95%) was calculated as follows34: MDD95% = z × SEM × √2, where z = 1.96 and SEM = SD√(1−ICC).
The Fisher exact test was used for comparisons between male and female specimens and between left and right arms for the AB, PB, and CT types, and multiple comparisons were performed through use of the Ryan nominal level for post hoc testing. Statistical analyses were performed using SPSS (Version 26.0; SPSS Japan Inc., Tokyo, Japan). The level of statistical significance was P < .05.
Results
Intrarater Reliability and MDD95% of Morphological Characteristics for Type I
The ICC(1,3) for the measurement of morphological characteristics for type I was 0.846 to 0.999 (Table 1). In this study, measurement of the morphological characteristics for type I showed almost perfect reliability, according to the criteria of Landis and Koch.26
Table 1.
Intrarater Reliability and MDD95% of Morphological Characteristics for Type Ia
| First Rater | Second Rater | ICC (1,3) | MDD95% | |
|---|---|---|---|---|
| Anterior bundle | ||||
| Length, mm | 21.8 | 21.6 | 0.948 | 2.1 |
| Width, mm | ||||
| Proximal | 3.9 | 3.9 | 0.928 | 0.5 |
| Middle | 4.8 | 4.8 | 0.948 | 0.6 |
| Distal | 6.5 | 6.5 | 0.967 | 0.8 |
| Thickness, mm | 1.9 | 1.8 | 0.872 | 0.4 |
| Footprint, mm2 | ||||
| Humeral | 24.8 | 25.2 | 0.980 | 3.3 |
| Ulnar | 79.9 | 80.6 | 0.992 | 5.3 |
| Posterior bundle | ||||
| Length, mm | 12.6 | 12.7 | 0.982 | 0.8 |
| Width, mm | ||||
| Proximal | 4.4 | 4.5 | 0.919 | 0.8 |
| Middle | 4.7 | 5.0 | 0.912 | 1.1 |
| Distal | 6.9 | 6.9 | 0.895 | 1.6 |
| Thickness, mm | 1.0 | 1.0 | 0.927 | 0.2 |
| Footprint, mm2 | ||||
| Humeral | 18.7 | 18.8 | 0.998 | 0.4 |
| Ulnar | 10.8 | 11.3 | 0.999 | 0.2 |
| Anterior common tendon | ||||
| Length, mm | 32.8 | 32.5 | 0.930 | 2.6 |
| Thickness, mm | 2.4 | 2.4 | 0.846 | 0.6 |
| Footprint, mm2 | ||||
| Humeral | 109.2 | 108.2 | 0.987 | 9.5 |
| Ulnar | 30.7 | 31.6 | 0.987 | 4.7 |
| Posterior common tendon | ||||
| Length, mm | 37.3 | 37.4 | 0.989 | 1.2 |
| Thickness, mm | 1.0 | 1.0 | 0.983 | 0.1 |
| Footprint, mm2 | ||||
| Humeral | 109.2 | 108.2 | 0.987 | 9.5 |
| Ulnar | 23.3 | 23.9 | 0.970 | 4.4 |
aICC, intraclass correlation coefficient; MDD95%, minimal detectable difference at the 95% CI.
Classification of the AB, PB, and CT
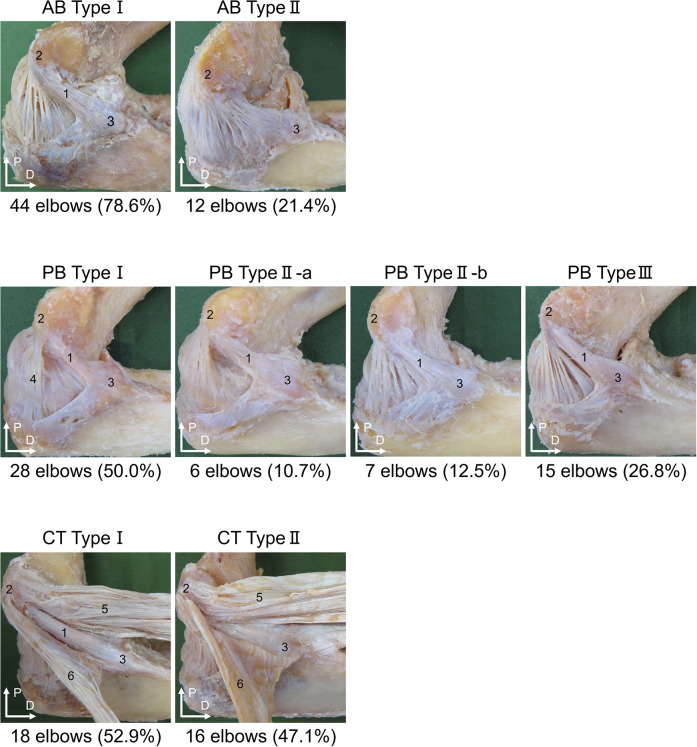
The AB was classified as follows: type I (44 elbows; 78.6%), the AB was located superficial to the PB and joint capsule and could be separated as a single bundle, or type II (12 elbows; 21.4%), the AB was located in the same layer as the PB and joint capsule and could not be separated from them (Figure 3). Regarding sex differences, AB type II was only observed in male specimens, with female specimens only demonstrating AB type I (Table 2). No significant differences were seen between right and left arms (P = .165).
Figure 3.
Classification of the anterior bundle (AB), posterior bundle (PB), and common tendon (CT) (left side, medial view). AB type I: The AB is located superficial to the PB and joint capsule and can be separated as a single bundle. AB type II: The AB is located in the same layer as the PB and joint capsule and cannot be separated from them. PB type I: The anterior and posterior edges of the PB are located on the surface of the joint capsule and can be separated as a single bundle. PB type IIa: The anterior edge of the PB can be separated from the joint capsule, but the posterior edge cannot. PB type IIb: The posterior edge of the PB can be separated from the joint capsule, but the anterior edge cannot. PB type III: The PB cannot be separated from the joint capsule. CT type I: The AB is located superficial to the anterior common tendon (ACT) and posterior common tendon (PCT), and the ligament and tendon can be separated from each other. CT type II: The AB is located in the same layer as and cannot be separated from the ACT and PCT. Anterior bundle (1); medial epicondyle of the humerus (2); sublime tubercle of the ulna (3); posterior bundle (4); anterior common tendon (5); posterior common tendon (6). D, distal; P, proximal.
Table 2.
Left and Right Differences and Sex Differences for Each Type of AB, PB, and CTa
| AB | PB | CT | ||||||
|---|---|---|---|---|---|---|---|---|
| Type I | Type II | Type I | Type IIa | Type IIb | Type III | Type I | Type II | |
| Male | 24 (66.7)b | 12 (33.3)b | 10 (27.8) | 6 (16.7) | 7 (19.4) | 13 (36.1)c,d | 11 (40.7)f | 16 (59.3)f |
| Female | 20 (100.0)b | 0 (0.0) | 18 (90.0)c,d,e | 0 (0.0) | 0 (0.0) | 2 (10.0) | 7 (100.0)g | 0 (0.0) |
| Right | 20 (71.4) | 8 (28.6) | 12 (42.9) | 2 (7.1) | 6 (21.4) | 8 (28.6) | 8 (50.0) | 8 (50.0) |
| Left | 24 (85.7) | 4 (14.3) | 16 (57.1) | 4 (14.3) | 1 (3.6) | 7 (25.0) | 10 (55.6) | 8 (44.4) |
| Total | 44 (78.6) | 12 (21.4) | 28 (50.0) | 6 (10.7) | 7 (12.5) | 15 (26.8) | 18 (52.9) | 16 (47.1) |
aValues are expressed as n (%). AB, Anterior bundle; CT, common tendon; PB, posterior bundle.
bP < .001 vs AB type II females.
cP < .001 vs PB type IIa females.
dP < .001 vs PB type IIb females.
eP < .001 vs PB type III females.
fP < .001 vs CT type II females.
gP = .023 vs CT type II females.
The PB was classified as follows: type I (28 elbows; 50.0%), anterior and posterior edges of the PB were located superficial to the joint capsule and could be separated as a single bundle; type IIa (6 elbows; 10.7%), the anterior edge of the PB could be separated from the joint capsule, but the posterior edge could not; type IIb (7 elbows; 12.5%), the posterior edge of the PB could be separated from the joint capsule, but the anterior edge could not; and type III (15 elbows; 26.8%), no parts of the PB could be separated from the joint capsule (Figure 3). Regarding sex differences, PB type IIa and type IIb were only observed in male specimens, with female specimens only demonstrating PB type I and PB type III (Table 2). No significant differences were seen between right and left arms (P = .192).
The CT was classified as follows: type I (18 elbows; 52.9%), the AB was superficial to the ACT and PCT, with structures separable from each other; and type II (16 elbows; 47.1%), the AB was located in the same layer as the ACT and PCT, and the structures could not be separated from each other (Figure 3). Regarding sex differences, CT type II was only observed in male specimens, with female specimens only demonstrating CT type I (Table 2). No significant differences were seen between right and left arms (P = .508).
Morphological Characteristics of the AB, PB, and CT
Morphological characteristics of AB type I, PB type I, and CT type I are shown in Table 3. AB type II, PB type IIa, PB type IIb, PB type III, and CT type II were unclear and could not be measured.
TABLE 3.
Morphological Features of the Anterior Bundle, Posterior Bundle, and Common Tendon in Present Study and Previous Studiesa
| Footprint, mm2 | ||||||
|---|---|---|---|---|---|---|
| N | Length, mm | Width, mm | Thickness, mm | Humeral | Ulnar | |
| Anterior bundle | ||||||
| Morrey28 (1985) | 10 | 27.1 ± 4.3 | M: 4.7 ± 1.2 | — | — | — |
| Regan32 (1991) | 7 | 21.13 ± 2.29 | 7.6 | — | — | — |
| Timmerman35 (1994) | 10 | — | 6 | 4-8 | — | — |
| Cage2 (1995) | 20 | — | 7.9 | 2.8 | — | — |
| Floris18 (1998) | 18 | — | 5.8 (5-7) | — | — | — |
| Beckett1 (2000) | 39 | 26.7 ± 3.7 | — | — | — | — |
| Eygendaal15 (2002) | 5 | 26 (24-31) | 5 (4-7) | — | — | — |
| Gurbuz22 (2005) | 20 | R: 21.1 ± 6.3 L: 21.7 ± 5.3 |
D (R): 12.7 ± 2.8 D (L): 13.9 ± 2.4 |
— | — | — |
| Safran33 (2005) | 6 | — | 7.2 ± 1.7 | — | — | — |
| Dugas9 (2007) | 13 | — | P: 6.8 ± 1.4 M: 6.8 ± 1.3 D: 9.2 ± 1.6 |
— | 45.5 ± 9.3 | 127.8 ± 35.7 |
| Farrow16 (2011) | 10 | 53.9 ± 0.7 | — | — | — | — |
| Farrow17 (2014) | 12 | 21.5 (16.7-27.6) | — | — | — | — |
| Otoshi30 (2014) | 52 | — | P: 8.3 ± 1.2 D: 11.7 ± 1.8 |
P: 10.0 ± 1.6 D: 1.1 ± 0.1 |
— | — |
| Camp3 (2018) | 10 | — | — | — | 32.3 ± 6.8 | 187.6 ± 47.3 |
| Frangiamore19 (2018) | 10 | 21.5 (20.0-23.0) | — | — | 17.0 (14.9-19.1) | 66.4 (54.0-78.7) |
| Dutton10 (2019) | 18 | — | — | — | — | 216.9 ± 42.1 |
| Present study | 44 | 21.8 ± 3.3 | P: 3.9 ± 0.7 M: 4.8 ± 0.9 D: 6.5 ± 1.2 |
M: 1.9 ± 0.4 | 24.8 ± 8.5 | 79.9 ± 21.2 |
| Posterior bundle | ||||||
| Morrey28 (1985) | 10 | 24.2 ± 4.3 | M: 5.3 ± 1.1 | — | — | — |
| Regan32 (1991) | 7 | 16.51 ± 1.52 | 8.8 | — | — | — |
| Timmerman35 (1994) | 10 | — | 8 | 2-3 | — | — |
| Beckett1 (2000) | 39 | 23.2 ± 3.7 | — | — | — | — |
| Camp3 (2018) | 10 | — | — | — | 25.9 ± 10.0 | 15.8 ± 7.2 |
| Frangiamore19 (2018) | 10 | 15.0 (13.5-16.5) | — | — | 18.5 (13.6-23.4) | 17.6 (14.7-20.6) |
| Present study | 28 | 12.6 ± 2.2 | P: 4.4 ± 1.0 M: 4.7 ± 1.4 D: 6.9 ± 1.8 |
M: 1.0 ± 0.3 | 18.7 ± 3.5 | 10.8 ± 2.3 |
| Anterior common tendon | ||||||
| Otoshi30 (2014) | 52 | 28.3 ± 4.3 | — | 2.5 ± 0.7 | — | — |
| Present study | 18 | 32.8 ± 3.6 | — | 2.4 ± 0.5 | 106.4 ± 28.7 | 30.3 ± 15.1 |
| Posterior common tendon | ||||||
| Otoshi30 (2014) | 52 | — | — | 0.9 ± 0.3 | — | — |
| Present study | 18 | 37.3 ± 4.3 | — | 1.0 ± 0.3 | 106.4 ± 28.7 | 23.6 ± 9.4 |
aValues represent mean ± SD or range. Dashes indicate data not reported. D, distal; L, left; M, middle; P, proximal; R, right.
Discussion
This study examined morphological features of the AB, PB, and CT using Japanese cadavers. Several studies have reported anatomic features of the medial elbow. However, none have classified the AB, PB, and CT by focusing on the positional relationship with surrounding structures and characterized these morphological features.
In this study, we saw AB type I in 78.6% of specimens, PB type I in 50.0%, and CT type I in 52.9%, as the independent forms of each. In contrast, we saw AB type II in 21.4% of specimens, PB type IIa in 10.7%, PB type IIb in 12.5%, PB type III in 26.8%, and CT type II in 47.1%, as the unclear forms. Davidson et al8 reported that AB forms were cord-shaped in 9 ligaments (82%) and fan-shaped in 2 ligaments (18%). Regarding the PB, Morrey and An28 reported the PB as a thickened posterior joint capsule, with no clear definition provided. Regarding the CT, Otoshi et al30 reported that the intermuscular fascia between the humeral heads of the PT, flexor carpi radialis, palmaris longus, and FDS converged and formed a common tendon at their proximal origin (ACT), which was attached to the medial epicondyle and anterior joint capsule, just anterior and parallel to the AB. Hoshika et al23 reported that the tendinous septa between the PT and FDS, the tendinous septa between the FDS and FCU, the medial part of the brachialis tendon, and the deep FDS and FCU aponeuroses formed a tendinous complex, with the traditional AB as part of the tendinous complex and joint capsule. In the current study, the AB, PB, and CT each showed an independent form and at least 1 unclear form, and this appears to be the first study to clarify these variations.
Based on the results of type classifications in this study, the frequencies of the AB, PB, and CT forms differed significantly between men and women and also within women. Sex differences related to the elbow joint include the carrying angle and range of motion of the elbow joint, both of which have been reported as greater in women than in men.21 A phenomenon specific to female patients is the menstrual cycle, which has been reported to affect relaxation of the ligaments.31 However, relationships between these factors and forms of the AB, PB, and CT remain unclear. Further study of these issues is needed in the future.
Measurement of morphological features was performed only for AB type I, PB type I, and CT type I as the independent forms, but reliability for these was almost perfect. In previous studies (Table 3),§ morphological features of the AB and PB were not consistent. This is because limb positions at the time of measurement have included the elbow joint in extension with the forearm in supination,3,19 elbow flexion at 25°,15 elbow flexion at 90°,9 maximum tension position,28 maximum tension position in neutral position of the forearm,1 neutral position of the forearm,10 and even undescribed positions2,16-18,22,30,32,33,35 that presumably varied widely among studies. In addition, the AB is generally measured as a single bundle originating from the anteroinferior aspect of the medial epicondyle of the humerus and inserting at the sublime tubercle of the ulna.28 However, a previous study reported that the traditional AB measurement might have included a mixed construct of the joint capsule and tendinous complex as the AB.23 In the present study, the AB, PB, and CT each showed an independent form and at least 1 unclear form. Previous studies could thus have potentially measured unclear forms along with independent forms of each structure.
This study has several limitations that need to be considered when interpreting the results. First, whether donors had been involved in overhead sports during their lifetime was unknown. A previous study reported that throwing athletes show changes in the morphology of the UCL, such as UCL thickening and calcifications due to repeated throwing motions.4 Therefore, if the donor had been involved in overhead sports during life, the form of the UCL would presumably have been influenced. Second, the cadavers included in this study were only from Japanese donors, and the results cannot be generalized to different ethnicities in the absence of additional investigations. Several anatomic studies have reported ethnic differences in skeletal muscles and tendons,11,12 and this may also apply to ligaments. In addition, the cadavers used in this study were embalmed specimens, and the embalming processes could potentially have affected the quality of ligaments and tendons so as to affect dissection and apparent interconnections. Third, this study did not investigate interrater reliability. Fourth, this study was an anatomic study; future biomechanical studies using these results as basic information are necessary to clarify the functional roles of the AB, PB, and CT.
Conclusion
The AB, PB, and CT were each classified into an independent form and at least 1 unclear form. Presence of the unclear form was suggested as one of the factors causing morphological variation. In the future, biomechanical studies using these research results as basic information are needed.
Acknowledgment
The authors acknowledge and thank those anonymous individuals who generously donated their bodies so that this study could be performed.
Footnotes
Final revision submitted March 30, 2020; accepted April 14, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI grant No. JP19K11358 and a grant-in-aid program from Niigata University of Health and Welfare. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Beckett KS, McConnell P, Lagopoulos M, Newman RJ. Variations in the normal anatomy of the collateral ligaments of the human elbow joint. J Anat. 2000;197(pt 3):507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cage DJ, Abrams RA, Callahan JJ, Botte MJ. Soft tissue attachments of the ulnar coronoid process: an anatomic study with radiographic correlation. Clin Orthop Relat Res. 1995;320:154–158. [PubMed] [Google Scholar]
- 3. Camp CL, Jahandar H, Sinatro AM, Imhauser CW, Altchek DW, Dines JS. Quantitative anatomic analysis of the medial ulnar collateral ligament complex of the elbow. Orthop J Sports Med. 2018;6(3):2325967118762751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ciccotti MG, Atanda A, Jr, Nazarian LN, Dodson CC, Holmes L, Cohen SB. Stress sonography of the ulnar collateral ligament of the elbow in professional baseball pitchers: a 10-year study. Am J Sports Med. 2014;42(3):544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ciccotti MG, Pollack KM, Ciccotti MC, et al. Elbow injuries in professional baseball: epidemiological findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2017;45(10):2319–2328. [DOI] [PubMed] [Google Scholar]
- 6. Ciccotti MG, Siegler S, Kuri JA II, Thinnes JH, Murphy DJ. Comparison of the biomechanical profile of the intact ulnar collateral ligament with the modified Jobe and the Docking reconstructed elbow: an in vitro study. Am J Sports Med. 2009;37(5):974–981. [DOI] [PubMed] [Google Scholar]
- 7. Cohen SB, Woods DP, Siegler S, Dodson CC, Namani R, Ciccotti MG. Biomechanical comparison of graft fixation at 30 degrees and 90 degrees of elbow flexion for ulnar collateral ligament reconstruction by the docking technique. J Shoulder Elbow Surg. 2015;24(2):265–272. [DOI] [PubMed] [Google Scholar]
- 8. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245–250. [DOI] [PubMed] [Google Scholar]
- 9. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657–660. [DOI] [PubMed] [Google Scholar]
- 10. Dutton PH, Banffy MB, Nelson TJ, Metzger MF. Anatomic and biomechanical evaluation of ulnar tunnel position in medial ulnar collateral ligament reconstruction. Am J Sports Med. 2019;47(14):3491–3497. [DOI] [PubMed] [Google Scholar]
- 11. Edama M, Kubo M, Onishi H, et al. Anatomical study of toe flexion by flexor hallucis longus. Ann Anat. 2016;204:80–85. [DOI] [PubMed] [Google Scholar]
- 12. Edama M, Kubo M, Onishi H, et al. The twisted structure of the human Achilles tendon. Scand J Med Sci Sports. 2015;25(5):e497–503. [DOI] [PubMed] [Google Scholar]
- 13. Edama M, Takabayashi T, Inai T, et al. Differences in the strain applied to Achilles tendon fibers when the subtalar joint is overpronated: a simulation study. Surg Radiol Anat. 2019;41(5):595–599. [DOI] [PubMed] [Google Scholar]
- 14. Edama M, Takabayashi T, Inai T, et al. The effect of differences in the number of fiber bundles of the anterior tibial ligament on ankle braking function: a simulation study. Surg Radiol Anat. 2019;41(1):69–73. [DOI] [PubMed] [Google Scholar]
- 15. Eygendaal D, Valstar ER, Sojbjerg JO, Rozing PM. Biomechanical evaluation of the elbow using roentgen stereophotogrammetric analysis. Clin Orthop Relat Res. 2002;396:100–105. [DOI] [PubMed] [Google Scholar]
- 16. Farrow LD, Mahoney AJ, Stefancin JJ, Taljanovic MS, Sheppard JE, Schickendantz MS. Quantitative analysis of the medial ulnar collateral ligament ulnar footprint and its relationship to the ulnar sublime tubercle. Am J Sports Med. 2011;39(9):1936–1941. [DOI] [PubMed] [Google Scholar]
- 17. Farrow LD, Mahoney AP, Sheppard JE, Schickendantz MS, Taljanovic MS. Sonographic assessment of the medial ulnar collateral ligament distal ulnar attachment. J Ultrasound Med. 2014;33(8):1485–1490. [DOI] [PubMed] [Google Scholar]
- 18. Floris S, Olsen BS, Dalstra M, Sojbjerg JO, Sneppen O. The medial collateral ligament of the elbow joint: anatomy and kinematics. J Shoulder Elbow Surg. 1998;7(4):345–351. [DOI] [PubMed] [Google Scholar]
- 19. Frangiamore SJ, Moatshe G, Kruckeberg BM, et al. Qualitative and quantitative analyses of the dynamic and static stabilizers of the medial elbow: an anatomic study. Am J Sports Med. 2018;46(3):687–694. [DOI] [PubMed] [Google Scholar]
- 20. Fuss FK. The ulnar collateral ligament of the human elbow joint: anatomy, function and biomechanics. J Anat. 1991;175:203–212. [PMC free article] [PubMed] [Google Scholar]
- 21. Golden DW, Jhee JT, Gilpin SP, Sawyer JR. Elbow range of motion and clinical carrying angle in a healthy pediatric population. J Pediatr Orthop B. 2007;16(2):144–149. [DOI] [PubMed] [Google Scholar]
- 22. Gurbuz H, Kutoglu T, Mesut R, Gurbuz H. Anatomical dimensions of anterior bundle of ulnar collateral ligament and its role in elbow stability. Folia Med (Plovdiv). 2005;47(1):47–52. [PubMed] [Google Scholar]
- 23. Hoshika S, Nimura A, Yamaguchi R, et al. Medial elbow anatomy: a paradigm shift for UCL injury prevention and management. Clin Anat. 2019;32(3):379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jackson TJ, Jarrell SE, Adamson GJ, Chung KC, Lee TQ. Biomechanical differences of the anterior and posterior bands of the ulnar collateral ligament of the elbow. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2319–2323. [DOI] [PubMed] [Google Scholar]
- 25. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158–1163. [PubMed] [Google Scholar]
- 26. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 27. Molenaars RJ, van den Bekerom MPJ, Eygendaal D, Oh LS. The pathoanatomy of the anterior bundle of the medial ulnar collateral ligament. J Shoulder Elbow Surg. 2019;28(8):1497–1504. [DOI] [PubMed] [Google Scholar]
- 28. Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop Relat Res. 1985;201:84–90. [PubMed] [Google Scholar]
- 29. Ochi N, Ogura T, Hashizume H, Shigeyama Y, Senda M, Inoue H. Anatomic relation between the medial collateral ligament of the elbow and the humero-ulnar joint axis. J Shoulder Elbow Surg. 1999;8(1):6–10. [DOI] [PubMed] [Google Scholar]
- 30. Otoshi K, Kikuchi S, Shishido H, Konno S. The proximal origins of the flexor-pronator muscles and their role in the dynamic stabilization of the elbow joint: an anatomical study. Surg Radiol Anat. 2014;36(3):289–294. [DOI] [PubMed] [Google Scholar]
- 31. Park SK, Stefanyshyn DJ, Loitz-Ramage B, Hart DA, Ronsky JL. Changing hormone levels during the menstrual cycle affect knee laxity and stiffness in healthy female subjects. Am J Sports Med. 2009;37(3):588–598. [DOI] [PubMed] [Google Scholar]
- 32. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170–179. [PubMed] [Google Scholar]
- 33. Safran MR, McGarry MH, Shin S, Han S, Lee TQ. Effects of elbow flexion and forearm rotation on valgus laxity of the elbow. J Bone Joint Surg Am. 2005;87(9):2065–2074. [DOI] [PubMed] [Google Scholar]
- 34. Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J. 2013;24(3):21–29. [PMC free article] [PubMed] [Google Scholar]
- 35. Timmerman LA, Andrews JR. Histology and arthroscopic anatomy of the ulnar collateral ligament of the elbow. Am J Sports Med. 1994;22(5):667–673. [DOI] [PubMed] [Google Scholar]
- 36. Wavreille G, Seraphin J, Chantelot C, Marchandise X, Fontaine C. Ligament fibre recruitment of the elbow joint during gravity-loaded passive motion: an experimental study. Clin Biomech (Bristol, Avon). 2008;23(2):193–202. [DOI] [PubMed] [Google Scholar]