Abstract
Background:
A 3-dimensional, scaffold-free, and completely autologous form of chondrocyte transplantation (ACT3D) has been developed and applied in clinical practice in the past decade to overcome disadvantages of previous-generation procedures.
Purpose:
To document and analyze the available literature on the results of ACT3D in the treatment of articular chondral lesions in the knee and hip joints.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
All studies published in English addressing ACT3D were identified and included those that fulfilled the following criteria: (1) level 1 through 4 evidence, (2) measures of radiological or functional/clinical outcome, and (3) outcome related to cartilage lesions of the knee and hip joints.
Results:
A total of 10 studies were selected: 2 randomized controlled trials, 1 cohort study, and 7 case series. The studies revealed significant increases in patients’ subjective quality of life, satisfaction, pain reduction, and improvement in joint function at short- to medium-term follow-up. Magnetic resonance imaging-assisted examination and second-look arthroscopy showed a hyaline-like repair tissue with a high degree of defect filling and integration.
Conclusion:
ACT3D shows promising results in the therapy of articular cartilage defects in the knee as well as in the hip, but well-designed, long-term studies are lacking. ACT3D might have relevant advantages over common matrix-associated autologous chondrocyte transplantation products, but systematic evaluation and randomized controlled studies are crucial to verify the potential of this tissue-engineered approach.
Keywords: autologous chondrocyte transplantation, Chondrosphere, tissue engineering, ACT3D, Spherox
Due to the limited regeneration capacity of hyaline cartilage in human joints, articular chondral lesions present a challenging therapeutic issue in orthopaedics. During recent decades, a few techniques for articular cartilage repair became established. Microfracture, mosaicplasty (osteochondral autograft transfer), and autologous chondrocyte transplantation (ACT) are the most commonly used and most discussed methods. Among those, high expectations are placed on the further development of ACT. Since its first description by Brittberg et al7 in 1994, ACT has been continuously improved, and it is currently used as a standard procedure for the treatment of large chondral defects (>3 cm2) of the knee.17,26 The evolution of ACT entailed chondrocyte injection covered by periosteum in first-generation techniques,7 the later use of artificial collagen membranes,6 and 3-dimensional (3D) matrices introducing the third generation of ACT.8,37 The scaffolds used in matrix-associated ACT (MACT) can be adapted to the size and form of the lesion and can reduce cell loss after implantation. The potential for improvement remains because most techniques require special conditions for the application. Minimally invasive implantation through arthroscopy can be challenging in some cases. Certain regions, such as the femoral head, preclude the use of rigid scaffolds,12 whereas areas such as the ankle joint require much more invasive approaches, including osteotomy of the malleolus.35 Furthermore, most of the matrices used in MACT have to be fixed at their destination area. The extraneous fixation material might cause immune response, contamination, or healing problems. Concerning this matter, the work of Hunziker and Stähli16 indicated that suturing of articular cartilage induced severe local damage and was associated with early stages of osteoarthritis.
In 2002, Anderer and Libera3 presented an innovative 3D but scaffold-free autologous chondrocyte culture system that completely excludes xenogenous material. The culture process generates 3D spheroids of neocartilage composed of redifferentiated autologous chondrocytes and cartilage-specific matrix (further named ACT3D). Based on that, a new ACT product called Spherox (formerly called Chondrosphere) was introduced by the German company co.don AG. Like other ACT techniques, ACT3D requires a 2-step surgical procedure. In the first step, a cartilage sample for in vitro expansion is harvested from the affected joint. In a second surgery, the expanded chondrocytes are re-implanted into the defect site. Because the spheroids are totally autologous, no foreign material is used for either production or implantation. The isolated chondrocytes are cultured in patient serum without any supplements. The aggregates’ spherical structure implies certain advantages compared with those of ACT or other MACT products. Because chondrocytes in the 3D structures have started to differentiate and produce extracellular matrix, they are already in a higher developmental state compared with that of the chondrocytes in cell suspension for ACT, which show no differentiation or matrix production before implantation. Given this process, Chondrosphere may lead to faster defect filling with hyaline cartilage.22 The spheroids can be applied easily to the defect by arthroscopy, and they adhere to the subchondral bone and native cartilage tissue without additional fixation material.21 ACT3D showed promising in vivo results in several animal models25,31 and has been in clinical use since 2004.
The aim of this systematic review is to document and analyze the available literature on the results of ACT3D in the treatment of articular chondral lesions in the knee and hip joints.
Methods
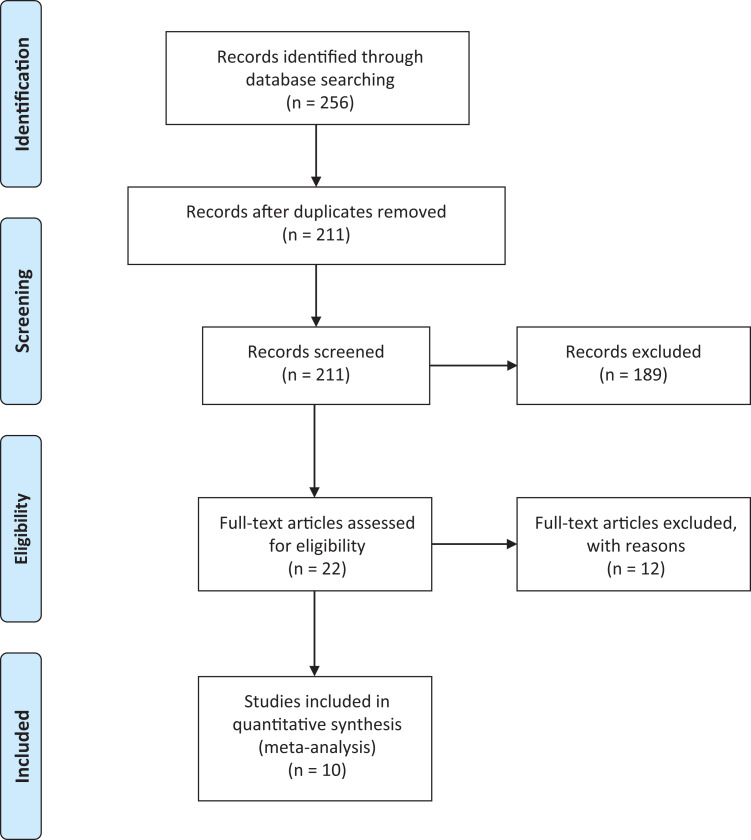
A systematic review of all studies on ACT3D using Chondrosphere and published in English was performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. We searched PubMed, Embase, and the Cochrane Library database between 2004 and January 2020, using the terms “chondrosphere,” “spherox,” “chondrocytes AND spheroid,” “ACI AND spheroids,” “ACI AND injectable,” “ACT3D,” and “3-dimensional autologous chondrocyte transplantation.” The search algorithm according to the PRISMA guidelines is shown in Figure 1. A total of 211 studies were reviewed by title and/or abstract to determine study eligibility. Studies were included in our systematic review if they fulfilled the following criteria: (1) level 1 through 4 evidence addressing the areas of interest outlined above, (2) measures of radiological or functional/clinical outcome, and (3) outcome related to joint cartilage lesions. Studies were excluded if any kind of xenogenous material or scaffold material was implanted in test group patients. Citations from relevant studies as well as any relevant articles captured by the search were also examined to determine whether they were suitable for inclusion.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2009 flow diagram.
Study Methodology Assessment
To assess the quality of the studies, we used the Modified Coleman Methodology Score (MCMS). The MCMS has a scaled potential score ranging from 0 to 100. High scores indicate that the studies largely avoid chance, various biases, and confounding factors. Scores ranging from 85 to 100 are excellent, 70 to 84 are good, 55 to 69 are fair, and <55 are poor.
Statistical Analysis
Variable data were presented as mean with the standard deviation if available. A weighted average was calculated for outcome scores based on the included studies. In all studies, P < .05 was considered statistically significant.
Results
The search included 10 studies11,12,14,15,19,20,29,33,34,36 that fulfilled the inclusion criteria: 2 randomized controlled trials,15,19 7 case series,11,12,19,20,33,34,36 and 1 cohort study.14 Of the 10 studies, 6 studies addressed ACT3D of articular cartilage defects in the knee joint,11,14,15,29,33,34 and 4 publications investigated the outcome of ACT3D therapy in affected hip joints.12,19,20,36 The mean defect size of the treated lesions ranged from 2 to 6 cm2. The included studies are presented in detail in Tables 1 through 3. For some investigations, data were published several times after different follow-up time points. We listed these works by the most recent publication and included the reference numbers for the previous articles.
Table 1.
Details of the 6 Studies Focusing on Clinical and Radiological Results of ACT3D in the Knee Jointa
| Lead Author (Year) | Joint | Patients, n | Follow-up, mo | Lesion Size, cm2 | Study Design (Level of Evidence) | MCMS |
|---|---|---|---|---|---|---|
| Hoburg (2020)15,28 | Knee | 52 ACT3D | 36 | 2.2 ± 0.7 | RCT (1) | 76 |
| 50 microfracture | 2.0 ± 0.8 | |||||
| Niemeyer (2020)5,27,29 | Knee | 75 | 48 | 5.0 ± 1.9 | RCT (1) | 86 |
| Hoburg (2019)14 | Knee | 29 adolescents | 63.3 | 4.6 ± 2.4 | Cohort study (3) | 47 |
| 42 adults | 48.4 | 4.7 ± 1.2 | ||||
| Siebold (2018)34 | Knee | 30 | 34.8 ± 10.3 | 6 ± 3.1 | Case series (4) | 71 |
| Siebold (2016)33 | Knee | 41 | 34 ± 19.2 | 4.3 ± 3.4 | Case series (4) | 61 |
| Fickert (2012)11 | Knee | 37 | 12 | 4.4 | Case series (4) | 71 |
aData are expressed as mean, with SD when available. ACT3D, 3-dimensional autologous chondrocyte transplantation; MCMS, Modified Coleman Methodology Score; RCT, randomized controlled trial.
Table 2.
Results of 3-Dimensional Autologous Chondrocyte Transplantation Performed in the Knee Joint at a Minimum Follow-up of 1 Yeara
| Score | Lead Author (Year) | Value |
|---|---|---|
| MOCART | Hoburg (2020)15,28 | 76.0 ± 16.0 (n = 46) |
| Niemeyer (2020)5,27,29 | 75.5 ± 13.1 (n = 75) | |
| Hoburg (2019) adolescents14 | 74.7 ± 12.0 (n = 29) | |
| Hoburg (2019) adults14 | 77.2 ± 11.2 (n = 42) | |
| Siebold (2018)34 | 70.9b (n = 30) | |
| Fickert (2012)11 | 70.0 (n = 37) | |
| Weighted average | 74.5 (n = 259) | |
| Tegner | Siebold (2018)34 | 5.0 (n = 30) |
| Siebold (2016)33 | 3.5 ± 1.2 (n = 31) | |
| Fickert (2012)11 | 4.0 (n = 37) | |
| Weighted average | 4.2 (n = 98) | |
| Lysholm | Hoburg (2019) adolescents14 | 87.5 ± 10.0 (n = 22) |
| Hoburg (2019) adults14 | 92.9 ± 7.9 (n = 30) | |
| Siebold (2018)34 | 77.7 ± 14.6 (n = 30) | |
| Siebold (2016)33 | 79.0 ± 18.0 (n = 31) | |
| Fickert (2012)11 | 82.5 (n = 37) | |
| Weighted average | 83.6 (n = 150) | |
| IKDC | Hoburg (2019) adolescents14 | 81.1 ± 17.7 (n = 22) |
| Hoburg (2019) adults14 | 80.5 ± 15.2 (n = 27) | |
| Siebold (2018)34 | 84.2 ± 5.6 (n = 30) | |
| Siebold (2016)33 | 63.0 ± 18.8 (n = 31) | |
| Fickert (2012)11 | 64.0 (n = 37) | |
| Weighted average | 73.5 (n = 147) | |
| KOOS (overall) | Hoburg (2020)15,28 | 83.2 ± 14.9 (n = 48) |
| Niemeyer (2020)5,27,29 | 77.1 ± 18.6 (n = 75) | |
| Hoburg (2019) adolescents14 | 82.6 ± 11.6 (n = 29) | |
| Hoburg (2019) adults14 | 84.6 ± 11.7 (n = 42) | |
| Siebold (2018)34 | 78.7 (n = 30) | |
| Weighted average | 80.7 (n = 224) |
aScores are expressed as mean, with SD when available. IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MOCART, magnetic resonance observation of cartilage repair tissue.
bSiebold et al34 used a maximum MOCART score of 85 rather than 100; to compare their results with those of the other studies, we used the percentage instead of the absolute value.
Table 3.
Details of the Studies Focusing on Clinical and Radiological Results of 3-Dimensional Autologous Chondrocyte Transplantation in the Hip Jointa
| Lead Author (Year) | Joint | Patients, n | Follow-up, mo | Lesion Size, cm2 | Study Design (Level of Evidence) | MCMS |
|---|---|---|---|---|---|---|
| Krueger (2018)20 | Hip | 32 | 35.5 | 4.9 | Case series (4) | 76 |
| Thier (2017)36 | Hip | 29 | 19 | 2.21 | Case series (4) | 69 |
| Körsmeier (2016)19 | Hip | 16 | 16.1 ± 5.3 | 4.5 ± 1.1 | Case series (3) | 67 |
| Fickert (2014)12 | Hip | 6 | 12 | 3.5 | Case series (4) | 57 |
aData are expressed as mean, with SD when available. MCMS, Modified Coleman Methodology Score.
Methodological Evaluation
MCMS was used to rate the methodological quality of the reviewed studies. The mean ± SD score was 68.1 ± 11.0. With exception of the 2 randomized controlled trials, the overall methodological rating was rather low. This is mainly because of the majority of small case series with low numbers of patients and relatively short follow-up times, and it illustrates the lack of highly evidential studies.
ACT3D in the Knee Joint
Fickert et al11 published the first clinical data on the innovative 3D autologous chondrocyte implantation (ACI) technique in 2012. In patients who underwent ACT3D, an overall statistically significant improvement for the evaluated patient-administered assessment scores was found 1 year after implantation (Table 2). Furthermore, magnetic resonance imaging (MRI) examinations revealed a very good integration of the repair tissue and an increase of the MRI-assessed MOCART (magnetic resonance observation of cartilage repair tissue) score. This publication was followed by several studies providing promising short- to medium-term results using ACT3D in the knee joint.
Investigation of the repair tissue using second-look arthroscopy and T2-weighted MRI mapping attested to the high defect filling and integration properties of ACT3D. The appearance of the repair tissue was found to resemble normal cartilage without significant differences to the corresponding healthy joint surface.33,34 ACT3D further proved to be a suitable therapy for children and adolescents with chondral defects.14
In 2020, Niemeyer et al29 published the 4-year outcomes of their European Medicines Agency (EMA)-driven, prospective, phase II study addressing the dose-dependent safety and efficacy of Chondrosphere. The study did not reveal any statistically significant differences between different concentrations of spheroids in the dose range of 10 to 70 spheroids/cm2. This broad therapeutic window reduces the risk of over- or underdosing. The incidence of adverse reactions did not show statistically significant differences between the dose groups. With a treatment failure rate of 4%, ACT3D was confirmed as a well-tolerated and efficient therapy for articular cartilage lesions with favorable overall results in MRI and clinical scores.
Most recently, Hoburg et al15 compared spheroid-based ACT versus microfracture, the currently recommended first-line treatment for small cartilage defects. The 3-year follow-up analysis of the randomized controlled trial using MRI and clinical scores revealed the equality of both therapy options for defects between 1 and 4 cm2.
ACT3D in the Hip Joint
ACT3D seems to be especially suitable for use in the hip joint because other ACT techniques have certain limitations for this application. The anatomic features of the hip restrict surgical access to the central joint, particularly when matrix-associated products are used, whereas Chondrosphere can easily be injected during arthroscopy.
In 2014, Fickert et al12 published the first short-term results for ACT3D in the hip joint. Several clinical outcome measures were used and included the Nonarthritic Hip Score, the modified Harris Hip Score, and the 36-Item Short Form Health Survey. An overall statistically significant improvement was observed for all assessment scores 12 months after the intervention. Good clinical results were also reported by Körsmeier et al19 in 2016. Statistically significant increases of the subjective assessment scores were reported as early as 6 weeks after implantation, and second-look arthroscopy showed good ingrowth of the repair tissue with macroscopic appearance of hyaline cartilage.
The work of Thier et al36 from 2017 might be the only study that directly compared ACT3D with another MACT product. The study included 10 cases treated using Chondrosphere and 19 cases treated using Novocart Inject (TETEC), a hydrogel of albumin and hyaluronic acid. An overall significant improvement was observed for Nonarthritic Hip Score, the International Hip Outcome Tool, and the Euro-Qol group score after an average follow-up of 19 months. However, the investigators did not find significant differences between the products.
In 2018, Krueger et al20 presented the largest study thus far addressing ACT3D in the hip joint with good midterm results. In that study, 32 patients underwent arthroscopic treatment of large, full-thickness, acetabular cartilage defects and were monitored for 3 years using pre- and postoperative scores. The modified Harris Hip Score improved significantly from 64 to 91 points; the International Hip Outcome Tool, from 44% to 86%; and the Subjective Hip Value, from 54% to 87%.
Discussion
During the past decade, several authors published promising short-term results for the innovative ACT3D procedure in the treatment of articular cartilage defects in the knee as well as in the hip. ACT3D might have relevant advantages over common MACT products, but systematic evaluation and randomized controlled studies are lacking to verify the potential of this therapy option.
Much scientific attention is being paid to the treatment of articular cartilage defects. This may be motivated by the massive prevalence of these defects in the population and their associated economic significance as well as by the improved possibilities for treatment. The introduction of ACT in 1994 seemed to be the crucial turning point in the field of articular cartilage repair. Since that time, the technique has been continuously developed and improved. One of the latest developments is the introduction of the innovative scaffold-free ACT3D technique using spheroids of redifferentiated autologous chondrocytes and cartilage-specific matrix. Even though MACT was an important improvement on the original ACT, there are still certain disadvantages of the procedure. The chondrocytes are mostly embedded in scaffolds made of different materials such as collagen9 or hyaluronic acid23; this is useful because the products can be perfectly adapted to the form and size of the defect, deliver a matrix similar to the chondral extracellular matrix, and fix the chondrocytes safely at the lesion site. However, the structure of bulky and stiff matrices complicates the operative handling and often requires a more invasive approach than arthroscopy. Many of the products additionally have to be fixed by sutures that permanently injure surrounding cartilage tissue.16 Furthermore, the xenogenous scaffold might interact with chondrocytes in a nonphysical way, impairing the defect repair,1 or could even cause immunologic responses.30 Chondrosphere addresses these disadvantages of synthetic scaffolds. Because chondrocyte spheroids are intended to be injected into the defect site, they can be applied arthroscopically even to joint areas that are difficult to access using matrices. After the injection, the spheroids immediately adhere to the subchondral bone without being fixed with sutures or other extraneous materials.21 Thus, the surrounding healthy cartilage is unaffected, and adverse events, such as immunological responses or infections, might be reduced because of the absence of xenogenous materials. However, it has to be considered that the failure rate after ACT in general is low and the biggest improvement concerning adverse events, such as hypertrophy or infections, was achieved by the step from first- to second-generation ACT and from arthrotomy to all-arthroscopic techniques.13 Furthermore, gel-based MACT products that feature the same advantages as ACT3D regarding application are already on the market. However, those products still contain allogenic material.36,38 Chondrocyte spheroids not only try to mimic the extracellular matrix of articular cartilage, as do synthetically produced scaffolds, but consist of neocartilage produced by autologous chondrocytes.
The studies we found mostly combined MRI-assisted examination with subjective patient-administered assessment scores to evaluate the success of the therapy. The MOCART score is a widely acknowledged instrument to assess the appearance of cartilage tissue on MRI scans. For this purpose, 9 variables are considered, including defect filling, integration into the surrounding cartilage, and structure and surface of the newly formed tissue.24 All studies we found achieved convincing results concerning the clinical outcome and quality of the repair tissue after a time period of 1 to 4 years. In summary, the results revealed a MOCART score between 70 and 77.2 out of 100 after a follow-up of at least 1 year. The subjective scores increased statistically significantly in every study after treatment using ACT3D. Second-look arthroscopies performed by Siebold et al showed repair tissue with hyaline-like appearance and strong integration and defect filling properties in the majority of the examined cases.33
Comparable studies that investigated the treatment success of articular cartilage lesions by other MACT products after short- to medium-term follow-up showed similar outcomes. Zak et al40 achieved a mean MOCART score of 73.2 in a 2-year follow-up of patients with chondral lesions of the knee treated using Novocart 3D (TETEC), a bilayered MACT product composed of collagen type I. All subjective scores increased statistically significantly.40 The combination of MACT using Novocart 3D and bone augmentation even showed a MOCART score of 82.6 at 1 year after treatment.41 Furthermore, long-term MACT results are available. Aldrian et al2 examined patients treated using Hyalocraft C (Anika Therapeutics), a hyaluronic acid-based scaffold, after a minimum period of 10 years. The MOCART score was still high at a mean value of 70.4, whereas a clinical score showed only partially statistically significant improvements compared with those at the baseline.2 Several systematic reviews have discussed the therapy of articular cartilage lesions using MACT.6,18,32 However, they did not include an evaluation of the MOCART score but focused more on the clinical outcomes. Schuette et al32 summarized the medium- to long-term results of MACT in the knee at a minimum follow-up of 5 years and determined a weighted average Knee injury and Osteoarthritis Outcome Score of 73.3 and a Tegner score of 4.5. These results accord with those we found for ACT3D. It has to be considered that only short-term outcomes are available for ACT3D, with a maximum follow-up of 4 years. However, this indicates that ACT3D might have no clinical advantage over MACT.
Recently, the British National Institute of Health and Care Excellence appraised the clinical effectiveness of co.don Chondrosphere based on the company’s randomized controlled trial COWISI (data not published), which compared the ACT3D product versus microfracture.4 The committee attested to the economic advantages of Chondrosphere compared with other appraised ACT techniques and recommended it as a treatment option for symptomatic articular cartilage defects >2 cm2 in knee joints. However, like most of the other studies, the review lacked direct comparison with other ACT procedures in terms of clinical and morphological outcomes. Thus, to verify the putative advantages of ACT3D compared with MACT, it would be necessary to perform a long-term study in the form of a randomized controlled clinical trial comparing ACT3D with a common MACT product.
Different opinions are available concerning the suitable study concept to investigate articular cartilage repair. The MRI-assisted MOCART score seems to be a popular tool to describe the appearance of articular cartilage tissue. However, we did not find evidence for a correlation between MRI and clinical outcome scores, which creates doubts about this choice of examination.2,10,41 For this purpose, functional (biochemical) MRI, such as delayed gadolinium-enhanced MRI of cartilage, quantitative T1rho, T2-weighted mapping, or chemical exchange saturation transfer on glycosaminoglycans (GagCest), might be more suitable for the evaluation of cartilage repair in concordance with the clinical situation.39,40 However, availability and applicability of these techniques are still limited, and the interpretation is complex and time-consuming.
Promising results have been reported with the use of ACT3D, but several limitations to this review prevent us from making a final statement regarding effectiveness and indication. The studies in our review had level 1 to 4 evidence, with a majority of small, less evidential case series. The data presented were overall short-time results, with a mean follow-up of 32.6 months over all studies. This is also reflected in the rather low methodological scoring in the MCMS. Of the defects compared, there was a wide range in defect size, which might affect comparability between single studies. The established study design using MRI outcome assessment might have to be reconsidered because the MOCART score did not correlate with clinical outcomes. Additionally, most of the publications lacked a suitable control group. One randomized controlled trial compared ACT3D with microfracture,15 which is an important step, but there are no works contrasting ACT3D with other ACT methods.
Conclusion
Minimally invasive, all-arthroscopic ACT3D using autologous chondrocyte spheroids shows promising results in the therapy of articular cartilage defects in the knee as well as in the hip. Several short- to medium-term clinical studies revealed significant increases in patients’ subjective quality of life, satisfaction, pain reduction, and improvement in knee function. MRI-assisted and arthroscopic examination showed convincing outcomes as well, but well-designed long-term studies are lacking. ACT3D might have relevant advantages over common MACT products, but systematic evaluation and randomized controlled studies are crucial to verify the potential of this tissue-engineered approach.
Footnotes
Final revision submitted June 4, 2020; accepted July 6, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Albrecht C, Reuter C-A, Stelzeneder D, et al. Matrix production affects MRI outcomes after matrix-associated autologous chondrocyte transplantation in the knee. Am J Sports Med. 2017;45(10):2238–2246. [DOI] [PubMed] [Google Scholar]
- 2. Aldrian S, Zak L, Wondrasch B, et al. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: a prospective follow-up at a minimum of 10 years. Am J Sports Med. 2014;42(11):2680–2688. [DOI] [PubMed] [Google Scholar]
- 3. Anderer U, Libera J. In vitro engineering of human autogenous cartilage. J Bone Min Res. 2002;17(8):1420–1429. [DOI] [PubMed] [Google Scholar]
- 4. Armoiry X, Cummins E, Connock M, et al. Autologous chondrocyte implantation with chondrosphere for treating articular cartilage defects in the knee: an evidence review group perspective of a NICE single technology appraisal. PharmacoEconomics. 2019;37(7):879–886. [DOI] [PubMed] [Google Scholar]
- 5. Becher C, Laute V, Fickert S, et al. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J Orthop Surg Res. 2017;12(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38(6):1259–1271. [DOI] [PubMed] [Google Scholar]
- 7. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 8. Brittberg M, Peterson L, Sjögren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation: a review of recent developments. J Bone Joint Surg Am. 2003;85(suppl 3):109–115. [DOI] [PubMed] [Google Scholar]
- 9. Crawford DC, Heveran CM, Cannon WD, Foo LF, Potter HG. An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. Am J Sports Med. 2009;37(7):1334–1343. [DOI] [PubMed] [Google Scholar]
- 10. De Windt TS, Welsch GH, Brittberg M, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41(7):1695–1702. [DOI] [PubMed] [Google Scholar]
- 11. Fickert S, Gerwien P, Helmert B, et al. One-year clinical and radiological results of a prospective, investigator-initiated trial examining a novel, purely autologous 3-dimensional autologous chondrocyte transplantation product in the knee. Cartilage. 2012;3(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fickert S, Schattenberg T, Niks M, Weiss C, Thier S. Feasibility of arthroscopic 3-dimensional, purely autologous chondrocyte transplantation for chondral defects of the hip: a case series. Arch Orthop Trauma Surg. 2014;134(7):971–978. [DOI] [PubMed] [Google Scholar]
- 13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779–791. [DOI] [PubMed] [Google Scholar]
- 14. Hoburg A, Löer I, Körsmeier K, et al. Matrix-associated autologous chondrocyte implantation is an effective treatment at midterm follow-up in adolescents and young adults. Orthop J Sports Med. 2019;7(4):2325967119841077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoburg A, Niemeyer P, Laute V, et al. Matrix-associated autologous chondrocyte implantation with spheroid technology is superior to arthroscopic microfracture at 36 months regarding activities of daily living and sporting activities after treatment. Cartilage. 2020;1947603519897290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hunziker EB, Stähli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis Cartilage. 2008;16(9):1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am. 2016;98(16):1332–1339. [DOI] [PubMed] [Google Scholar]
- 18. Kon E, Filardo G, Di Matteo B, Perdisa F, Marcacci M. Matrix assisted autologous chondrocyte transplantation for cartilage treatment: a systematic review. Bone Joint Res. 2013;2(2):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Körsmeier K, Claßen T, Kamminga M, Rekowski J, Jäger M, Landgraeber S. Arthroscopic three-dimensional autologous chondrocyte transplantation using spheroids for the treatment of full-thickness cartilage defects of the hip joint. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2032–2037. [DOI] [PubMed] [Google Scholar]
- 20. Krueger DR, Gesslein M, Schuetz M, Perka C, Schroeder JH. Injectable autologous chondrocyte implantation (ACI) in acetabular cartilage defects—three-year results. J Hip Preserv Surg. 2018;5(4):386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lehmann M, Martin F, Mannigel K, Kaltschmidt K, Sack U, Anderer U. Three-dimensional scaffold-free fusion culture: the way to enhance chondrogenesis of in vitro propagated human articular chondrocytes. Eur J Histochem. 2013;57(4):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Libera J, Luethi U, Alasevic OJ. Co.don Chondrosphere (co.don AG): autologous matrix-induced engineered cartilage transplantation In: Basic Science, Clinical Repair and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Vol 1 Timeo Editore; 2006. [Google Scholar]
- 23. Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I. Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc. 2002;10(3):154–159. [DOI] [PubMed] [Google Scholar]
- 24. Marlovits S, Striessnig G, Resinger CT, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310–319. [DOI] [PubMed] [Google Scholar]
- 25. Meyer U, Wiesmann H-P, Libera J, Depprich R, Naujoks C, Handschel J. Cartilage defect regeneration by ex vivo engineered autologous microtissue—preliminary results. Vivo. 2012;26(2):251–257. [PubMed] [Google Scholar]
- 26. Niemeyer P, Albrecht D, Andereya S, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee. 2016;23(3):426–435. [DOI] [PubMed] [Google Scholar]
- 27. Niemeyer P, Laute V, John T, et al. The effect of cell dose on the early magnetic resonance morphological outcomes of autologous cell implantation for articular cartilage defects in the knee: a randomized clinical trial. Am J Sports Med. 2016;44(8):2005–2014. [DOI] [PubMed] [Google Scholar]
- 28. Niemeyer P, Laute V, Zinser W, et al. A prospective, randomized, open-label, multicenter, phase III noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. 2019;7(7):2325967119854442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niemeyer P, Laute V, Zinser W, et al. Safety and efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology is independent of spheroid dose after 4 years. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1130–1143. [DOI] [PubMed] [Google Scholar]
- 30. Revell CM, Athanasiou KA. Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Eng Part B Rev. 2009;15(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schubert T, Anders S, Neumann E, et al. Long-term effects of chondrospheres on cartilage lesions in an autologous chondrocyte implantation model as investigated in the SCID mouse model. Int J Mol Med. 2009;23(4):455–460. [DOI] [PubMed] [Google Scholar]
- 32. Schuette HB, Kraeutler MJ, McCarty EC. Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid- to long-term clinical outcomes. Orthop J Sports Med. 2017;5(6):2325967117709250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siebold R, Karidakis G, Feil S, Fernandez F. Second-look assessment after all-arthroscopic autologous chondrocyte implantation with spheroides at the knee joint. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1678–1685. [DOI] [PubMed] [Google Scholar]
- 34. Siebold R, Suezer F, Schmitt B, Trattnig S, Essig M. Good clinical and MRI outcome after arthroscopic autologous chondrocyte implantation for cartilage repair in the knee. Knee Surg Sports Traumatol Arthrosc. 2018;26(3):831–839. [DOI] [PubMed] [Google Scholar]
- 35. Thermann H, Driessen A, Becher C. Autologous chondrocyte transplantation in the treatment of articular cartilage lesions of the talus. Article in German. Orthopade. 2008;37(3):232–239. [DOI] [PubMed] [Google Scholar]
- 36. Thier S, Weiss C, Fickert S. Arthroscopic autologous chondrocyte implantation in the hip for the treatment of full-thickness cartilage defects—a case series of 29 patients and review of the literature. SICOT J. 2017;3:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trattnig S, Pinker K, Krestan C, Plank C, Millington S, Marlovits S. Matrix-based autologous chondrocyte implantation for cartilage repair with Hyalograft C: two-year follow-up by magnetic resonance imaging. Eur J Radiol. 2006;57(1):9–15. [DOI] [PubMed] [Google Scholar]
- 38. Vadalà G, Russo F, Musumeci M, et al. Clinically relevant hydrogel-based on hyaluronic acid and platelet rich plasma as a carrier for mesenchymal stem cells: rheological and biological characterization. J Orthop Res. 2017;35(10):2109–2116. [DOI] [PubMed] [Google Scholar]
- 39. Welsch GH, Mamisch TC, Zak L, et al. Morphological and biochemical T2 evaluation of cartilage repair tissue based on a hybrid double echo at steady state (DESS-T2d) approach. J Magn Reson Imaging. 2011;34(4):895–903. [DOI] [PubMed] [Google Scholar]
- 40. Zak L, Albrecht C, Wondrasch B, et al. Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold: an analysis of clinical and radiological data. Am J Sports Med. 2014;42(7):1618–1627. [DOI] [PubMed] [Google Scholar]
- 41. Zellner J, Grechenig S, Pfeifer CG, et al. Clinical and radiological regeneration of large and deep osteochondral defects of the knee by bone augmentation combined with matrix-guided autologous chondrocyte transplantation. Am J Sports Med. 2017;45(13):3069–3080. [DOI] [PubMed] [Google Scholar]