Abstract
Since the beginning of the SARS-CoV-2 outbreak, few cases of COVID-19 pneumonia in patients with pulmonary arterial hypertension have been reported. We present four patients with known history of PAH admitted to our hospital with SARS-CoV-2 pneumonia to analyze the impact of this disease on their clinical outcome.
Keywords: COVID-19, pulmonary circulation, pulmonary hypertension
The Lombardy region in Northern Italy was one of the most affected areas by the recent Coronavirus Disease 2019 (COVID-19) outbreak. Since the outset of the pandemic wave, almost 1900 COVID-19 patients with different comorbidities and underlying diseases were admitted at our Hospital in the province. Among them, the clinical cases of four patients with known history of pulmonary arterial hypertension (PAH) and Severe Acute Respiratory Syndrome – Coronavirus 2 (SARS-CoV-2) pneumonia were reported.
All patients have provided written informed consent for the anonymized collection of data and figures.
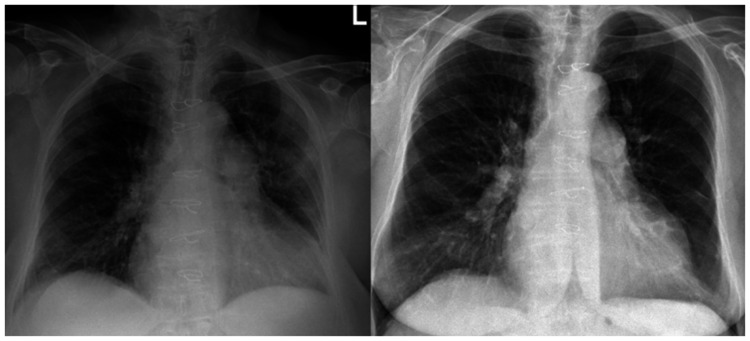
The first patient was a 66-year-old woman treated for an advanced form of idiopathic PAH in World Health Organization (WHO) functional class III, REVEAL score 15,1 treated by macitentan 10 mg, tadalafil 40 mg and long-term oxygen therapy (LTOT) 5 L/min (Vitamin-K Antagonists (VKA) was withheld due to recent gastrointestinal bleeding). A recent right heart catheterization (RHC) showed a mean pulmonary artery pressure (mPAP) of 45 mmHg, pulmonary vascular resistance (PVR) of 13.4 WU/m2, a cardiac index (CI) of 2.8 L/min/m2 and a wedge pressure (WP) of 8 mmHg. The patient reported that dry cough and fever with chills started seven days before the admission. When admitted to the Emergency Room (ER), the oxygen saturation (SaO2) was 90% and X-ray showed typical signs of SARS-CoV-2 infection (Fig. 1) with a positive nasopharyngeal swab. C-reactive Protein (CRP) was 10.3 mg/dl, lactate dehydrogenase (LDH) was 523 U/L and hemochrome showed lymphopenia (1.04 × 109/L) with normal white blood cells (WBC) (5.24 × 109/L). The blood gas showed a pH of 7.42, PaCO2 of 33, PaO2 of 73, HCO3− of 22.4, lactate of 3 mmol/l and a ratio of arterial oxygen partial pressure (PaO2) on fractional inspired oxygen (FiO2) (PaO2/FiO2 ratio) of 110. Due to respiratory distress, the patient was treated by Continuous Airway Positive Pressure (C-PAP) with FiO2 of 60% and Positive End-Expiratory Pressure (PEEP) of 10 cm H2O, obtaining a 94% SaO2 without signs of hemodynamic deterioration. In addition, ceftriaxone 2 g, azithromycin 500 mg, hydroxychloroquine 200 mg b.i.d. and ritonavir 50/lopinavir 200 mg b.i.d. were administered for seven days. Remdesivir was introduced at day 12 and administered for an additional 10 days. The patient improved after seven days and was discharged in 19 days with LTOT 12 L/min (Fig. 1).
Fig. 1.
On the left, chest X-ray showing bilateral interstitial infiltrates with accentuation of the vascular plot, in the absence of pleural effusion. On the right, the same patient after COVID-19 pneumonia resolution.
The second patient was a 79-year-old woman with a chronic thromboembolic pulmonary hypertension undergone surgical endarterectomy four years earlier, in WHO functional class II-III, REVEAL score 10, in treatment with riociguat 2.5 mg t.i.d. because of residual PH after surgery and VKA (RHC after surgery showed a mPAP of 26 mmHg, WP of 9 mmHg, CI of 3.2 L/min/m2 and PVR of 5.3 WU).
The patient was admitted to our hospital for fever (38.4℃) and dyspnea, SaO2 = 87% with typical X-ray bilateral interstitial infiltrates and a positive nasopharyngeal swab for SARS-CoV-2 infection. CRP was 2.2 mg/dl, hemochrome showed leukopenia (3.12 × 109/L) and lymphopenia (0.66 × 109/L). The blood gas showed a pH of 7.48, pCO2 of 33, pO2 of 53, lactate of 1.13 mmol/l and PaO2/FiO2 ratio of 250. The patient was stable on oxygen via nasal cannula at 3 L/min (SaO2 95%). Concomitant therapy with ceftriaxone 2 g, azithromycin 500 mg daily, hydroxychloroquine 200 mg b.i.d., darunavir 800/cobicistat 150 mg and methylprednisolone (1 mg/kg day) was started. The patient responded well to the treatments with clinical improvement, and then she was discharged after seven days on riociguat, VKA, oral steroid and LTOT 3 L/min.
The third patient was a 78-year-old man with PAH, WHO functional class III, REVEAL score 12, LTOT 5 L/min, therapy with macitentan 10 mg and dual antiplatelet therapy for recent coronary stenting. A recent RHC showed mPAP of 55 mmHg, WP of 14 mmHg, CI of 1.95 L/min/m2 and PVR of 20 WU/m2. He was admitted for fever and worsening dyspnea with SaO2 of 88%. CRP was 1.8 mg/dl, hemochrome showed leukopenia (3.5 × 109/L) and lymphopenia (0.5 × 109/L). The blood gas was: pH 7.53, PCO2 33, PO2 43, lactate 1.53 mmol/l, PaO2/FiO2 was 190. High flow respiratory mask therapy at 12 L/min was initiated. Therapy with levofloxacine 500 mg iv daily, hydroxychloroquine 200 mg b.i.d. and ritonavir 50/lopinavir 200 mg b.i.d. was given for seven days and the patient was discharged after 13 days.
The fourth patient was a 36-year-old woman with systemic sclerosis and PAH (at RHC: mPAP 36 mmHg, PVR 8.5 WU/m2, CI 3.66 L/min/m2, WP 4 mmHg ), REVEAL score 7 and WHO class II-III in treatment with bosentan 125 mg b.i.d., aspirin 100 mg and prednisone 2.5 mg b.i.d. She had fever and typical X-ray interstitial infiltrates with a positive nasopharyngeal swab for SARS-CoV-2 infection. CRP was 1.8 mg/dl, hemochrome showed leukocytosis (13.67 × 109/L), normal lymphocytes (2.65 × 109/L) and LDH (213 U/L) but elevated D-dimer (951 ng/mL) and interleukine-6 (6.67 pg/mL). The blood gas was: pH 7.45, PCO2 30.1 PO2 89.4, HCO3− 22.4, lactate 1.46 mmol/l, PaO2/FiO2 424. Treatment with oral steroid was increased up to 25 mg daily. Enoxaparin 4000 IU was started and the patient was not hospitalized. In all four cases the PAH therapy was not discontinued.
None of these patients developed acute right heart failure and all of them are still alive despite having been managed with different COVID-19 treatments. Importantly, all patients had no pre-existent relevant pulmonary disease. Furthermore, it is important to consider that the common denominator for these patients is that they were all under treatment for PAH. These unexpected favorable outcomes in chronically ill patients with PAH should lead to make a series of considerations. It is difficult to estimate the risk of adverse events in these patients. In this phase of the pandemic, data are characterized by inaccuracy and heterogeneity, making the estimates complicated. According to Zhao's meta-analysis2 three out of four patients have an increased risk of death if they are more than 50 years old (OR 2.6 95% CI 2.29–2.98) and of male sex (OR = 1.348, 95% CI, 1.195–1.521). Considering PAH as any co-morbidity, our patients had a moderately increased risk of death (OR 2.63 95% CI 2.09–3.30). In addition, if we consider the PAH disease as a risk factor like chronic obstructive pulmonary disease (COPD), the risk of death would have been severely increased (OR 5.32 95% CI 2.6–10.85). Despite all these considerations no fatal events were observed in our patients, although no risk stratification is available for PAH. Mechanical ventilation is recommended for patients with moderate or severe acute respiratory distress syndrome (ARDS) who remains hypoxemic or profoundly symptomatic despite supplemental oxygen. At present, there are no studies examining specific ventilatory strategies in patients with COVID-19.3 Several hypotheses could be considered to explain this unexpected favorable outcome. Firstly, our patients had chronic adjustments to hypoxic states. Secondly, chronic pulmonary vascular disease treated by endothelin antagonists’ therapy may have produced a protective effect on vascular inflammatory respiratory distress. In addition, another interesting theory concerns the potential benefit of anticoagulant or antiplatelet therapy in patients with COVID-19 infection.4,5 Furthermore, in late March 2020, experts from over 32 U.S. PAH Centers responded to a Pulmonary Hypertension Association query: 13 COVID-19 cases were reported, with only one death.6
As described, the impact of COVID-19 in PAH patients has not been as dramatic as originally thought. These results may arise from a complex interaction between chronic exposure to hypoxia together with the effects with PH therapy on pulmonary circulation and coagulation profile. Our observations have many limitations, first of all, heterogeneous characteristics of PH etiology and severity which may have affected the clinical outcomes of these patients, together with the effects of antiviral therapy. In addition to the above limits, it is right to underline the exceptional nature of the case studies in a truly emergency context. The Lombardy region, the province in which Bergamo hospital is located, was the western region most affected by the virus in the world. This supports the hypothesis of a potential protective role of this type of lung disease.
Author contributions
All authors were involved in the conception of the study, the collection and interpretation of the data, revised manuscript critically and approved the submitted manuscript.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding in the public, commercial or not-for-profit sectors.
References
- 1.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 2.Zhao X, Zhang B, Li P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. medRxiv. DOI: 10.1101/2020.03.17.20037572.
- 3.Gage A, Higgins A, Ran L et al. Reacquainting cardiology with mechanical ventilation in response to the COVID-19 pandemic. DOI: 10.1016/j.jaccas.2020.03.007. [DOI] [PMC free article] [PubMed]
- 4.Kollias A, Kyriakoulis KG, Dimakakos E, et al. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020; 189: 846–847. [DOI] [PMC free article] [PubMed]
- 5.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. Epub ahead of print 6 May 2020. DOI: 10.1016/j.jacc.2020.05.00. [DOI] [PMC free article] [PubMed]
- 6.Horn E, Chakinala MM, Oudiz R, et al. Could pulmonary arterial hypertension (PAH) patients be at a lower risk from severe COVID-19? Pulm Circ 2020; 10: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]