Abstract
Background: Melanoma is the most lethal skin cancer with a mortality rate of 262 cases per 100.000 cases. The sentinel lymph node (SLN) is the first lymph node draining the tumor. SLN biopsy is a widely accepted procedure in the clinical setting since it provides important prognostic information, which helps patient management, and avoids the side effects of complete lymph node dissection. The rationale of identifying and removing the SLN relies on the low probability of subsequent metastatic nodes in case of a negative histological exam performed in the SLN.
Discussion: Recently, new analytical approaches, based on the evaluation of scintigraphic images are also exploring the possibility to predict the metastatic involvement of the SLN. 99mTc-labeled colloids are still the most commonly used radiotracers but new promising radiotracers, such as 99mTc-Tilmanocept, are now on the market. In the last decades, single photon emission computed tomography-computerized tomography (SPECT/CT) has gained wider diffusion in clinical departments and there is large evidence about its superior diagnostic accuracy over planar lymphoscintigraphy (PL) in the detection of SLN in patients with melanoma. Scientists are also investigating new hybrid techniques combining functional and anatomical images for the depiction of SLN but further evidence about their value is needed.
Conclusion: This review examined the predictive and prognostic factors of lymphoscintigraphy for metastatic involvement of SLN, the currently available and emerging radiotracers and the evidence of the additional value of SPECT/CT over PL for the identification of SLN in patients with melanoma. Finally, the review discussed the most recent technical advances in the field.
Keywords: Melanoma, sentinel lymph node biopsy, 99mTc-Tilmanocept, 99mTc-colloids, SPECT/CT, hybrid imaging
1. INTRODUCTION
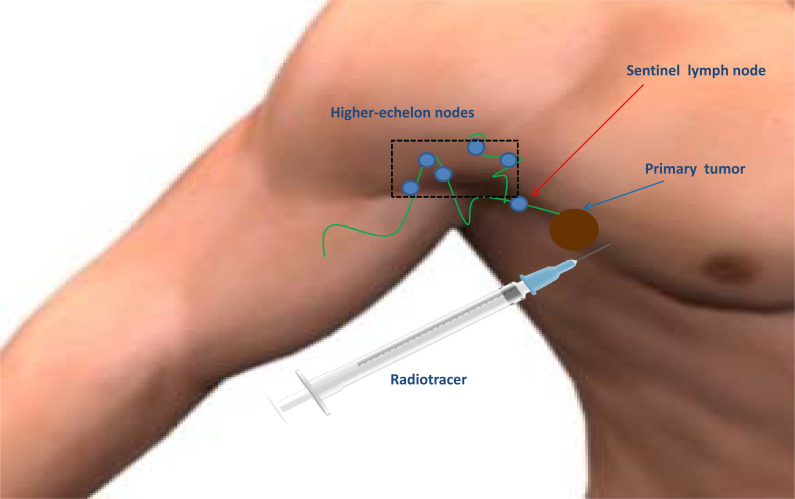
Melanoma is the least frequent type of skin cancer but the deadliest one, with an annual global incidence of five cases per 100.000 persons and a mortality rate of 262 cases per 100.000 [1-3]. The Sentinel Lymph Node (SLN) is the putative first lymph node draining the tumor [4, 5]. The rationale of identifying and removing the first lymph node in the lymphatic chain draining the primary tumor relies on the low probability of subsequent metastatic nodes in case of a negative histological exam performed in the SLN (Fig. 1) [4]. The removal of the SLN avoids the side effects of complete lymph node dissection (CLND); furthermore, prospective trials demonstrated no improvement in survival for patients with melanoma undergoing CLND [6, 7]. CLND should, therefore, be preferentially suggested for patients with positive SLN [8].
Fig. (1).
Schematic representation of the procedure and rationale of lymphoscintigraphy for the identification of the SLN in a patient with melanoma.
Sentinel Lymph Node Biopsy (SLNB) is indicated in patients with melanoma (T1b-T4), without evidence of clinically evident locoregional or distant mestastases, once excision with a narrow margin of the primary lesion has been carried out [5, 9]. According to the last available version of the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual, in patients with T1a melanoma (Breslow thickness <0.8 mm without ulceration) SLNB is not recommended, whereas in T1b melanoma (Breslow thickness = 0.8-1 mm with or without ulceration) [10, 11] SLNB should be considered, especially in the presence of adverse prognostic features [12]. In patients with T4 melanoma or with a Breslow thickness>4 mm, SLNB may be required for correct staging and also justified by the important prognostic information provided by the technique [13, 14]. Of note, the reported false-negative rates for patients with melanoma undergoing SLNB, range, approximately, from 5% to 20%, and are considerably higher than for breast cancer (0-3%) [15]. It has been advocated that part of this discrepancy may be related to a shorter follow-up period of the studies involving patients with breast cancer, different physiology of the lymphatic flow and difference in the biology of the two cancers [16].
Lymphoscintigraphy has become, over the last decades, a routine procedure for the identification of SLN [5]. Lymphoscintigraphy is based on the detection of γ emission originating from the radiotracer, which is commonly administered employing an intradermal or subdermal paracicatricial/perilesional injection [4, 17-19]. The procedure requires a dynamic acquisition to track the transit of the radiotracer from the primary tumor to the SLN; the dynamic acquisition is followed by early static acquisitions and eventually delayed static acquisitions, including body regions of interest; the procedure can be completed carrying out a whole-body scan (from the neck to the groin) [5]. The main interpretation criterion to define a lymph node as SLN is the presence of a lymphatic channel connecting the primary tumor to the lymph node; usually, the lymph node becoming visible first has to be defined as SLN and this is often the hottest node. It should be mentioned that, in patients with melanoma undergoing lymphoscintigraphy, the pattern of lymphatic drainage may be occasionally unpredictable and there could be multiple SLNs demonstrating radiotracer uptake [4, 5, 20]. Recently, single photon emission computed tomography (SPECT) and SPECT/CT have been reported to provide additional value to the diagnostic procedure [5, 21, 22]. In the operation room, the surgeon, with the help of the gamma probe, identifies the region with the highest count rate and removes the radioactive nodes. Despite the lack of international consensus, removal of additional lymph nodes within a certain threshold of radioactivity (e.g. 10% or 20% of the hottest lymph node), has been suggested [23-25].
Historically, it has been largely demonstrated that the risk of developing lymph node metastases increases with Breslow thickness [4]. A large multicentre trial reported nodal metastases in 20% of cases in 765 with intermediate-thickness (1.20-3.50 mm) melanoma and in 32.9% of cases in 173 patients with thick melanoma (>3.50 mm) [26]. For thin thickness melanoma (<1 mm) risk of lymph node metastasis is extremely lower: 5% for thickness ranging between 0.76 mm and 1 mm and 1% for a thickness<0.75 mm [27]. Although there is no sufficient evidence from the literature concerning the advantages of performing SLNB in thin melanoma (Breslow thickness<1mm), the procedure is also acceptable and performed in several European centres. In subjects with a Breslow thickness < 1 mm, two subgroups deserve particular attention: patients with Breslow thickness 0.75 - 1 mm and patients with Breslow thickness < 0.75 mm. For the first subgroup (Breslow thickness: 0.75-1 mm) SLNB is proposed in the presence of at least one high-risk feature, such as age < 40 years, positive deep margins, lymphovascular invasion, ulceration, regression of more than 50-75% of the whole pigmented lesions and mitotic rate [5]. For melanoma ≤ 0.75 mm thick, owing the very low risk of SLN metastasis (2.7% [10]) and favourable outcome (10-year survival rate of 98% [28]), SLNB is not generally recommended except in case of significant uncertainty about the adequacy of microstaging [21] and after multidisciplinary team discussion.
In patients with recurrent melanoma still a few data exist in literature, but there is some evidence that the SLNB is a feasible procedure, helping patient management and providing also the important prognostic information [26, 29, 30].
This review will first discuss the predictive and prognostic factors of lymphoscintigraphy for metastatic sentinel lymph node involvement. Second, the currently available and emerging radiotracers will be exposed. Furthermore, the manuscript will explore the evidence of the additional value of SPECT/CT over planar imaging for the identification of SLN in patients with melanoma. Finally, the review will discuss the most recent technical advances in the field.
2. PREDICTIVE AND PROGNOSTIC FACTORS OF LYMPHOSCINTIGRAPHY FOR METASTATIC SLN IN PATIENTS WITH CUTANEOUS MELANOMA
The prognostic value of SLNB in patients with cutaneous malignant melanoma is very reliable, showing an improvement to predict the survival as compared with classical histologic factors markers, such as Breslow and ulceration [31-33]. Although using SLNB for an early lymph node dissection does not improve the overall survival, however, it delays time to the first event [34]. While the technical details of lymphoscintigraphy are well standardized [24, 35], some information derived from lymphoscintigraphy is still debated.
The study of the lymphatic flow rate may be interesting to understand the time needed to reach the regional lymph nodes from the injection sites around the location of a previously excised melanoma [36-39]. Indeed, in animal melanoma models, some authors have found a direct correlation between increasing lymphatic flow rate and a larger incidence of metastases [40-42]. The most accepted hypothesis concerns the intense lymphatic neoangiogenesis in more severe tumors that could explain the more rapid mapping [43, 44]. Some studies have shown that a long scintigraphic appearance time (SAT), defined as the time between radiopharmaceutical injection and first lymphoscintigraphic visualization of the SLN, is a negative predictor for nodal metastasis in patients with melanoma [45]. Hence, Maza et al. and Cammilleri et al. showed a correspondence between fast appearance time of SLN and lymphogenic metastastic spread. Based on previous clinical observations, Maza et al. set a threshold of 20 min for SLN visualization after injection of 99mTc-nanocolloids to discriminate between slow and fast drainage, while Cammilleri et al. used a threshold of 30 min [45, 46]. On the other hand, Toubert et al. [47] did not find any difference regarding the rate of SLN metastasis in patients with slow or fast kinetics. Several reasons could be explained why the authors found different results. First, the methods used to measure the SAT and lymphatic flow rate by the authors were different [36-40, 45, 46, 48-51]. Differences among the protocols for the identification of SLN in the clinical setting are determined by the specific properties of the radiopharmaceutical, such as the particle size, the preparation method, the distance between the scar of a previously excised melanoma and the injection site and the number and volume of the injections [52-54]. Finally, muscle contractions, lymphatic pulse, temperature and anatomic location could influence SAT [39, 55].
Recently, a more accurate methodology, which estimates the SAT and lymphatic flow rate, was proposed by Fujiwara et al. to predict nodal metastases based on the appearance time of SLN. Furthermore, using dynamic lymphoscintigraphy, the authors determined a new parameter, the scintigraphic saturation time (SST), defined as the time at which the tracer counts reached a plateau in the SLN, proposed as an alternative to the conventional SAT [56]. Hence, the predictive parameter called lymphatic transit rate (LTR) representing the scintigraphic saturation velocity was defined as the value between the primary lesion and the SLN divided by SST. In this study, Fujiwara et al. showed the LTR was significantly higher SLN with metastatic spread than in non-metastatic SLNs [57]. Apart from the lymphatic flow rate, the SST could be influenced also by the peritumoral lymphatic vessel density and lumen diameter [58]. Fujiwara et al. observed that the peritumoral lymphatic vessel density, measured by means of immunostaining of the peritrumoral area for D2‐40, a typical lymphatic duct, marker, was higher in patients with a metastatic SLN than in those without metastasis [56]. In the lower limb melanoma, the mean LTR was 3.49 cm/min in non-metastatic SLNs and 4.49 cm/min in metastatic SLNs. While, in the melanoma of the upper limb, LTR was 2.59cm/min in non-metastatic SLNs and 3.94cm/min in metastatic SLNs. The authors concluded therefore that LTR could be a useful predictive indicator for SLN metastasis in melanoma patients.
In 2009, Solari et al. proposed a new lymphoscintigraphy score (from L1 to L5) based on the ratio of radiotracer concentration within SLNs compared to the injection site: L1=no visualization of SLN; L2=hardly recognizable SLN; L3=faint visualization of SLN; L4=normal visualization with regular-shaped SLN; L5=very high uptake of SLN (similar to the site of injection). The mentioned score suggested that patients with thin melanoma (T1b-T2) showed 91% and 100% chances of being free of metastasis within the SLNs based, respectively on the preoperative lymphoscintigraphy score (L1-L2-L3 vs. L4-L5). Based on these data, the authors stated that frozen-section examination should not be performed in thin melanoma due to the low rate of SLNs metastasis. Conversely, patients with thick melanomas (T3-T4) and a high preoperative (L4-L5) showed a 17% likelihood of finding a metastatic SLNs, while those with a low preoperative score (L1-L2-L3) have a high likelihood (90%) to present metastatic SLNs. Hence, the authors suggested treating this category of patients in ‘one-stage’ procedure (SLNB and intraoperative frozen-section examination) [59].
Recently, Ho et al. investigated whether lymphoscintigraphy could predict if the most radioactive lymph node at the moment of surgery will be non-metastatic at pathology in patients with melanoma [60]. The authors found high specificity (91%) and negative predictive value (85%) for predicting whether the most radioactive lymph node at surgery would be non-metastatic at histology, however, modest sensitivity (31%) and positive predictive value (44%) was observed when the SLN was not the most radioactive lymph node. Finally, the authors concluded saying that dynamic lymphoscintigraphy has a role in surgical planning but that the imaging protocol needs to be implemented.
In cutaneous melanoma, the prognostic significance of multiple drainage basins is still unclear [61]. As recommended, SLNB is performed for each basin identified during lymphoscintigraphy [62]. Although previous studies have shown that the number of drainage basins does not affect survival in primary cutaneous melanoma [63, 64], a recent study investigated overall survival (OS), disease-specific survival (DSS), and disease-free survival (DFS) between multiple versus single drainage basins in patients who underwent preoperative lymphoscintigraphy for SLNB for cutaneous melanoma. The results showed that the number of drainage basins did not affect rates of SLN metastasis, OS, DSS, overall recurrence, locoregional recurrence, and distant recurrence. In conclusion, this study demonstrated that as identified by lymphoscintigraphy multiple basin drainage is not an independent biological or prognostic factor in primary cutaneous melanoma [61].
3. PRESENT STATUS AND NEW SPECT RADIOTRACERS FOR THE IDENTIFICATION OF SLN IN MELANOMA
The currently available and most commonly used radiotracers for the identification of SLN in patients with melanoma may be schematically distinguished in 99mTc-bound colloids and receptor-based radiotracers [4] (Table 1).
Table 1.
List of main SPECT radiotracers for SLN detection in patients with melanoma.
| Class of Tracer | Radiotracer | Listed Trade Names | Particle Size (nm) | Additional Notes |
|---|---|---|---|---|
| Radiolabelled colloids | 198Au-Colloid | N/A | 9-15 | β-emitter and unfavorable γ emission |
| 99mTc-Human serum albumin | Nanocoll, Nanoalbumon, Nanotop | 7-23 | Registered in Europe; large clinical experience. |
|
| 99mTc-Rhenium sulphide | Nanocis | 8-68 | Registered in Europe | |
| 99mTc-Sulphur colloid | TechneScan, TechneColl | 50-1000 | Registered in USA | |
| 99mTc-Antimony trisulphide | Lymph-Flo | 2-23 | Registered in Australia | |
| 99mTc-Calcium Phytate | Technephyte | 150-200 | Mostly used in Japan | |
| ICG-99mTc-nanocolloid | N/A | 700-900 | Clinically introduced in 2009; it can be detected using nuclear medicine modalities and (near-infrared) fluorescence imaging |
|
| Receptor-based radiotracers | 99mTc-Tilmanocept | Lymphoseek | 7 (mean) | Registered in USA and Europe |
| 99mTc- mannosyl neoglycoalbumin/ 99mTc galactosyl neoglycoalbumin | N/A Galactoscint |
9 | Commercially available in Japan | |
| Other | 99mTc-Isosulfan blue | N/A | 639 nm | Radiolabelled dye; only preclinical experience |
Examples of 99mTc-labeled colloids include Sulphur colloid (TechneScan®, TechneColl®), Antimony trisulphide (Lymph-Flo®), Sulphide nanocolloid (Lymphoscint®), Nanocolloidal albumin (Nanocoll®, Nanoalbumon®, Nanotop®) and Rhenium sulphide (Nanocis®) [5, 65]. In the clinical setting, the choice of the radiopharmaceutical is influenced by the local availability, with 99mTc-albumin nanocolloids being present in Europe, 99mTc-antimony trisulphide in Australia and Canada and 99mTc-sulphur colloid in the United States [5].
99mTc-pertechnetate-labeled colloids are particles with size ranging from 5 to 5000 nm [4]. The mechanism of tracer accumulation of these particles is dictated by their dimensions; the tracer drains from the injection site to the SLN, where it stops due to particle size and is phagocytized by the macrophages [51], although a quote of the injected radiotracer proceeds to the higher echelon nodes [8]. The smaller the particle size is, the faster the migration to the SLN will be, although a quote of small particles may drain to non-SLN tissue [5, 51]. An example of 99mTc-pertechnetate-labeled colloid, characterized by small particle size, is 99mTc-pertechnetate-labeled colloids which allow visualization of the SLN with 1 h. With colloids with medium particle size, such as Nanocis®, lymph nodes may occasionally not been identified within a short time interval from radiopharmaceutical administration, requiring delayed acquisition at 4-6 h or even the following day. Larger particles (>200 nm) are mainly retained in lymph nodes but present very slow migration from the injection site [66]. When a node is already clinically evident and there is a massive metastatic involvement, the physiological clearance function mediated by macrophages may be impaired, leading to difficulties in detecting the SLN [8]. Particles with size < 5 nm may penetrate capillary membranes without showing significant retention in the lymph nodes; dimensions of 100-200 nm are commonly considered the most adequate for the identification of the SLN [67]. Persico et al. evaluated Nanocoll®, Nanoalbumon® and Nanotop® demonstrating that the highest radioactivity is vehiculated by particles with size ranging from 30 to 50 nm (representing only 0.02-0.05% of the total) [65].
99mTc-tilmanocept (Lymphoseek®; [mannosyl diethylene-triaminepentaacetic acid (DTPA) dextran]) is a radiotracer recently approved by the Food and Drug Administration and the European Medicines Agency for SLN protocols in the breast, melanoma (in particular for the head and neck melanoma) and head and neck squamous cell carcinoma [4]. Of note, it is important to highlight the higher cost of this radiotracer compared to radiolabeled nanocolloid. The accumulation of this tracer is not size-dependent (molecular size = 7 nm) and binds the mannose receptor CD206, expressed in the surface of macrophages and dendritic cells [4, 5]. This radiotracer presents quick clearance from the injection site, higher retention in the SLN, negligible accumulation in second-echelon nodes compared to radiocolloids and acceptable biodistribution [5, 68]. Importantly, these characteristics determine decreased radiation exposure and mapping time for the patients with an equivalent sensitivity in detecting the SLN [69]. Wallace, first, demonstrated in a small group of 24 melanoma patients equivalent SLN uptake for Lymphoseek® and 99mTc-sulfur colloid, but a faster mean clearance half-time for Lymphoseek® (2.17 ± 0.96 h vs.14.7 ± 6.3 h) [70]. These results are in keeping with a subsequent study performed by another group, who demonstrated a high detection rate of SLN (97.9%) in 47 patients with melanoma [71]. In a multicentre study, including 154 patients, Sondak et al. defined as “hot” nodes the lymph nodes with 3 SD above the normal tissue background count [72]. 99mTc-tilmanocept has also been compared to vital blue dye detecting at least one node in more patients than blue dye (150 vs. 138; p=0.002) [72].
4. SPECT/CT VS. PLANAR IMAGING
The introduction of SPECT and SPECT/CT in protocols for the identification of SLN in patients with melanoma has been advocated due to the relatively recent scientific evidence showing additional value over planar imaging [5] (Table 2). In complex anatomical regions, SPECT or SPECT/CT may act as complementary multimodal techniques in the identification of an SLN located in the proximity of the primary tumor (e.g. head and neck) or in case of multiple LN basins (ubiquitary drainage in case of melanoma of the posterior trunk. Additional anatomical sites that may benefit from the complementary acquisition of SPECT may be shoulder, abdomen and pelvis [8]. SPECT/CT may show superior diagnostic performance than planar imaging, discriminating the LN from the injection site [4, 21] and body regions close to the expected site of lymphatic drainage [4, 5, 8]. In this latter case, a further advantage arises from the possible use of CT which enables the correction for issue attenuation and scattering and better morphologic evaluation, improving the visualization of nodes [8].
Table 2.
Summary of studies published in the last decade including patients with melanoma undergoing planar imaging and SPECT/CT for the identification of SLN. Patient-basis = rate of patients with additional LNs detected by SPECT/CT. SLN-basis = % of additional SLNs detected by SPECT/CT compared to planar imaging. NR=not reported.
| Authors | Year |
Multimodal
Technique |
Anatomical Region | Nr. of Patients | Additional SLNs Detected by SPECT/CT Over Planar Imaging | Refs. | |
|---|---|---|---|---|---|---|---|
| Patient-basis (%) | SLN-basis (%) | ||||||
| Benke | 2018 | SPECT/CT | Trunk | 255 | 16.9 | 18.6 | [1] |
| Borbon-Arce | 2014 | SPECT/CT | Head and neck | 16 | 32 | 20 | [2] |
| Chapman | 2016 | SPECT/CT | Head and neck | 176 | NR | NR | [3] |
| Doepker | 2017 | SPECT/CT | All regions | *351 | 49 | 43 | [4] |
| Fairbairn | 2013 | SPECT/CT | All regions | 32 | 12.5 | 3.1 | [5] |
| Jimenez-Heffernan | 2015 | SPECT/CT | All regions | 264 | 20.2 | 13.2 | [6] |
| Kraft | 2012 | SPECT/CT | All regions | 107 | 43.4 | 32 | [7] |
| Martinez Castillo | 2014 | SPECT/CT | All regions | 63 | 42.9 | 19.8 | [8] |
| Tew | 2017 | SPECT/CT | All regions | 86 | 0 | NR | [9] |
| Trinh | 2018 | SPECT/CT | All regions | 73 | NR | 26.3 | [10] |
| van der Ploeg | 2009 | SPECT/CT | All regions | 85 | 8.2 | 5.6 | [11] |
| Veenstra | 2012 | SPECT/CT | All regions | 35 | 20 | 11.6 | [12] |
| Vermeeren | 2011 | SPECT/CT | Head and neck | 38 | 16 | 6.4 | [13] |
| Zender | 2014 | SPECT/CT | Head and neck | 14 | 28.6 | 23.5 | [14] |
*351 patients: 300 = Melanoma, 33 = Merkel cell carcinoma, 8 = Squamous cell carcinoma, 2 = Sarcoma, Other = 8.
**In the 5/2 patients with additional SLNs.
SPECT/CT has been shown to improve the accuracy and the sensitivity of the procedure, allowing the identification of a larger number of SLNs [5]. Benke et al. [73] demonstrated in patients with trunk melanoma that SPECT/CT located significantly more SLNs than planar lymphoscintigraphy; furthermore, the average number of lymph nodes detected in a patient-based analysis was significantly higher when SPECT/CT was performed. Analysis of planar imaging revealed a 95.1% sensitivity (SS - P < 0.05, CI 90-95.1%), positive predictive value (PPV) of 95.6% (p < 0.05, CI 93.4-97.3%) and an accuracy (ACC) of 89.4% (p < 0.05, CI 86.5-91.9%). For the SPECT-CT test SS was 98.6% (p < 0.05, CI 97.1-99.4%), PPV was 99.8% (p < 0.05 CI 98.9-99.9%) and ACC was 98.4% (p < 0.05, CI 96.8-99.3%).
The use of SPECT/CT provides important anatomical information and may reduce the false-negative rate (a sentinel lymph node excision - SLNE - followed by primary recurrence developed within 12 months after SLNE in the lymph node basin from which a tumor-free SLN had been removed) [5]. Stoffels et al. [74] evaluated two groups of patients (149 patients undergoing standard SLN excision and 254 undergoing SPECT/CT-aided SLN excision) and demonstrated that the false-negative rate was 6.8% (3/41 subjects) in the SPECT/CT cohort and 23.8% (15/48 patients) in the standard cohort (P=.03). Furthermore, among subjects with clinically lymph node-negative melanoma, patients undergoing SPECT/CT-aided SLN excision demonstrated a higher incidence of metastatic involvement and a higher rate of disease-free survival, compared to patients undergoing SLN excision alone.
SPECT/CT is also able to modify clinical decision making. In fact, the large prospective multicenter International Atomic Energy Agency Sentinel Node Trial (21), demonstrated that SPECT/CT had modified the surgical approach in 97 patients with melanoma (37% of the patient population): 41.6%, 39.7%, 33.3%, and 30.2% of subjects with head and neck, trunk, lower limb, and upper limb lesions, respectively [75].
Lastly, SPECT/CT is also useful to identify “in-transit” or “interval” nodes (incidence 3-12%). They are nodes lying along a lymphatic channel between the primary melanoma site and a common basin and that must be considered SLNs because they receive direct lymphatic drainage from a primary tumor site [21].
5. TECHNOLOGICAL ADVANCEMENTS: PORTABLE GAMMA-CAMERAS, FREEHAND SPECT NAVIGATION SYSTEMS AND HYBRID INTRAOPERATIVE IMAGING TECHNIQUES
The conventional method for intraoperative detection of SLN involves the exploration of the surgical bed using a gamma probe to depict areas with higher count rates by means of increasing volumes of an acoustic signal [5]. In the last two decades, new devices for intraoperative detection of SLN have been launched and have been proved by some authors to depict additional SLNs [76, 77]. A common drawback of these techniques may be represented by the reduction of the accuracy in case of deep SLNs but the main advantage is the gain of anatomical information.
Intraoperative small field of view (SFOV) gamma cameras, for example, have been demonstrated to improve localization for SLNB, especially for nodes close to high-activity injection sites [78]. Another interesting device is the freehand SPECT (fhSPECT) which provides intraoperative three-dimensional imaging and encompasses a tracking infrared optical system and data extracted from a conventional gamma probe [8]. This tool may be particularly useful for melanoma located in anatomically complex regions such as the head and neck district [76, 79].
Another technique which appears to be very attractive, due to high spatial and temporal resolution, is the near-infrared (NIR) fluorescence which exploits a tracer emitting optical photons when hit by an external light source. Indocyanine-green (ICG - peak emission at 820 nm -) is the most promising tracer in this field but is a more expensive procedure compared to lymphoscintigraphy, due to the high cost of the equipment. Furthermore, the technique requires several minutes to collect a statistically significant number of photon counts [80], the accuracy of the technique is dependent on the experience of the user and the surgeon can visualize the SLN, only if the probe is placed upon the putative anatomical region containing the SLN. An evolution of this technique is the hybrid gamma-NIR fluorescence intraoperative guidance employing the combined use of ICG and 99mTc [81].
Amongst the newly available techniques, also gamma-ultrasound imaging deserves to be mentioned. Gamma-ultrasound imaging combines functional and anatomical data and has already been evaluated in patients’ head and neck district undergoing fine-needle aspiration cytology [82], but warrants still validation in patients with melanoma [83].
Further hybrid techniques in development include β-Optical coherence tomography, Gamma-β imaging, Gamma-Cerenkov luminescence imaging and Gamma-magnetic resonance imaging [83].
CONCLUSION
SLNB is currently deemed considerably valuable in the management of patients with melanoma, especially in case of intermediate-thickness melanoma. The study of the lymphatic flow rate may yield additional predictive information in terms of lymphogenic metastatic spread. 99mTc-Tilmanocept seems to be the most promising alternative to radiocolloids for melanoma located in certain anatomical regions; further large investigational studies are warranted to confirm the limited evidence available in the literature. The use of SPECT/CT improves the diagnostic accuracy in the identification of the SLN. New intraoperative tools for the identification of SLN, such as portable gamma-cameras, freehand SPECT navigation systems and hybrid intraoperative imaging techniques, may be helpful for the surgeons; however, further technical validation is warranted.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ACC
Accuracy
- CI
Confidence Interval
- CLND
Complete Lymph Node Dissection
- DFS
Disease-Free Survival
- DSS
Disease-Specific Survival
- fh
Freehand
- h
Hour
- ICG
Indocyanine-Green
- LTR
Lymphatic Transit Rate
- NIR
Near-Infrared
- OS
Overall Survival
- PL
Planar Lymphoscintigraphy
- PPV
Positive Predictive Value
- SAT
Scintigraphic Appearance Time
- SD
Standard Deviation
- SFOV
Small Field of View
- SLN
Sentinel Lymph Node
- SLNB
Sentinel Lymph Node Biopsy
- SLNE
Sentinel Lymph Node Excision
- SPECT/CT
Single Photon Emission Computed Tomography-Computerized Tomography
- SS
Sensitivity
- SST
Scintigraphic Saturation Time
- vs.
Versus
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Karimkhani C., Green A.C., Nijsten T., Weinstock M.A., Dellavalle R.P., Naghavi M., Fitzmaurice C. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br. J. Dermatol. 2017;177(1):134–140. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnone G. Dermatologia e medicina interna. II. Palminteri, G.; Lotti; Scerrato; Brai, Eds. Mattioli 1885; 1998. Ruolo clinico dell’imaging integrato nel melanoma. pp. 1021–1029. [Google Scholar]
- 3.Prado G., Svoboda R.M., Rigel D.S. What’s New in Melanoma. Dermatol. Clin. 2019;37(2):159–168. doi: 10.1016/j.det.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Moncayo V.M., Aarsvold J.N., Alazraki N.P. Lymphoscintigraphy and sentinel nodes. J. Nucl. Med. 2015;56(6):901–907. doi: 10.2967/jnumed.114.141432. [DOI] [PubMed] [Google Scholar]
- 5.Bluemel C., Herrmann K., Giammarile F., Nieweg O.E., Dubreuil J., Testori A., Audisio R.A., Zoras O., Lassmann M., Chakera A.H., Uren R., Chondrogiannis S., Colletti P.M., Rubello D. EANM practice guidelines for lymphoscintigraphy and sentinel lymph node biopsy in melanoma. Eur. J. Nucl. Med. Mol. Imaging. 2015;42(11):1750–1766. doi: 10.1007/s00259-015-3135-1. [DOI] [PubMed] [Google Scholar]
- 6.Sim F.H., Taylor W.F., Ivins J.C., Pritchard D.J., Soule E.H. A prospective randomized study of the efficacy of routine elective lymphadenectomy in management of malignant melanoma. Preliminary results. Cancer. 1978;41(3):948–956. doi: 10.1002/1097-0142(197803)41:3<948:AID-CNCR2820410324>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U., Adamus J., Bandiera D.C., Brennhovd O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R.L., Javorski V.V., Kirov S., Kulakowski A., Lacour J., Lejeune F., Mechl Z., Morabito A., Rodé I., Sergeev S., van Slooten E., Szczygiel K., Trapeznikov N.N., Wagner R.I. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982;49(11):2420–2430. doi: 10.1002/1097-0142(19820601)49:11<2420:AID-CNCR2820491133>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Tardelli E., Mazzarri S., Rubello D., Gennaro M., Fantechi L., Duce V., Romanini A., Chondrogiannis S., Volterrani D., Colletti P.M., Manca G. Sentinel Lymph Node Biopsy in Cutaneous Melanoma: Standard and New Technical Procedures and Clinical Advances. A Systematic Review of the Literature. Clin. Nucl. Med. 2016;41(12):e498–e507. doi: 10.1097/RLU.0000000000001370. [DOI] [PubMed] [Google Scholar]
- 9.Wong S.L., Faries M.B., Kennedy E.B., Agarwala S.S., Akhurst T.J., Ariyan C., Balch C.M., Berman B.S., Cochran A., Delman K.A., Gorman M., Kirkwood J.M., Moncrieff M.D., Zager J.S., Lyman G.H. Sentinel Lymph Node Biopsy and Management of Regional Lymph Nodes in Melanoma: American Society of Clinical Oncology and Society of Surgical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36(4):399–413. doi: 10.1200/JCO.2017.75.7724. [DOI] [PubMed] [Google Scholar]
- 10.Andtbacka R.H., Gershenwald J.E. Role of sentinel lymph node biopsy in patients with thin melanoma. J. Natl. Compr. Canc. Netw. 2009;7(3):308–317. doi: 10.6004/jnccn.2009.0023. [DOI] [PubMed] [Google Scholar]
- 11.Cordeiro E., Gervais M.K., Shah P.S., Look Hong N.J., Wright F.C. Sentinel Lymph Node Biopsy in Thin Cutaneous Melanoma: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2016;23(13):4178–4188. doi: 10.1245/s10434-016-5137-z. [DOI] [PubMed] [Google Scholar]
- 12.Gershenwald J.E., Scolyer R.A., Hess K.R., Sondak V.K., Long G.V., Ross M.I., Lazar A.J., Faries M.B., Kirkwood J.M., McArthur G.A., Haydu L.E., Eggermont A.M.M., Flaherty K.T., Balch C.M., Thompson J.F. American Joint Committee on Cancer Melanoma Expert, P.; the International Melanoma, D.; Discovery, P., Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–792. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gyorki D.E., Sanelli A., Herschtal A., Lazarakis S., McArthur G.A., Speakman D., Spillane J., Henderson M.A. Sentinel Lymph Node Biopsy in T4 Melanoma: An Important Risk-Stratification Tool. Ann. Surg. Oncol. 2016;23(2):579–584. doi: 10.1245/s10434-015-4894-4. [DOI] [PubMed] [Google Scholar]
- 14.Borgognoni L., Sestini S., Gerlini G., Brandani P., Chiarugi C., Gelli R., Giannotti V., Crocetti E. Sentinel Lymph Node Status is a Main Prognostic Parameter Needful for the Correct Staging of Patients with Melanoma Thicker than 4 mm: Single-Institution Experience and Literature Meta-Analysis. Journal of investigative surgery : the official journal of the Academy of Surgical Research. 2017. pp. 1–11. [DOI] [PubMed]
- 15.Sondak V.K., Zager J.S. Who is to blame for false-negative sentinel node biopsies in melanoma? Ann. Surg. Oncol. 2010;17(3):670–673. doi: 10.1245/s10434-009-0857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieweg O.E. False-negative sentinel node biopsy. Ann. Surg. Oncol. 2009;16(8):2089–2091. doi: 10.1245/s10434-009-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappalardo M., Cheng M-H. Abstract: A New Lymphoscintigraphy Staging for Unilateral Extremity Lymphedema Validation and Correlation between Nuclear Images and Clinical Findings. Plast. Reconstr. Surg. Glob. Open. 2018;6(8S):74–75. [Google Scholar]
- 18.Cheng M.H., Pappalardo M., Lin C., Kuo C.F., Lin C.Y., Chung K.C. Validity of the Novel Taiwan Lymphoscintigraphy Staging and Correlation of Cheng Lymphedema Grading for Unilateral Extremity Lymphedema. Ann. Surg. 2018;268(3):513–525. doi: 10.1097/SLA.0000000000002917. [DOI] [PubMed] [Google Scholar]
- 19.Pappalardo M., Cheng M.H. Lymphoscintigraphy for the diagnosis of extremity lymphedema: Current controversies regarding protocol, interpretation, and clinical application. J. Surg. Oncol. 2019 doi: 10.1002/jso.25526. Jun 18 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Valdes Olmos R.A., Vidal-Sicart S., Manca G., Mariani G., Leon-Ramirez L.F., Rubello D., Giammarile F. Advances in radioguided surgery in oncology. The quarterly journal of nuclear medicine and molecular imaging: official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR). Section of the So. 2017;61(3):247–270. doi: 10.23736/S1824-4785.17.02995-8. [and]. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez Paez A.M., Brouwer O.R., Veenstra H.J., van der Hage J.A., Wouters M., Nieweg O.E., Valdés-Olmos R.A. Decisive role of SPECT/CT in localization of unusual periscapular sentinel nodes in patients with posterior trunk melanoma: three illustrative cases and a review of the literature. Melanoma Res. 2012;22(3):278–283. doi: 10.1097/CMR.0b013e32835312b1. [DOI] [PubMed] [Google Scholar]
- 22.Chapman B.C., Gleisner A., Kwak J.J., Hosokawa P., Paniccia A., Merkow J.S., Koo P.J., Gajdos C., Pearlman N.W., McCarter M.D., Kounalakis N. SPECT/CT Improves Detection of Metastatic Sentinel Lymph Nodes in Patients with Head and Neck Melanoma. Ann. Surg. Oncol. 2016;23(8):2652–2657. doi: 10.1245/s10434-016-5175-6. [DOI] [PubMed] [Google Scholar]
- 23.Manca G., Romanini A., Pellegrino D., Borsò E., Rondini M., Orlandini C., Zucchi V., Pasqualetti F., Mariani G. Optimal detection of sentinel lymph node metastases by intraoperative radioactive threshold and molecular analysis in patients with melanoma. J. Nucl. Med. 2008;49(11):1769–1775. doi: 10.2967/jnumed.108.055350. [DOI] [PubMed] [Google Scholar]
- 24.Martin R.C., II, Edwards M.J., Wong S.L., Tuttle T.M., Carlson D.J., Brown C.M., Noyes R.D., Glaser R.L., Vennekotter D.J., Turk P.S., Tate P.S., Sardi A., Cerrito P.B., McMasters K.M. For the University of Louisville Breast Cancer Study Group. Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. Surgery. 2000;128(2):139–144. doi: 10.1067/msy.2000.108064. [DOI] [PubMed] [Google Scholar]
- 25.McMasters K.M., Reintgen D.S., Ross M.I., Wong S.L., Gershenwald J.E., Krag D.N., Noyes R.D., Viar V., Cerrito P.B., Edwards M.J. Sentinel lymph node biopsy for melanoma: how many radioactive nodes should be removed? Ann. Surg. Oncol. 2001;8(3):192–197. doi: 10.1007/s10434-001-0192-4. [DOI] [PubMed] [Google Scholar]
- 26.Morton D.L., Thompson J.F., Cochran A.J., Mozzillo N., Nieweg O.E., Roses D.F., Hoekstra H.J., Karakousis C.P., Puleo C.A., Coventry B.J., Kashani-Sabet M., Smithers B.M., Paul E., Kraybill W.G., McKinnon J.G., Wang H-J., Elashoff R., Faries M.B. MSLT Group. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014;370(7):599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakera A.H., Hesse B., Burak Z., Ballinger J.R., Britten A., Caracò C., Cochran A.J., Cook M.G., Drzewiecki K.T., Essner R., Even-Sapir E., Eggermont A.M., Stopar T.G., Ingvar C., Mihm M.C., Jr, McCarthy S.W., Mozzillo N., Nieweg O.E., Scolyer R.A., Starz H., Thompson J.F., Trifirò G., Viale G., Vidal-Sicart S., Uren R., Waddington W., Chiti A., Spatz A., Testori A. European Association of Nuclear Medicine-European Organisation for Research and. EANM-EORTC general recommendations for sentinel node diagnostics in melanoma. Eur. J. Nucl. Med. Mol. Imaging. 2009;36(10):1713–1742. doi: 10.1007/s00259-009-1228-4. [DOI] [PubMed] [Google Scholar]
- 28.Marghoob A.A., Koenig K., Bittencourt F.V., Kopf A.W., Bart R.S. Breslow thickness and clark level in melanoma: support for including level in pathology reports and in American Joint Committee on Cancer Staging. Cancer. 2000;88(3):589–595. doi: 10.1002/(SICI)1097-0142(20000201)88:3<589:AID-CNCR15>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Beasley G.M., Hu Y., Youngwirth L., Scheri R.P., Salama A.K., Rossfeld K., Gardezi S., Agnese D.M., Howard J.H., Tyler D.S., Slingluff C.L., Jr, Terando A.M. Sentinel Lymph Node Biopsy for Recurrent Melanoma: A Multicenter Study. Ann. Surg. Oncol. 2017;24(9):2728–2733. doi: 10.1245/s10434-017-5883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beasley G.M., Speicher P., Sharma K., Seigler H., Salama A., Mosca P., Tyler D.S. Efficacy of repeat sentinel lymph node biopsy in patients who develop recurrent melanoma. J. Am. Coll. Surg. 2014;218(4):686–692. doi: 10.1016/j.jamcollsurg.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balch C.M., Soong S.J., Gershenwald J.E., Thompson J.F., Reintgen D.S., Cascinelli N., Urist M., McMasters K.M., Ross M.I., Kirkwood J.M., Atkins M.B., Thompson J.A., Coit D.G., Byrd D., Desmond R., Zhang Y., Liu P.Y., Lyman G.H., Morabito A. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J. Clin. Oncol. 2001;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 32.Gershenwald J.E., Thompson W., Mansfield P.F., Lee J.E., Colome M.I., Tseng C.H., Lee J.J., Balch C.M., Reintgen D.S., Ross M.I. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J. Clin. Oncol. 1999;17(3):976–983. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]
- 33.Statius Muller M.G., van Leeuwen P.A., de Lange-De Klerk E.S., van Diest P.J., Pijpers R., Ferwerda C.C., Vuylsteke R.J., Meijer S. The sentinel lymph node status is an important factor for predicting clinical outcome in patients with Stage I or II cutaneous melanoma. Cancer. 2001;91(12):2401–2408. doi: 10.1002/1097-0142(20010615)91:12<2401:AID-CNCR1274>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 34.Morton D.L., Thompson J.F., Cochran A.J., Mozzillo N., Elashoff R., Essner R., Nieweg O.E., Roses D.F., Hoekstra H.J., Karakousis C.P., Reintgen D.S., Coventry B.J., Glass E.C., Wang H.J. MSLT Group. Sentinel-node biopsy or nodal observation in melanoma. N. Engl. J. Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 35.Uren R.F., Howman-Giles R., Chung D., Thompson J.F. Guidelines for lymphoscintigraphy and F18 FDG PET scans in melanoma. J. Surg. Oncol. 2011;104(4):405–419. doi: 10.1002/jso.21770. [DOI] [PubMed] [Google Scholar]
- 36.Mahieu-Renard L., Cammilleri S., Giorgi R., Gaudy-Marqueste C., Mundler O., Richard M.A., Grob J.J. Slow dynamics of lymphoscintigraphic mapping is associated to the negativity of the sentinel node in melanoma patients. Ann. Surg. Oncol. 2008;15(10):2878–2886. doi: 10.1245/s10434-008-0080-2. [DOI] [PubMed] [Google Scholar]
- 37.Nathanson S.D., Nelson L., Karvelis K.C. Rates of flow of technetium 99m--labeled human serum albumin from peripheral injection sites to sentinel lymph nodes. Ann. Surg. Oncol. 1996;3(4):329–335. doi: 10.1007/BF02305661. [DOI] [PubMed] [Google Scholar]
- 38.Uren R.F., Hawman-Giles R., Thompson J.F. Variation in cutaneous lymphatic flow rates. Ann. Surg. Oncol. 1997;4(3):279–281. doi: 10.1007/BF02306624. [DOI] [PubMed] [Google Scholar]
- 39.Uren R.F., Howman-Giles R.B., Thompson J.F., Roberts J., Bernard E. Variability of cutaneous lymphatic flow rates in melanoma patients. Melanoma Res. 1998;8(3):279–282. doi: 10.1097/00008390-199806000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Nathanson S.D., Anaya P., Avery M., Hetzel F.W., Sarantou T., Havstad S. Sentinel lymph node metastasis in experimental melanoma: relationships among primary tumor size, lymphatic vessel diameter and 99mTc-labeled human serum albumin clearance. Ann. Surg. Oncol. 1997;4(2):161–168. doi: 10.1007/BF02303800. [DOI] [PubMed] [Google Scholar]
- 41.Harrell M.I., Iritani B.M., Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 2007;170(2):774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruddell A., Harrell M.I., Minoshima S., Maravilla K.R., Iritani B.M., White S.W., Partridge S.C. Dynamic contrast-enhanced magnetic resonance imaging of tumor-induced lymph flow. Neoplasia (New York, N.Y.) 2008;10(7):706–713. doi: 10.1593/neo.08342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol. (Dordr.) 2016;39(5):397–410. doi: 10.1007/s13402-016-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shields J.D., Borsetti M., Rigby H., Harper S.J., Mortimer P.S., Levick J.R., Orlando A., Bates D.O. Lymphatic density and metastatic spread in human malignant melanoma. Br. J. Cancer. 2004;90(3):693–700. doi: 10.1038/sj.bjc.6601571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maza S., Valencia R., Geworski L., Sandrock D., Zander A., Audring H., Dräger E., Winter H., Sterry W., Munz D.L. Influence of fast lymphatic drainage on metastatic spread in cutaneous malignant melanoma: a prospective feasibility study. Eur. J. Nucl. Med. Mol. Imaging. 2003;30(4):538–544. doi: 10.1007/s00259-003-1114-4. [DOI] [PubMed] [Google Scholar]
- 46.Cammilleri S., Jacob T., Rojat-Habib M.C., Hesse S., Berthet B., Giorgi R., Bonerandi J.J., Mundler O. High negative predictive value of slow lymphatic drainage on metastatic node spread detection in malignant limb and trunk cutaneous melanoma. Bull. Cancer. 2004;91(7-8):E225–E228. [PubMed] [Google Scholar]
- 47.Toubert M.E., Just P.A., Baillet G., Kerob D., Hindié E., Verola O., Revol M., Servant J.M., Basset-Seguin N., Lebbé C., Banti E., Rubello D., Moretti J.L. Slow dynamic lymphoscintigraphy is not a reliable predictor of sentinel-node negativity in cutaneous melanoma. Cancer Biother. Radiopharm. 2008;23(4):443–450. doi: 10.1089/cbr.2008.0468. [DOI] [PubMed] [Google Scholar]
- 48.Akhras V., Stanton A.W., Levick J.R., Mortimer P.S. A quantitative examination of lymph drainage from perilesion skin in human melanoma. Lymphat. Res. Biol. 2012;10(3):107–111. doi: 10.1089/lrb.2012.0009. [DOI] [PubMed] [Google Scholar]
- 49.Richtig E., Komericki P., Trapp M., Ott A., Bisail B., Egger J. W., Zalaudek I. Ratio of marked and excised sentinel lymph nodes and scintigraphic appearance time in melanoma patients with negative sentinel lymph node. Eur. J. Surg. Oncol.; The journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2010;36(8):783–8. doi: 10.1016/j.ejso.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Conway W.C., Faries M.B., Nicholl M.B., Terando A.M., Glass E.C., Sim M., Morton D.L. Age-related lymphatic dysfunction in melanoma patients. Ann. Surg. Oncol. 2009;16(6):1548–1552. doi: 10.1245/s10434-009-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glass E.C., Essner R., Morton D.L. Kinetics of three lymphoscintigraphic agents in patients with cutaneous melanoma. J. Nucl. Med. 1998;39(7):1185–1190. [PubMed] [Google Scholar]
- 52.Nathanson S.D. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98(2):413–423. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 53.Ogasawara Y., Ikeda H., Takahashi M., Kawasaki K., Doihara H. Evaluation of breast lymphatic pathways with indocyanine green fluorescence imaging in patients with breast cancer. World J. Surg. 2008;32(9):1924–1929. doi: 10.1007/s00268-008-9519-7. [DOI] [PubMed] [Google Scholar]
- 54.Uren R.F., Howman-Giles R.B., Chung D., Thompson J.F. Role of lymphoscintigraphy for selective sentinel lymphadenectomy. Cancer Treat. Res. 2005;127:15–38. doi: 10.1007/0-387-23604-X_2. [DOI] [PubMed] [Google Scholar]
- 55.Mariani G., Gipponi M., Moresco L., Villa G., Bartolomei M., Mazzarol G., Bagnara M.C., Romanini A., Cafiero F., Paganelli G., Strauss H.W. Radioguided sentinel lymph node biopsy in malignant cutaneous melanoma. J. Nucl. Med. 2002;43(6):811–827. [PubMed] [Google Scholar]
- 56.Fujiwara M., Sawada M., Kasuya A., Matsushita Y., Yamada M., Fukamizu H., Magata Y., Tokura Y., Sakahara H. Measurement of cutaneous lymphatic flow rates in patients with skin cancer: area extraction method. J. Dermatol. 2014;41(6):498–504. doi: 10.1111/1346-8138.12506. [DOI] [PubMed] [Google Scholar]
- 57.Fujiwara M., Suzuki T., Takiguchi T., Fukamizu H., Tokura Y. Lymphatic transit rate as a novel predictive parameter for nodal metastasis in primary truncal skin cancers. J. Dermatol. 2016;43(2):170–174. doi: 10.1111/1346-8138.13033. [DOI] [PubMed] [Google Scholar]
- 58.Fujiwara M., Suzuki T., Kasuya A., Shimauchi T., Fukamizu H., Tokura Y. Lymphatic transit rate as a predictive parameter for nodal metastasis in primary limb malignant melanoma. J. Dermatol. Sci. 2018;90(1):27–34. doi: 10.1016/j.jdermsci.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Solari N., Gipponi M., Stella M., Queirolo P., di Somma C., Villa G., Piccardo A., Gualco M., Cardinale F., Cafiero F. Predictive role of preoperative lymphoscintigraphy on the status of the sentinel lymph node in clinically node-negative patients with cutaneous melanoma. Melanoma Res. 2009;19(4):243–251. doi: 10.1097/CMR.0b013e32832e0b9a. [DOI] [PubMed] [Google Scholar]
- 60.Ho A.M., Avery R., Krupinski E.A., Warneke J., Kuo P.H. Predictive role of imaging in sentinel lymph node dissection for melanoma. Lymphology. 2014;47(3):134–141. [PubMed] [Google Scholar]
- 61.Howard J.H., Ozao-Choy J.J., Hiles J.M., Sim M.S., Faries M.B. Prognostic Value of Multiple Draining Lymph Node Basins in Melanoma: A Matched-Pair Analysis Based on the John Wayne Cancer Institute Experience. Front. Oncol. 2017;7:172. doi: 10.3389/fonc.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dale P.S., Foshag L.J., Wanek L.A., Morton D.L. Metastasis of primary melanoma to two separate lymph node basins: prognostic significance. Ann. Surg. Oncol. 1997;4(1):13–18. doi: 10.1007/BF02316805. [DOI] [PubMed] [Google Scholar]
- 63.Federico A. C., Chagpar A. B., Ross M. I., Martin R. C., Noyes R. D., Goydos J. S., Beitsch P. D., Urist M. M., Ariyan S., Sussman J. J., McMasters K. M., Scoggins C. R. Effect of multiple- nodal basin drainage on cutaneous melanoma. rchives of surgery (Chicago, Ill. : 1960) 2008;632(7):632–7. doi: 10.1001/archsurg.143.7.632. discussion 637-8. [DOI] [PubMed] [Google Scholar]
- 64.McHugh J.B., Su L., Griffith K.A., Schwartz J.L., Wong S.L., Cimmino V., Chang A.E., Johnson T.M., Sabel M.S. Significance of multiple lymphatic basin drainage in truncal melanoma patients undergoing sentinel lymph node biopsy. Ann. Surg. Oncol. 2006;13(9):1216–1223. doi: 10.1245/s10434-006-9014-z. [DOI] [PubMed] [Google Scholar]
- 65.Persico M.G., Lodola L., Buroni F.E., Morandotti M., Pallavicini P., Aprile C. (99m)Tc-human serum albumin nanocolloids: particle sizing and radioactivity distribution. J. Labelled Comp. Radiopharm. 2015;58(9):376–382. doi: 10.1002/jlcr.3317. [DOI] [PubMed] [Google Scholar]
- 66.Cheng G., Kurita S., Torigian D.A., Alavi A. Current status of sentinel lymph-node biopsy in patients with breast cancer. Eur. J. Nucl. Med. Mol. Imaging. 2011;38(3):562–575. doi: 10.1007/s00259-010-1577-z. [DOI] [PubMed] [Google Scholar]
- 67.AGENCY. I. A. E., Radiopharmaceuticals for Sentinel Lymph Node Detection: Status and Trends. Vienna: INTERNATIONAL ATOMIC ENERGY AGENCY; 2015. [Google Scholar]
- 68.Hoh C.K., Wallace A.M., Vera D.R. Preclinical studies of [99mTc]DTPA-mannosyl-dextran☆☆☆Supported in part by National Cancer Institute Grant CA72751 and University of California Breast Cancer Research Program Grants 2RB-0018 and 4IB-0051. Nucl. Med. Biol. 2003;30(5):457–464. doi: 10.1016/S0969-8051(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 69.Silvestri C., Christopher A., Intenzo C., Kairys J.C., Kim S., Willis A., Berger A.C. Consecutive case series of melanoma sentinel node biopsy for Lymphoseek compared to sulfur colloids. J. Surg. Res. 2019;233:149–153. doi: 10.1016/j.jss.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 70.Wallace A.M., Hoh C.K., Ellner S.J., Darrah D.D., Schulteis G., Vera D.R. Lymphoseek: a molecular imaging agent for melanoma sentinel lymph node mapping. Ann. Surg. Oncol. 2007;14(2):913–921. doi: 10.1245/s10434-006-9099-4. [DOI] [PubMed] [Google Scholar]
- 71.Leong S.P., Kim J., Ross M., Faries M., Scoggins C.R., Metz W.L., Cope F.O., Orahood R.C. A phase 2 study of (99m)Tc-tilmanocept in the detection of sentinel lymph nodes in melanoma and breast cancer. Ann. Surg. Oncol. 2011;18(4):961–969. doi: 10.1245/s10434-010-1524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sondak V.K., King D.W., Zager J.S., Schneebaum S., Kim J., Leong S.P., Faries M.B., Averbook B.J., Martinez S.R., Puleo C.A., Messina J.L., Christman L., Wallace A.M. Combined analysis of phase III trials evaluating [99mTc]tilmanocept and vital blue dye for identification of sentinel lymph nodes in clinically node-negative cutaneous melanoma. Ann. Surg. Oncol. 2013;20(2):680–688. doi: 10.1245/s10434-012-2612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benke M., Wocial K., Lewandowska W., Rutkowski P., Teterycz P., Jarek P., Dedecjus M. Value of planar lymphoscintigraphy (PL) versus SPECT/CT in evaluation of sentinel lymph node in trunk melanoma - one center, large series retrospective study. Nucl. Med. Rev. Cent. East. Eur. 2018;21(2):79–84. doi: 10.5603/NMR.a2018.0022. [DOI] [PubMed] [Google Scholar]
- 74.Stoffels I., Boy C., Pöppel T., Kuhn J., Klötgen K., Dissemond J., Schadendorf D., Klode J. Association between sentinel lymph node excision with or without preoperative SPECT/CT and metastatic node detection and disease-free survival in melanoma. JAMA. 2012;308(10):1007–1014. doi: 10.1001/2012.jama.11030. [DOI] [PubMed] [Google Scholar]
- 75.Jimenez-Heffernan A., Ellmann A., Sado H., Huić D., Bal C., Parameswaran R., Giammarile F., Pruzzo R., Kostadinova I., Vorster M., Almeida P., Santiago J., Gambhir S., Sergieva S., Calderon A., Young G.O., Valdes-Olmos R., Zaknun J., Magboo V.P., Pascual T.N. Results of a Prospective Multicenter International Atomic Energy Agency Sentinel Node Trial on the Value of SPECT/CT Over Planar Imaging in Various Malignancies. J. Nucl. Med. 2015;56(9):1338–1344. doi: 10.2967/jnumed.114.153643. [DOI] [PubMed] [Google Scholar]
- 76.Vermeeren L., Valdés Olmos R.A., Klop W.M., Balm A.J., van den Brekel M.W. A portable gamma-camera for intraoperative detection of sentinel nodes in the head and neck region. J. Nucl. Med. 2010;51(5):700–703. doi: 10.2967/jnumed.109.071407. [DOI] [PubMed] [Google Scholar]
- 77.Vermeeren L., Valdés Olmos R.A., Klop W.M., van der Ploeg I.M., Nieweg O.E., Balm A.J., van den Brekel M.W. SPECT/CT for sentinel lymph node mapping in head and neck melanoma. Head Neck. 2011;33(1):1–6. doi: 10.1002/hed.21392. [DOI] [PubMed] [Google Scholar]
- 78.Vidal-Sicart S., Paredes P., Zanón G., Pahisa J., Martinez-Román S., Caparrós X., Vilalta A., Rull R., Pons F. Added value of intraoperative real-time imaging in searches for difficult-to-locate sentinel nodes. J. Nucl. Med. 2010;51(8):1219–1225. doi: 10.2967/jnumed.110.074880. [DOI] [PubMed] [Google Scholar]
- 79.KleinJan. G.H.; Karakullukçu, B.; Klop, W.M.C.; Engelen, T.; van den Berg, N.S.; van Leeuwen, F.W.B. Introducing navigation during melanoma-related sentinel lymph node procedures in the head-and-neck region. EJNMMI Res. 2017;7(1):65. doi: 10.1186/s13550-017-0312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marshall M.V., Rasmussen J.C., Tan I.C., Aldrich M.B., Adams K.E., Wang X., Fife C.E., Maus E.A., Smith L.A., Sevick-Muraca E.M. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open surgical oncology journal (Online) 2010;2(2):12–25. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frontado L.M., Brouwer O.R., van den Berg N.S., Mathéron H.M., Vidal-Sicart S., van Leeuwen F.W., Valdés Olmos R.A. Added value of the hybrid tracer indocyanine green-99mTc-nanocolloid for sentinel node biopsy in a series of patients with different lymphatic drainage patterns. Rev. Esp. Med. Nucl. Imagen Mol. 2013;32(4):227–233. doi: 10.1016/j.remnie.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 82.de Bree R., Pouw B., Heuveling D.A., Castelijns J.A. Fusion of Freehand SPECT and Ultrasound to Perform Ultrasound-Guided Fine-Needle Aspiration Cytology of Sentinel Nodes in Head and Neck Cancer. AJNR Am. J. Neuroradiol. 2015;36(11):2153–2158. doi: 10.3174/ajnr.A4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bugby S.L., Lees J.E., Perkins A.C. Hybrid intraoperative imaging techniques in radioguided surgery: present clinical applications and future outlook. Clin. Transl. Imaging. 2017;5(4):323–341. doi: 10.1007/s40336-017-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]