Abstract
Background: Chitinases are the evolutionary conserved glycosidic enzymes that are characterized by their ability to cleave the naturally abundant polysaccharide chitin. The potential role of chitinases has been identified in the manifestation of various allergies and inflammatory diseases. In recent years, chitinases inhibitors are emerging as an alluring area of interest for the researchers and scientists and there is a dire need for the development of potential and safe chitinase antagonists for the prophylaxis and treatment of several diseases.
Objective: The present review expedites the role of chitinases and their inhibitors in inflammation and related disorders.
Methods: At first, an exhaustive survey of literature and various patents available related to chitinases were carried out. Useful information on chitinases and their inhibitor was gathered from the authentic scientific databases namely SCOPUS, EMBASE, PUBMED, GOOGLE SCHOLAR, MEDLINE, EMBASE, EBSCO, WEB OF SCIENCE, etc. This information was further analyzed and compiled up to prepare the framework of the review article. The search strategy was conducted by using queries with key terms “ chitin”, “chitinase”, “chitotrisidase”, “acidic mammalian chitinase”, “chitinase inhibitors”, “asthma” and “chitinases associated inflammatory disorders”, etc. The patents were searched using the key terms “chitinases and uses thereof”, “chitinase inhibitors”, “chitin-chitinase associated pathological disorders” etc. from www.google.com/patents, www.freepatentsonline.com, and www.scopus.com.
Results: The present review provides a vision for apprehending human chitinases and their participation in several diseases. The patents available also signify the extended role and effectiveness of chitinase inhibitors in the prevention and treatment of various diseases viz. asthma, acute and chronic inflammatory diseases, autoimmune diseases, dental diseases, neurologic diseases, metabolic diseases, liver diseases, polycystic ovary syndrome, endometriosis, and cancer. In this regard, extensive pre-clinical and clinical investigations are required to develop some novel, potent and selective drug molecules for the treatment of various inflammatory diseases, allergies and cancers in the foreseeable future.
Conclusion: In conclusion, chitinases can be used as potential biomarkers in prognosis and diagnosis of several inflammatory diseases and allergies and the design of novel chitinase inhibitors may act as key and rational scaffolds in designing some novel therapeutic agents in the treatment of variety of inflammatory diseases.
Keywords: Acidic Mammalian Chitinases (AMCase), allergy, allosamidin (Allo), chitinases, chitotriosidase (CHIT1), inflammatory disorders
1. INTRODUCTION
Chitinases (EC 3.2.1.14) are the enzymes which belong to the 18 glycosyl hydroxylase (GH18) family, a class of ancient, conserved evolutionary enzymes [1]. They are distributed in a variety of organisms that include bacteria, viruses, fungi, parasites, insects, plants and mammals. They undergo the cleavage of glycosidic bond by the hydrolysis of chitin via a substrate assisted mechanism [2].
Chitin is a linear, insoluble polysaccharide of β-1,4-linked polymer containing N-acetyl glucosamine as the monosaccharide units [3-5]. Although humans do not synthesize chitin of their own, yet their genome expresses certain chitinases which can specifically degrade chitin upon confrontation during inhalation or ingestion, thereby exhibiting an anti-pathogenic function [6, 7].
Recently, chitinases have emerged as an area of interest owing to their significant role in human health care [8, 9]. In the recent times, researchers have come across with various evidence suggesting the potential role of chitinases not only in the human defense system against parasites but also act as biomarkers for inflammatory diseases, asthma, acute and chronic inflammatory diseases, autoimmune diseases, dental diseases, neurologic diseases, metabolic diseases, liver diseases, polycystic ovary syndrome, endometriosis and cancer [10].
In this review, we intend to illuminate several chitinases and their inhibitors along with the related recent patents for the development of specific therapeutic approaches in the management of various diseases in particular, allergy and inflammatory disorders [11].
2. CHITINASES
Chitinases are the glycosidases which have been isolated from the stomachs of certain mammals, including humans [12]. Chitinase activity can also be detected in human blood [13, 14] and possibly cartilage [15]. Their sizes range from 20kDa to about 90kDa [2].
In humans, chitinases or chitin degrading enzymes consists of both enzymatically active chitinases and chitinase-like proteins also referred to as chi-lectins which are devoid of enzymatic activity [16]. The chitinases can be further classified as endochitinases and exochitinases based upon their mechanism of action i.e. whether it is endolytic or exolytic [17]. The endochitinases include Chitotriosidase or Chitinase 1(CHIT1) [18, 19] and Acidic Mammalian Chitinase (AMCase) [20, 21]. They cleave the chitin polymer randomly at internal sites [22]. The exochitinases referred to as Chitobiase (CTBS), catalyze the hydrolysis of chitin polymer in a progressive manner. Also identified are a set of enzymes that lack the catalytic site partially or completely [8]. However, they have retained carbohydrate binding pockets [23, 24]. They are collectively termed as Chitinase-Like Proteins (CLPs) or Chi- Lectins (ChiLs) [25]. The ChiLs so far identified in humans include Chitinase-3-Like 1 (CHI3L1), CHI3L2, Oviductin (OVGP1) and Stabilin-1 interacting Chitinase-Like Protein (SI-CLP) or CHID [26]. The phylogenetic studies have revealed that the active chitinases resulted from an early gene duplication event and the later duplication events followed by gene mutations resulted in the evolution of CLPs or ChiLs [27].
Chitinases exhibit their roles in both innate and adaptive immunity [28]. They are essential for the following three different functions: Chitinases are expressed in certain organisms during development to aid in the remodeling of their biological matrices to accommodate changes in body size and shape [29]. Secondly, chitinases help some organisms digest chitin containing food. Chitinases are also expressed in mammals, including humans and mice, that are prone to cause some reactions with chitin-containing pathogens (e.g. house dust mites, fungi, parasites) to degrade the chitin protective covering on the infectious microorganisms. This way the inner core of chitin is susceptible to attacks by chitinases that ultimately lead to death and expulsion from the body of the chitin-containing organisms [30].
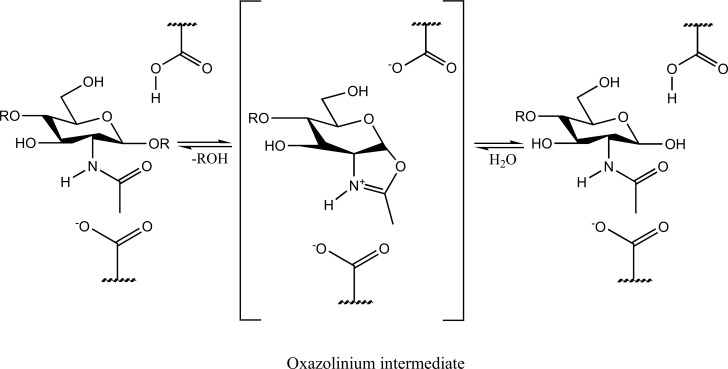
The mechanism of action of family 18 chitinases is an unusual substrate assisted reaction mechanism, in which oxazolinium ion intermediate is formed as described in Fig. (1). Conserved glutamate protonates the glycosidic bond and the oxygen of the N-acetyl group on the -1 sugar (the sugar on the non-reducing end of the glycosidic bond) [31].
Fig. (1).
Mechanism of action of chitinases undergoing chitin hydrolysis. Here, R is β-1,-linked N-acetylglucosamine residue. Chitinases hydrolyze the glycosidic linkage of the repeating β -1,-linked N-acetylglucosamine-containing polymer chitin. The hydrolysis is accompanied with the formation of an oxazolinium intermediate followed by the degradation of chitin in simpler monosaccharides.
3. CHITOTRIOSIDASES
Chitotriosidase (CHIT1) is the first mammalian chitinase to be discovered. The gene encoding this is located on chromosome 1q31-q32. Boot et al. showed that CHIT1 has 12 exons [32]. It is synthesized as a 50kDa protein containing a 39kDa N-terminal catalytic triose-phosphate isomerase fold, which is characterized by the (β/α)δ barrel. It is a true chitinase having trans-glycosylation activity towards chitin [2].
Hollak et al. showed that it is both a diagnostic hallmark of Gaucher’s Disease (GD) and a marker for monitoring the efficacy of various therapeutic approaches for the treatment of GD [33]. Weisner et al. demonstrated that recombinant CHIT1 plays a physiological role in the host defense mechanism by inhibiting fungal hyphal growth [34]. Di Rosa et al. suggested that chitinases regulate the immune response to chitin [35]. Boot et al. depicted that its activity is very low in healthy population [32]. On the other hand, Malaguarnera et al. reported that its activity increases a great deal during acute and chronic inflammatory disorders [36]. Van Ejik et al. unveiled the fact that it is induced in neutrophils by TLR signaling and in macrophages by NOD-2 signaling. They also depicted that the people having the mutant allele exhibited an increased susceptibility to various infections like Candida albicans, Wuchereria bancrofti, Cryptococcus neoformans and Plasmodium falciparum [37].
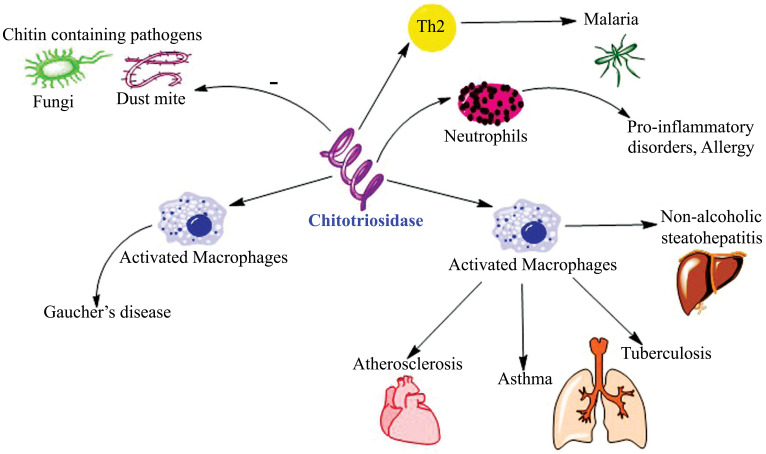
Malaguarnera et al. confirmed that CHIT1 provides innate protection against malaria. They also stressed on the fact that in fibroblastic hepatic tissue, tissue remodeling is modulated by CHIT1 over expression in Kupffer cells [38]. Kim et al. proved that CHIT1 A442G polymorphism is linked to increased childhood atopy [39]. Conversely, Di Rosa et al. demonstrated that a functional CHIT1 gene polymorphism retards progression of NAFLD [40]. Artieda et al. reported that atherosclerotic plaque formation and thrombosis are enhanced by chitotriosidases produced by macrophages [41]. Bargagli et al. [42] and Iyer et al. [43] proposed that CHIT1 is elevated specifically in the later inflammatory conditions like tuberculosis and leprosy. Therefore, they are implicated in the progression of many such diseases as depicted in Fig. (2).
Fig. (2).
Chitotriosidase (CHIT1) and cell type specificity and its role in associated pathological disorders. CHIT1 is the first mammalian chitinase to be discovered and characterized. It is a potential biomarker of activated macrophages in tissues hence exhibits a significant role in the manifestation of both innate and adaptive immunity. It exhibits hydrolysis as well as trans-glycosylation as its chitinolytic activity.
Sotgiu et al. showed the correlation between chitotriosidase activity and stroke severity [44]. Di Rosa et al. depicted that chitotriosidase mRNA expression was elevated in Alzheimer’s disease [45]. Ries et al. showed that evaluation of CHIT1 activity helps in guiding further confirmatory assays for NPC (Niemann Pick disease type- C) and SMD (Sphingomyelinase deficiency) [46]. As far as male Fabry disease patients are concerned, the only marker discovered till date for monitoring the efficacy of enzyme replacement therapy in them is CHIT1. Barone et al. pointed to the increased secretion of CHIT1 in response to the lysozomal system damage mediated by iron [47].
Di Rosa et al. depicted the increased mRNA CHIT1 expression in monocytes on treatment with IL-4. The Th2 cytokine IL-4 promotes immune responses to parasites. This finding helps us to envision clearly the association of increased CHIT1 secretion with the pathophysiological conditions mediated by Th2 cells [35]. Gavala et al. proposed the role of CHIT1 in asthma and airway hyper responsiveness [48]. Lee et al. demonstrated that CHIT1 play a significant role in IL-13 driven alveolar fibrosis by accentuating MAPK and TGFβ signaling in mice [49].
Overall it can be confirmed that CHIT1 play a role in both innate and adaptive immune responses to inflammatory stimuli and that their inhibition would definitely have beneficial effects in various diseased states.
4. ACIDIC MAMMALIAN CHITINASES
Acidic Mammalian Chitinases (AMCase) are true chitinases which are located on chromosome 1 (1q13.1e21.3) [50]. They contain a 30-kDa N-terminal catalytic domain just like CHIT1 and are expressed mainly in lungs (airway epithelial cells) and gastrointestinal system of humans and mice [51]. They also contain a C-terminal chitinase binding domain (CBM). The enzymes are acid stable and have an optimum pH of 2.0; therefore, they are able to function well in the harsh environment of the stomach [50, 52].
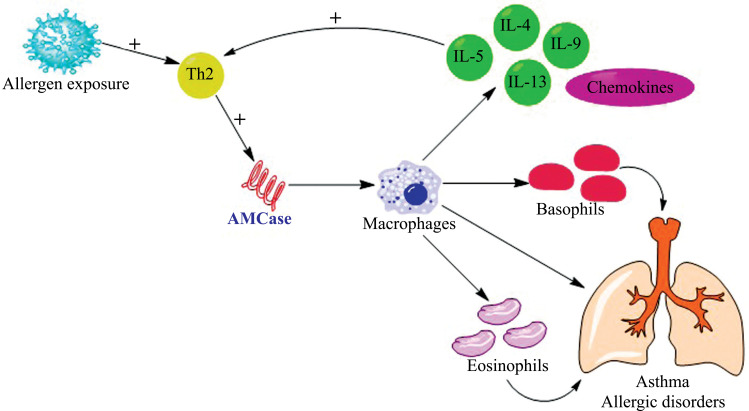
Zhu et al. [51] and Zimmermann et al. [53] showed that AMCases are specifically up regulated in response to Th2 inflammation in the lungs (Fig. 3). Di Rosa et al. demonstrated that AMCases modulate tissue inflammation and immunity by either directly acting as chemotactic agents or by indirectly inducing other chemokines that attract T cells and eosinophils to the sites of parasitic infection [54]. AMCase is closely associated with most of the pathophysiological conditions that are mediated by Th2 cells. Now-a-days, the use of antimicrobials and antibiotics has increased a lot, especially in industrialized countries [55]. They suppress bacterial populations which produce chitinases therefore the levels of chitins are going up. In such settings, production of a hyper functional AMCase enzyme would prove beneficial to the subjects [7]. Matsumoto et al. showed that the prevalence of new onset asthma is high in individuals employed in industries, in which the exposure to chitin is anticipated to be high, like in shellfish processors [56]. Seibold et al. reported that pre-treatment of chitin with recombinant AMCase decreased chitin’s inflammatory effects to a great deal [57].
Fig. (3).
Acidic mammalian chitinase (AMCase), cell type specificity and immune response mechanism. AMCase exhibits its true chitinase activity via hydrolysis of chitin polymer. It manifests its potential role in Th2 mediated pathophysiological conditions such as asthma and allergies. Higher expression of AMCases is detected in the lung tissues of asthmatic patients.
They have also identified an AMCase isoform which shows significant activity at almost all pH values; the haplotype encoding it is found to be protective against asthma in various ethnic populations of the United States. Bierbaum et al. suggested that AMCases help airway epithelial cells in evading apoptosis by AKT signaling and PI3K stimulation [58].
5. CHITINASE INHIBITORS
Zhu et al. demonstrated chitinase inhibitors to have anti-inflammatory potential against asthma and allergic diseases, including atopic dermatitis and allergic rhinitis [51].
With an intent of identifying a useful chitinase inhibitor, Sunazuka et al. have developed a potent 1,5- Disubstituted triazole inhibitor of Serratia marcescens chitinase (SmChi) B from an azide-bearing arginin fragment and application of the chitinase template utilizing the in situ click chemistry approach [59]. The new inhibitor expressed a 300 fold increase of inhibition against SmChiB compared to that of arginin [60]. Allosamidin, a pseudo trisaccharide first isolated from the mycelium of Streptomyces sp., is the most extensively studied chitinase inhibitor [61]. Various biological properties have been reported associated with its activity as a chitinase inhibitor, including inhibition of cell separation in fungi, toxicity towards insect larvae and blocking of malaria parasite penetration into the mosquito mid gut [62, 63]. Zhu et al. additionally reported that this compound was shown to decrease the number of inflammatory cells in a mouse model of asthma by targeting the Acidic Mammalian Chitinase (AMCase) [51]. Although total synthesis of allosamidin has been reported previously in Berecibar et al., 1999 [64], the length and complexity of the synthetic routes involved severely limit both the availability of this compound and the scope for structure-based design of novel allosamidin-derived inhibitors [65]. A similar type of problem appears with argifin because its molecular weight and number of hydrogen-bond donors/ acceptors well exceed the Lipinski criteria for drug-likeliness [66].
Matsumoto et al. reported that treatment with chitinase inhibitor- Demethylallosamidin (Dma) attenuated IL-13- induced Airway Hyper Responsiveness (AHR) [67] even at a lower dose than that of Allosamidin (Allo), a metabolite of Streptomyces. It also suppressed allergen-induced AHR. These findings suggest that DMA is a more effective inhibitor of asthmatic responses than Allo and could be a potential therapeutic target for asthma [68].
Andersen et al. underwent the progressive dissection of argifin, a natural product cyclopentapeptide. The peptide fragments inhibited chitinase B1 from Aspergillus fumigatus (AfChiB1), the human chitotriosidase and chitinase activity in lung homogenates from a murine model of chronic asthma, with potencies ranging from high nano molar to high micro molar inhibition [66]. Withers et al. described the proficient and readily scalable synthesis of novel, potent chitobiose and chitotriose thiazolines, especially in their thioamide forms possessing broad spectrum inhibitory activities, which could be used as probes for the modulation of chitinase activity in the biological systems [69].
van Aalten et al. identified methyl xanthine derivatives such as caffeine, theophylline and pentoxifylline to be the possible human chitinase inhibitor leads after screening a drug library of marketed drug molecules and devised their therapeutic role as anti-inflammatory agents, with pleiotropic mechanism of action thereby providing synthetically accessible scaffolds for further optimization [70]. van Aalten et al. also demonstrated the inhibitory effects of four new cyclic peptides (CI-4) derivatives by undertaking their structural optimization and hence providing further structural insights and new scaffolds to understand the mechanism of inhibition of chitinases [71]. In another study, they described the enzymological and structural analysis of the interaction between the enzyme chitinase ChiB from S. marcescens and the previously designed and synthesized N-N- diacetyl-chitobionoxime-N-phenylcarbamate (HM508), a GlcNAc2-derivative and identified their efficient inhibitory role [72].
6. RECENT PATENTS
In the past two decades, several other chitinase inhibitors have been identified and patented as shown in Table 1.
Table 1.
Patents of Novel Inventions in the Treatment of Various Chitinase Associated Disorders and Inhibitors Thereof.
| S. No. | Disease to be Treated | Patent Title | Patent Number | Inventors |
Date of
Publication |
Legal Status | References |
|---|---|---|---|---|---|---|---|
| 1. | Asthma, acute and chronic inflammatory diseases, autoimmune diseases, dental diseases, neurologic diseases, metabolic diseases, liver diseases, polycystic ovary syndrome, endometriosis, and cancer | Substituted amino triazoles useful as human chitinase inhibitors | US10208020 | Mazur, M., Koralewski, R., Borek, B., Olezniczak, S., Czestkowski, W., Piotrowicz, M.C., Olczack, J.P., Golebiowski, A.A., Bartoszewicz, A. Maziarez, E., Kowalski, M.I. | 19.02.2019 | Granted | [73] |
| 2. | Renal injury and renal failure | Methods for evaluating renal injury and renal failure using urine levels of chitinase - 3 - like protein 1 | US20190120858 | Anderberg, J., Gray, J., McPherson, P., Nakamura, K., Kampf, J.P. | 25.04.2019 | Granted | [74] |
| 3. | Epithelial and cutaneous disorders | Cosmetic use of chitinase - type proteins | US9926587 | Bernard, D., Berville, M.D. | 27.03.2018 | Granted | [75] |
| 4. | Inflammation and age- related pulmonary fibrosis | Chitinase administration to the airway to treat inflammation and age-related pulmonary fibrosis | WO2018191379 | Richard, L., Steven, V.D. | 18.10.2018 | Granted | [76] |
| 5. | Fungal infection | New macrocyclic amidinourea derivatives, methods of preparation and uses thereof as chtinase inhibitors | US20160137617 | Sanfilippo, S., Posteraro, B., Sanguinetti, M., Botta, M., Maccari, G., De Luca, F., Docquier, J., Deodato, D. | 19.05.2016 | Granted | [77] |
| 6. | Asthma and allergic responses | Substituted amino triazoles useful as human chitinase inhibitors | US2016176843 | Golebiowski, A.A., Koralewski, R., Czestkowski, W., Matyszewski, K., Olejniczak, S., Olczak, J., Beckett, P. | 23.06. 2016 | Granted | [78] |
| 7. | Asthma and allergic responses | Substituted amino triazoles useful as acidic mammalian chitinase inhibitors | US20160368894 | Golebiowski, A.A., Koralewski, R., Czestkowski, W., Matyszewski, K., Olejniczak, S., Olczak, J., Beckett, P. | 22.12.2016 | Granted | [79] |
| 8. | Asthma | Substituted amino triazoles, and methods using same | WO2015095701 | Corman, M.M., Gollebiowski, A., Beckett, R.P., Mazur, M., Olezniczak, S. | 25.06.2015 | Granted | [80] |
| 9. | IL-13 mediated inflammation | C/CLP antagonists and methods of use thereof | US20110059100 | Reed, J.L., White, W., Coyle, A., Kozhich, A., Elias, J., Doncki, N., Gao, C., Wu, H. | 10.03.2011 | Granted | [81] |
| 10. | Inflammatory disorders | Novel human acidic mammalian chitinase and use thereof. | US20090191552 | Vora, K.A., Demartino, J.A., Mudgett, J.S., Poster, J., Wolfe, G. |
30.07.2009 | Granted | [82] |
| 11. | Th2-driven and/or IL-mediated inflammatory diseases | C/CLP antagonists and methods of use thereof | WO2007027748 | Reed, J.L., White, W., Coyle, A., Kozhich, A., Elias, J. | 08.03.2007 | Granted | [83] |
| 12. | Various degenerative diseases involving inflammation, tissue repair and tissue remodeling | Use of chitooligomer formulations to modify abnormal activity of chitinase like proteins | WO2006054319 | Olafur, B.E., Jon, M.E., Johannes, G., Finnbogi, T. | 26.05.2006 | Granted | [84] |
| 13. | Asthma | Inhibitors of Acidic Mammalian Chitinase as asthma therapeutics | WO2004092404 | Maximillian, T.F. | 28.10.2004 | Granted | [85] |
| 14. | Inflammatory disease | Methods, compositions and kits relating to chitinases and chitin like molecules and inflammatory disease | WO03009808 | Elias, J., Zhu, Z. | 06.02.2003 | Granted | [86] |
| 15. | Fungal infection | Purified chitinases and use thereof | US6251390 | Harman, G.E., Broadway, R.M., Tronsmo, A., Lorito, M., Hayes, C.K., Pietro, A.D. | 26.06.2001 | Granted | [87] |
Mazur et al. disclosed the unexpected discovery owing to the chemical modification of amino-triazole-4-amino piperdine small compounds with a heterocyclic ring substitution (morpholine or piperazine) leading to the better fixation of their molecular geometry and increasing the molecular rigidity that resulted in an unexpected enhancement in their inhibitory efficacy towards acidic mammalian chitinase. Hence, the substituted amino -triazole compounds can be used in the treatment of various disorders associated with the up regulation or dysregulation of AMCase activity and CHIT1 activity such as asthma reactions caused by allergens as well as acute and chronic inflammatory diseases, auto-immune, metabolic, neurologic diseases and cancer US10208020 (2019) [73].
Anderberg et al. disclosed that chitinase-3-like protein 1 can be used as a biomarker in a subject with renal injury and hence the present invention described different assays, methods and compositions to measure the chitinase-3-like protein 1 for the prognosis, diagnosis, risk-stratification, stage determination, monitoring and providing effective therapeutic regimen to a subject suffering from or suspected of a renal injury US20190120858 (2019) [74].
Bernard et al. in their invention disclosed the cosmetic and therapeutic use of YKL-40 protein that belongs to the family of chitinase- type proteins or of peptides derived from this protein or of analogs thereof, of a nucleic acid sequences encoding such a polypeptide or an agent that modulates the expression of such a polypeptide especially for the stimulation of terminal epithelial differentiation US9926587 (2018) [75].
Richard et al. disclosed the novel therapeutic delivery of chitinases in the aerosolized form promoting the clearance of environmentally derived chitins and hence exerting a beneficial role in the prevention and treatment of pulmonary fibrosis. Also, the invention encompassed novel diagnostic methods for the detection of chitin and chitinase activity WO2018191379 (2018) [76].
Sanfilippo et al. described the synthesis of a new series of amidinourea derivatives for the treatment of fungal infections of candida species, IL-13 and Th2 mediated inflammation, allergic airway diseases and asthma. The disclosure also provided pharmaceutical compositions and formulations for the treatment of fungal infections to be used as chitinase inhibitors US20160137617 (2016) [77].
Golebiowski et al. in their patents described the synthesis of a novel series of amino triazole compounds substituted with carboxylate group or bioisosteric polar functional group as therapeutically potent Acidic mammalian chitinase inhibitors for the treatment of asthma caused by different allergens. They also disclosed methods for the treatment of fungal or parasitic infections that comprises of administration of therapeutically effective amount of synthesized compounds under consideration US20160368894, US2016176843 (2016) [78, 79].
Corman et al. disclosed the novel substituted amino triazoles and their respective pharmaceutically acceptable salts, hydrates and solvates as potential inhibitors of Acidic mammalian chitinases. The invention also provided different methods for the treatment of asthma and allergies and to monitor the asthma treatment as well WO2015095701 (2015) [80].
Reed et al. in their disclosure described the novel molecules, compounds and methods of use of the same as C/CLP antagonists for the treatment of disorders associated with the up regulation of one or more C/CLPs, Th-2 driven inflammatory disorders of the lungs, inflammatory arthritis and IL-13 mediated inflammation. These antagonists include any suitable molecules that disrupt one or more activities of C/CLPs including antibodies, antibody fragments, having specificity for C/CLP ad their fragments. The patent also revealed isolated antagonists identified via the disclosed methods and medicaments and therapeutic compositions comprising the same US20110059100 (2011) [81].
Vora et al. disclosed in their patent the novel variant acidic mammalian chitinase with improved stability and an isolated nucleic acid sequence that is responsible for encoding the variant enzyme. The invention further provided the nucleic acid constructs, vectors and host cells comprising of nucleic acid sequences and methods for the production of recombinant variant polypeptide. Also, the potential role of AMCase in high throughput screening assays to identify whether a test compound is capable of modulating its chitinolytic activity was described US20090191552 (2009) [82].
Reed et al. in their patent disclosed the novel C/CLP antagonists, their compositions and various methods for the treatment or amelioration and management of Th-2 driven and IL-13 mediated inflammatory disorders of lungs and inflammatory arthritis associated with the up regulation of C/CLPs WO2007027748 (2007) [83].
Olafur et al. described the use of novel chiti oligomer formulations for the modulation of inflammation in patients suffering from connective tissue, cardiovascular tissue associated diseases, central nervous system or degenerative disorders thereby reducing the abnormally high expression of chitinase-like proteins. It was also revealed that chitoligomeric formulations promote tissue regeneration instead of tissue repair and do not allow the fibroblasts to invade the tissue sites WO2006054319 (2006) [84].
Maximillian et al. described different methods for screening of agents involved in the exhibition of certain symptoms and pathological complications involved in asthma. The administration of therapeutic mount of these agents which include AMCase inhibitors, IL-13 antagonists and also the soluble antagonists of IL-13 receptors led to the decrease in the expression or production of AMCase protein that can be useful in treating asthma. The disclosure also included a method for monitoring the efficacy of treatment of asthma WO2004092404 (2004) [85].
Elias et al. stated in their invention that administration of an effective amount of chitinase-like molecule inhibitor to a mammal resulted in the treatment of inflammatory diseases. The chitinase-like molecule inhibitor is selected from a group consisting of a chemical compound, an antibody, a nucleic acid, a ribozyme and an anti-sense nucleic acid molecule. The disclosure also provided methods to identify new chemical compounds for the treatment of asthma, COPD, allergies and inflammatory diseases as for the first time the expression of IL-13 and chitinase-like molecule was found to be associated with inflammatory diseases WO03009808 (2003) [86].
Harman et al. in their patent provided the biologically pure compositions of chitinases containing an endochitinase and an exochitinase that can be used for the treatment of fungal infections and various insect related infections by inhibiting chitin containing fungi and insects US6251390 (2001) [87].
7. CHITIN AND CHITINASE EXPOSURE - ASSOCIATED PATHOGENIC MECHANISMS
Human chitinases play a significant role in mediating different allergic reactions and auto-immune diseases. They cause several allergic reactions in atopic individuals or in individuals with chitin exposure in high doses or for longer duration of time [88]. The females are at higher risk of attaining the chitinase allergies since they possess the hormone estrogen [89]. Cytokines or chemokines are secreted upon the recognition of chitins via pattern recognition receptors (pprs) [90]. Bacterial clearance is then promoted by CXCL8 which is a neutrophil attractant at the site of infection. Glycosylation of important host proteins takes place rendering them either active or inactive. The glycol moieties are then cleaved from these glycol proteins by the pathogenic or allergen acquires chitinases as they are glycosidic in nature, leading the alteration of their functions and hence resulting in flustered protein cascade precipitating various diseases like auto-immune diseases, asthma, cystic fibrosis and cancer [91- 94].
The chitin binding domains are present in lethal viruses such as Ebola and hepatic C virus and Zika virus, which are used by them to manipulate their hosts and carrier organisms [95]. In addition to this, the mosquito vector Aedea aegypti that is held responsible for transmitting dengue and yellow fever in humans [96].
The pathogenic bacterium, Vibrio cholera also expresses chitinases that causes gut inflammation by triggering the immune responses [97].
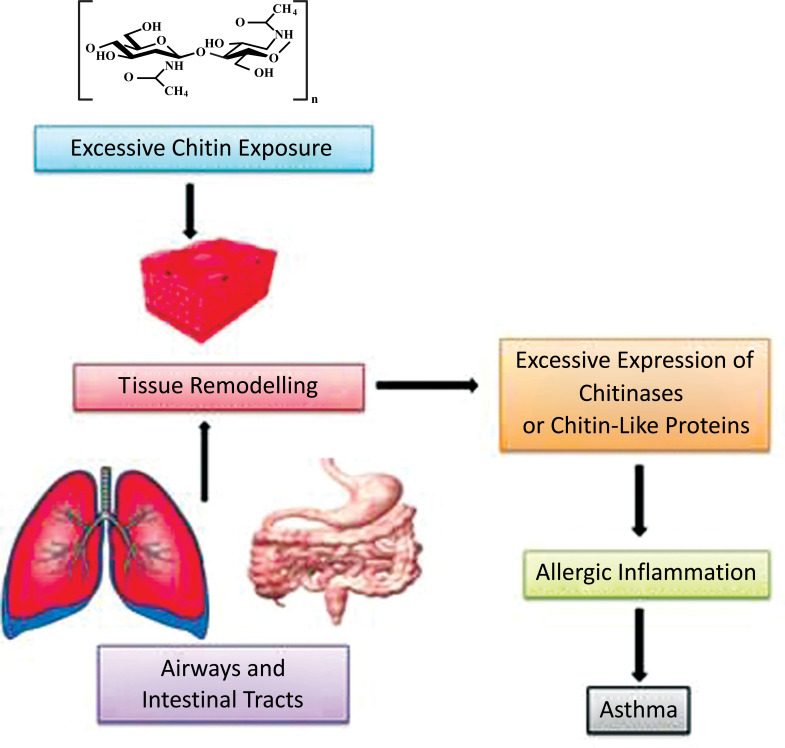
The chitin-chitinase allergies could be referred to as occupational allergies since they exhibit a major dependency on the occupation of individuals. For instance, prohevein, a cysteine rich protein present in rubber (Hevea brasiliensis), bears high homology with human chitin-binding proteins, causes IgE-associated allergy in workers wearing gloves in laboratories or healthcare practices [98]. The carpenters who work with woods like chestnut are more prone to asthma and rhinitis since they are exposed to the tree chitinases for longer durations [99, 100]. Similarly, people working in seafood processing industries are susceptible to allergies associated with chitinases of fish and crustaceans [101-103] (Fig. 4).
Fig. (4).
Chitin-chitinase- associated occupational allergies. Chitinase associated allergies are often associated with the population of workers working for an occupation that involves consistent exposure with chitins and homologous human chitin binding proteins.
The potential immune modulatory role of chitinases has been studied in house-dust mite allergic patients where they induce basophil activation in IgE dependent manner [104]. The findings of a murine model administered with chitin has demonstrated the ability of chitinaes to induce the infiltration of tissues of IL-4 expressing innate immune responses with Interlukin (IL)-4- and IL-13- expressing cells, including basophils, eosinophils and T-helper cells thereby expressing the significant role of chitinases in the modulation of allergic and parasitic worm immunity [105, 106].
CONCLUSION
In conclusion, chitinases can be used as significant biomarkers in prognosis and diagnosis of several inflammatory diseases and allergies. Therefore, by utilizing the findings of experiments performed with knockout animal models and interpretation of different mechanisms involved in their physiological as well as pathological roles, they could serve as potential candidates in the development of novel, potent and selective drug molecules. Hence, further extensive pre- clinical and clinical investigations are required to develop some novel chitinase inhibitors to treat various inflammatory diseases, allergies and cancers in the foreseeable future.
CURRENT & FUTURE DEVELOPMENTS
The present review article summarized the applications of chitinases in biomedical and environmental field. Chitinases are glycosal hydrolases that are widely expressed in mammals. Their diversity is not only restricted to GH 18 and GH 19 family of enzymes as previously anticipated. In the recent years, chitinase research has attained unparalleled growth at the molecular levels. Current studies have revealed their effective role in both adaptive and innate immunity and therefore, they are being used as biomarkers in several inflammatory and malignant disorders. Promising results have been achieved in the direct application of parenteral chitinase preparations to treat systemic fungal infections. Chitinases have attained prominence as bio control agents for insect vectors of certain human diseases. Also, the production of synthetic polysaccharides as prophylactic and therapeutic agents and neoglycoproteins as significant tools for structure function analysis with the aid of chitinases has drawn special attention in the recent times.
In the future, additional studies and experiments are required to explore the role of specific cell types involved in the secretion of chitinases via protein engineering and to better apprehend the biology and immune biology of chitinases along with the identification of their active sites for the enhancement of human immunity. Also, there stands a possibility of utilizing chitinases as food preservatives to increase the shelf life of certain food products. At last, chitinases can be studied further for their environmental applications as they serve as anti-pollutants to control the water pollution since they convert the chitinaeous waste of marine organisms into simpler compounds. Therefore, they can act as environment conservers by remediation of various organic contaminants. Also, it is hoped that this review will encourage the researchers to explore chitinases at a broad scale in order to develop new methodologies and therapeutic approaches for the betterment of mankind
ACKNOWLEDGEMENTS
Authors pay sincere thanks to Prof. Aditya Shastri, Vice Chancellor, Banasthali Vidyapith, Rajasthan, India and Indian Council of Medical Research (ICMR), India, for providing the necessary support for the compilation of this study in a convenient manner.
LIST OF ABBREVIATIONS
- GD
Gaucher’s Disease
- TLR
Toll Like Receptor
- NOD-2
Nucleotide-binding Oligomerization Domain-containing protein 2
- CHIT1
Chitotriosidase
- NAFLD
Non-Alcoholic Fatty Liver Disease
- CBM
C-Terminal Chitinase Binding Domain
- AHR
Airway Hyperresponsiveness
- NPC
Niemann Pick Disease Type-C
- SMD
Sphingomyelinase Deficiency
- IL-13
Interleukin-13
- MAPK
Mitogen Activated Protein Kinase
- TGF-β
Transforming Growth Factor Beta
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this review.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
Authors are grateful to Indian Council of Medical Research (ICMR), India, (45/74/2018/PHA/BMS/OL) for providing the financial support.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Henrissat B., Bairoch A. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1993;293(Pt 3):781–788. doi: 10.1042/bj2930781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamid R., Khan M.A., Ahmad M., Ahmad M.M., Abdin M.Z., Musarrat J., et al. Chitinases: An update. J. Pharm. Bioallied Sci. 2013;5(1):21–29. doi: 10.4103/0975-7406.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barikani M., Oliaei E., Siddiqi H., Honarkar H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014;23:307–326. doi: 10.1007/s13726-014-0225-z. [DOI] [Google Scholar]
- 4.Anitha A. Chitin and chitosan in selective biomedical applications. Prog. Polym. Sci. 2014;39:1644–1667. doi: 10.1016/j.progpolymsci.2014.02.008. [DOI] [Google Scholar]
- 5.Solairaj D., Rameshthangam P., Muthukumaran P., Wilson J. Studies on electrochemical glucose sensing, antimicrobial activity and cytotoxicity of fabricated copper nanoparticle immobilized chitin nanostructure. Int. J. Biol. Macromol. 2017;101:668–679. doi: 10.1016/j.ijbiomac.2017.03.147. [DOI] [PubMed] [Google Scholar]
- 6.Tang W.J., Fernandez J., Sohn J.J., Amemiya C.T. Chitin is endogenously produced in vertebrates. Curr. Biol. 2015;25(7):897–900. doi: 10.1016/j.cub.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Rosa M., Distefano G., Zorena K., Malaguarnera L. Chitinases and immunity: Ancestral molecules with new functions. Immunobiology. 2016;221(3):399–411. doi: 10.1016/j.imbio.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Adrangi S., Faramarzi M.A. From bacteria to human: A journey into the world of chitinases. Biotechnol. Adv. 2013;31(8):1786–1795. doi: 10.1016/j.biotechadv.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Gooday G.W. Aggressive and defensive roles for chitinases. EXS. 1999;87:157–169. doi: 10.1007/978-3-0348-8757-1_11. [DOI] [PubMed] [Google Scholar]
- 10.Nagpure A., Choudhary B., Gupta R.K. Chitinases: In agriculture and human healthcare. Crit. Rev. Biotechnol. 2014;34(3):215–232. doi: 10.3109/07388551.2013.790874. [DOI] [PubMed] [Google Scholar]
- 11.Lee C.G., Da Silva C.A., Dela Cruz C.S., Ahangari F., Ma B., Elias J.A., et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paoletti M.G., Norberto L., Damini R., Musumeci S. Human gastric juice contains chitinase that can degrade chitin. Ann. Nutr. Metab. 2007;51(3):244–251. doi: 10.1159/000104144. [DOI] [PubMed] [Google Scholar]
- 13.Renkema G.H., Boot R.G., Muijsers A.O., Donker-Koopman W.E., Aerts J.M. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J. Biol. Chem. 1995;270(5):2198–2202. doi: 10.1074/jbc.270.5.2198. [DOI] [PubMed] [Google Scholar]
- 14.Escott G.M., Adams D.J. Chitinase activity in human serum and leukocytes. Infect. Immun. 1995;63(12):4770–4773. doi: 10.1128/IAI.63.12.4770-4773.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakala B.E., White C., Recklies A.D. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 1993;268(34):25803–25810. [PubMed] [Google Scholar]
- 16.Kuddus M., Ahmad I.Z. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J Genet Eng Biotechnol. 2013;11:39–46. doi: 10.1016/j.jgeb.2013.03.001. [DOI] [Google Scholar]
- 17.Rathore A.S., Gupta R.D. Chitinases from bacteria to human: Properties, applications and future perspectives. Enzyme Res. 2015;2015: 791907. doi: 10.1155/2015/791907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D-H., Park H-J., Lim S., Lee H-G., Choi J.O., Oh J.H., et al. Regulation of chitinase-3-like-1 in T cell elicites Th1 nad cytotoxic responses to inhibit lung metastasis. Nat. Commun. 2018;9(503):1–14. doi: 10.1038/s41467-017-02731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidibule P.E., Sanlos-Moriano P., Jimimez-Ortego E., Rmiriz-Escudero M., Limon M.C., Rimacha M., et al. Use of chitin and chitosan to produce new chitooligosaccharides by chitinase Chit42: Enzymatic activity and structural basis of protein specificity. Microb. Cell Fact. 2018;17(1):47. doi: 10.1186/s12934-018-0895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata Y., Metzger W.J., Myrvik Q.N. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: Mannose receptor-mediated phagocytosis initiates IL-12 production. J. Immunol. 1997;159(5):2462–2467. [PubMed] [Google Scholar]
- 21.Synstad B., Gåseidnes S., Van Aalten D.M., Vriend G., Nielsen J.E., Eijsink V.G. Mutational and computational analysis of the role of conserved residues in the active site of a family 18 chitinase. Eur. J. Biochem. 2004;271(2):253–262. doi: 10.1046/j.1432-1033.2003.03923.x. [DOI] [PubMed] [Google Scholar]
- 22.Dahiya N., Tewari R., Hoondal G.S. Biotechnological aspects of chitinolytic enzymes: A review. Appl. Microbiol. Biotechnol. 2006;71(6):773–782. doi: 10.1007/s00253-005-0183-7. [DOI] [PubMed] [Google Scholar]
- 23.Stoykov Y.M., Pavlo A.I., Krastanov A.I. Chitinase biotechnology: Production, purification and application. Eng. Life Sci. 2015;15(1):30–38. doi: 10.1002/elsc.201400173. [DOI] [Google Scholar]
- 24.Kabir S.R., Rehman M.M., Tasnim S., Karim M.R., Khatun N., Hasan I., et al. Purification and characterization of a novel chitinase from Trichosanthes dioica seed with antifungal activity. Int. J. Biol. Macromol. 2016;84:62–68. doi: 10.1016/j.ijbiomac.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Vega K., Kalkum M. Chitin, chitinase responses, and invasive fungal infections. Int. J. Microbiol. 2012;2012: 920459. doi: 10.1155/2012/920459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roopavathy A.S., Vigneshwari R., Jayapradha R. Chitinase production and applicatons. J. Chem. Pharm. Res. 2015;7(5):924–931. [Google Scholar]
- 27.Bussink A.P., Speijer D., Aerts J.M., Boot R.G. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics. 2007;177(2):959–970. doi: 10.1534/genetics.107.075846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elias J.A., Homer R.J., Hamid Q., Lee C.G. Chitinases and chitinase-like proteins in T(H)2 inflammation and asthma. J. Allergy Clin. Immunol. 2005;116(3):497–500. doi: 10.1016/j.jaci.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Rameshthangam P., Solairaj D., Arunachalam G., Ramasamy P. Chitin and chitinases: Biomedical and environmental applications of chitin and its derivatives. J Enz. 2018;1(1):20–43. [Google Scholar]
- 30.Ober C., Chupp G.L. The chitinase and chitinase-like proteins: A review of genetic and functional studies in asthma and immune-mediated diseases. Curr. Opin. Allergy Clin. Immunol. 2009;9(5):401–408. doi: 10.1097/ACI.0b013e3283306533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald J.M., Tarling C.A., Taylor E.J., Dennis R.J., Myers D.S., Knapp S., et al. Chitinase inhibition by chitobiose and chitotriose thiazolines. Angew. Chem. Int. Ed. Engl. 2010;49(14):2599–2602. doi: 10.1002/anie.200906644. [DOI] [PubMed] [Google Scholar]
- 32.Boot R.G., Renkema G.H., Verhoek M., Strijland A., Bliek J., de Meulemeester T.M. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J. Biol. Chem. 1998;273(40):25680–25685. doi: 10.1074/jbc.273.40.25680. [DOI] [PubMed] [Google Scholar]
- 33.Hollak C.E., van Weely S., van Oers M.H., Aerts J.M. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Invest. 1994;93(3):1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiesner D.L., Specht C.A., Lee C.K., Smith K.D., Mukaremera L., Lee S.T., et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015;11(3): e1004701. doi: 10.1371/journal.ppat.1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Rosa M., Malaguarnera G., De Gregorio C., D’Amico F., Mazzarino M.C., Malaguarnera L. Modulation of chitotriosidase during macrophage differentiation. Cell Biochem. Biophys. 2013;66(2):239–247. doi: 10.1007/s12013-012-9471-x. [DOI] [PubMed] [Google Scholar]
- 36.Malaguarnera L., Di Rosa M., Zambito A.M. dell’ Ombra N, Di Marco R, Malaguarnera M. Potential role of chitotriosidase gene in non alcoholic fatty liver disease evolution. Am. J. Gastroenterol. 2006;101:2060–2069. doi: 10.1111/j.1572-0241.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 37.van Eijk M., Scheij S.S., van Roomen C.P., Speijer D., Boot R.G., Aerts J.M. TLR- and NOD2-dependent regulation of human phagocyte-specific chitotriosidase. FEBS Lett. 2007;581(28):5389–5395. doi: 10.1016/j.febslet.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 38.Malaguarnera L., Simporè J., Prodi D.A., Angius A., Sassu A., Persico I., et al. A 24-bp duplication in exon 10 of human chitotriosidase gene from the sub-Saharan to the Mediterranean area: Role of parasitic diseases and environmental conditions. Genes Immun. 2003;4(8):570–574. doi: 10.1038/sj.gene.6364025. [DOI] [PubMed] [Google Scholar]
- 39.Kim K.W., Park J., Lee J.H., Lee H.S., Lee J., Lee K.H., et al. Association of genetic variation in chitotriosidase with atopy in Korean children. Ann. Allergy Asthma Immunol. 2013;110(6):444–449.e1. doi: 10.1016/j.anai.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Di Rosa M., Mangano K., De Gregorio C., Nicoletti F., Malaguarnera L. Association of chitotriosidase genotype with the development of non-alcoholic fatty liver disease. Hepatol. Res. 2013;43(3):267–275. doi: 10.1111/j.1872-034X.2012.01063.x. [DOI] [PubMed] [Google Scholar]
- 41.Artieda M., Cenarro A., Gañán A., Lukic A., Moreno E., Puzo J., et al. Serum chitotriosidase activity, a marker of activated macrophages, predicts new cardiovascular events independently of C-reactive protein. Cardiology. 2007;108(4):297–306. doi: 10.1159/000099099. [DOI] [PubMed] [Google Scholar]
- 42.Bargagli E., Margollicci M., Nikiforakis N., Luddi A., Perrone A., Grosso S., et al. Chitotriosidase activity in the serum of patients with sarcoidosis and pulmonary tuberculosis. Respiration. 2007;74(5):548–552. doi: 10.1159/000100555. [DOI] [PubMed] [Google Scholar]
- 43.Iyer A., van Eijk M., Silva E., Hatta M., Faber W., Aerts J.M., et al. Increased chitotriosidase activity in serum of leprosy patients: Association with bacillary leprosy. Clin. Immunol. 2009;131(3):501–509. doi: 10.1016/j.clim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Sotgiu S., Barone R., Zanda B., Arru G., Fois M.L., Arru A., et al. Chitotriosidase in patients with acute ischemic stroke. Eur. Neurol. 2005;54(3):149–153. doi: 10.1159/000089935. [DOI] [PubMed] [Google Scholar]
- 45.Di Rosa M., Dell’Ombra N., Zambito A.M., Malaguarnera M., Nicoletti F., Malaguarnera L. Chitotriosidase and inflammatory mediator levels in Alzheimer’s disease and cerebrovascular dementia. Eur. J. Neurosci. 2006;23(10):2648–2656. doi: 10.1111/j.1460-9568.2006.04780.x. [DOI] [PubMed] [Google Scholar]
- 46.Ries M., Schaefer E., Lührs T., Mani L., Kuhn J., Vanier M.T., et al. Critical assessment of chitotriosidase analysis in the rational laboratory diagnosis of children with Gaucher disease and Niemann-Pick disease type A/B and C. J. Inherit. Metab. Dis. 2006;29(5):647–652. doi: 10.1007/s10545-006-0363-3. [DOI] [PubMed] [Google Scholar]
- 47.Barone R., Di Gregorio F., Romeo M.A., Schilirò G., Pavone L. Plasma chitotriosidase activity in patients with beta-thalassemia. Blood Cells Mol. Dis. 1999;25(1):1–8. doi: 10.1006/bcmd.1999.0221. [DOI] [PubMed] [Google Scholar]
- 48.Gavala M.L., Kelly E.A., Esnault S., Kukreja S., Evans M.D., Bertics P.J., et al. Segmental allergen challenge enhances chitinase activity and levels of CCL18 in mild atopic asthma. Clin. Exp. Allergy. 2013;43(2):187–197. doi: 10.1111/cea.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee C.G., Herzog E.L., Ahangari F., Zhou Y., Gulati M., Lee C.M., et al. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-β1 signaling. J. Immunol. 2012;189(5):2635–2644. doi: 10.4049/jimmunol.1201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boot RG, Blommaart EF, Swart E, Ghauharali-Vander Vlugt K. Bij lN, Moe C, et alIdentification of a novel acidic mammalian chitinase distinct from chitotriosidase. J. Biol. Chem. 2001;276(9):6770–6778. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Z., Zheng T., Homer R.J., Kim Y.K., Chen N.Y., Cohn L., et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304(5677):1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 52.Chou Y.T., Yao S., Czerwinski R., Fleming M., Krykbaev R., Xuan D., et al. Kinetic characterization of recombinant human acidic mammalian chitinase. Biochemistry. 2006;45(14):4444–4454. doi: 10.1021/bi0525977. [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann N., Mishra A., King N.E., Fulkerson P.C., Doepker M.P., Nikolaidis N.M., et al. Transcript signatures in experimental asthma: Identification of STAT6-dependent and -independent pathways. J. Immunol. 2004;172(3):1815–1824. doi: 10.4049/jimmunol.172.3.1815. [DOI] [PubMed] [Google Scholar]
- 54.Di Rosa M., Brundo V.M., Malaguarnera L. New insights on chitinases immunologic activities. World J. Immunol. 2016;6(2):96–104. doi: 10.5411/wji.v6.i2.96. [DOI] [Google Scholar]
- 55.Refos J.M., Vonk A.G., Ten Kate M.T., Verbrugh H.A., Bakker-Woudenberg I.A.J.M., van de Sande W.W.J. Chitinase induction prior to caspofungin treatment of experimental invasive Aspergillosis in neuropenic rats does not chance survival. Antimicrob. Agents Chemother. 2017;62(1):1–11. doi: 10.1128/AAC.00960-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumoto T., Inoue H., Sato Y., Kita Y., Nakano T., Noda N., et al. Demethylallosamidin, a chitinase inhibitor, suppresses airway inflammation and hyperresponsiveness. Biochem. Biophys. Res. Commun. 2009;390(1):103–108. doi: 10.1016/j.bbrc.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 57.Seibold M.A., Reese T.A., Choudhry S., Salam M.T., Beckman K., Eng C., et al. Differential enzymatic activity of common haplotypic versions of the human acidic mammalian chitinase protein. J. Biol. Chem. 2009;284(29):19650–19658. doi: 10.1074/jbc.M109.012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bierbaum S. Nicke lR, Koch A, Lau S, Deichmann KA, Wahn U, et al Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am. J. Respir. Crit. Care Med. 2005;172(12):1505–1509. doi: 10.1164/rccm.200506-890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shahidi F., Abuzaytoun R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005;49:93–135. doi: 10.1016/S1043-4526(05)49003-8. [DOI] [PubMed] [Google Scholar]
- 60.Sunazuka T., Hirose T., Omura S. Rapid Identification via in situ click chemistry of a novel chitinase inhibitor. J Synt Org Chem. 2016;74(11):62–68. [Google Scholar]
- 61.Sakuda S., Isogai A., Matsumoto S., Suzuki A., Koseki K. Structure of allosamisin, a novel insect chitinase inhibitor, produced by Streptomyces sp. Tetrahedron Lett. 1986;27:2475–2478. doi: 10.1016/S0040-4039(00)84560-8. [DOI] [Google Scholar]
- 62.Kuranda M.J., Robbins P.W. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 1991;266(29):19758–19767. [PubMed] [Google Scholar]
- 63.Tsai Y.L., Hayward R.E., Langer R.C., Fidock D.A., Vinetz J.M. Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect. Immun. 2001;69(6):4048–4054. doi: 10.1128/IAI.69.6.4048-4054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berecibar A., Grandjean C., Siriwardena A. Synthesis and biological activity of natural amino cyclopentitol glycosidase inhibitors: Mannostatins, trehazolin, allosamidins and their analogues. Chem. Rev. 1999;99(3):779–844. doi: 10.1021/cr980033l. [DOI] [PubMed] [Google Scholar]
- 65.Sakuda S. Studies on the chitinase inhibitors, allosamidins Chitin enzymology. 1996. 2.
- 66.Andersen O.A., Nathubhai A., Dixon M.J., Eggleston I.M., van Aalten D.M. Structure-based dissection of the natural product cyclopentapeptide chitinase inhibitor argifin. Chem. Biol. 2008;15(3):295–301. doi: 10.1016/j.chembiol.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gooyit M., Tricoche N., Lustigman S., Janda K.D. Dual protonophore-chitinase inhibitors dramatically affect O. volvulus molting. J. Med. Chem. 2014;57(13):5792–5799. doi: 10.1021/jm5006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho S.J., Weiden M.D., Lee C.G. Chitotriosidase in the pathogenesis of inflammation, intestinal lung diseases and COPD. Allergy Asthma Immunol. Res. 2015;7(1):14–21. doi: 10.4168/aair.2015.7.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Withers S.G., MacDonald J.M., Tarling C.A., Taylor E.J., Dennis R.J., Myers D.S., et al. Chitinase inhibition by chitobiose and chitotriose thiazolines. Angew. Chem. Int. Ed. 2010;49:2599–2602. doi: 10.1002/anie.200906644. [DOI] [PubMed] [Google Scholar]
- 70.Rao F.V., Andersen O.A., Vora K.A., Demartino J.A., van Aalten D.M. Methylxanthine drugs are chitinase inhibitors: Investigation of inhibition and binding modes. Chem. Biol. 2005;12(9):973–980. doi: 10.1016/j.chembiol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Houston DR1,Synstad B, Eijsink VG, Stark MJ, Eggleston IM, van Aalten DM. Structure-based exploration of cyclic dipeptide chitinase inhibitors. J. Med. Chem. 2004;47:5713–5720. doi: 10.1021/jm049940a. [DOI] [PubMed] [Google Scholar]
- 72.Van Aalten D.M., Vasella A., Peter M.G., Netter C., Houston D.R., Westereng B., et al. Interactions of a family 18 chitinase with the designed inhibitor HM508 and its degradation product, chitobiono-δ-lactone. J. Biol. Chem. 2004;279(5):3612–3619. doi: 10.1074/jbc.M310057200. [DOI] [PubMed] [Google Scholar]
- 73.Mazur M., Koralewski R., Borek B., Olezniczak S., Czestkowski W., Piotrowicz M.C., Olczack J.P., Golebiowski A.A., Bartoszewicz A. Substituted amino triazoles useful as human chitinase inhibitors.US10208020 (2019).
- 74.Anderberg J., Gray J., McPherson P., Nakamura K., Kampf J.P. Methods for evaluating renal injury and renal failure using urine levels of chitinase - 3 - like protein 1. US20190120858 (2019).
- 75.Bernard D., Berville M.D. Cosmetic use of chitinase - type proteins.US9926587 (2018).
- 76.Richard L., Steven V.D. Chitinase administration to the airway to treat inflammation and age-related pulmonary fibrosis.WO2018191379 (2018).
- 77.Sanfilippo S., Posteraro B., Sanguinetti M., Botta M., Maccari G., De Luca F., Docquier J., Deodato D. New macrocyclic amidinourea derivatives, methods of preparation and uses thereof as chtinase inhibitors. US20160137617 (2016).
- 78.Golebiowski A.A., Koralewski R., Czestkowski W., Matyszewski K., Olejniczak S., Olczak J., Beckett P. Matyszewski, K., Olejniczak, S., Olczak, J., Beckett, P. Substituted amino triazoles useful as human chitinase inhibitors. US2016176843 (2016)
- 79.Golebiowski A.A., Koralewski R., Czestkowski W., Matyszewski K., Olejniczak S., Olczak J., Beckett P. Matyszewski, K., Olejniczak, S., Olczak, J., Beckett, P. Substituted amino triazoles useful as acidic mammallian chitinase inhibitors. US20160368894 (2016).
- 80.Corman M.M., Gollebiowski A., Beckett R.P., Mazur M., Olezniczak S. Substituted amino triazoles, and methods using same. WO2015095701 (2015).
- 81.Reed J.L., White W., Coyle A., Kozhich A., Elias J., Doncki N., Gao C., Wu H. C/CLPantagonists and methods of use there of. US20110059100 (2011).
- 82.Vora K.A., Demartino J.A., Mudgett J.S., Poster J., Wolfe G. Novel human acidic mammalian chitinase and use thereof.US20090191552 (2009).
- 83.Reed J.L., White W., Coyle A., Kozhich A., Elias J. C/CLP antagonists and methods of use thereof. WO2007027748 (2007).
- 84.Olafur B.E., Jon M.E., Johannes G., Finnbogi T. Use of chitooligomer formulations to modify abnormal activity of chitinase like proteins. WO2006054319 (2006).
- 85.Maximillian T.F. Inhibitors of acidic mammalian chitinase as asthma therapeutics WO2004092404. (2004).
- 86.Elias J., Zhu Z. Methods, compositions and kits relating to chitinases and chitin like molecules and inflammatory disease. WO03009808 (2003).
- 87.Harman G.E., Broadway R.M., Tronsmom A., Lorito M., Hayes C.K., Pietr A.D. Purified chitinases and use thereof. US6251390 (2001).
- 88.Levitz S.M. Innate recognition of fungal cell walls. PLoS Pathog. 2010;6(4): e1000758. doi: 10.1371/journal.ppat.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edwards D.R.V., Gallins P., Polk M., Ayala-Haedo J., Schwartz S.G., Kovach J.L., et al. Inverse association of female hormone replacement therapy with age-related macular degeneration and interactions with ARMS2 polymorphisms. Invest. Ophthalmol. Vis. Sci. 2010;51(4):1873–1879. doi: 10.1167/iovs.09-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koller B., Müller-Wiefel A.S., Rupec R., Korting H.C., Ruzicka T. Chitin modulates innate immune responses of keratinocytes. PLoS One. 2011;6(2): e16594. doi: 10.1371/journal.pone.0016594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mack I, Hector A, Ballbach M. Kohlhäuf lJ, FuchsKJ, Weber A, etal.The role of chitin, chitinases, and chitinase-like proteins in pediatric lung diseases. Mol. Cell Pediatr. 2015;2(3):1–8. doi: 10.1186/s40348-015-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shuhui L., Mok Y.K., Wong W.S.F. Role of mammalian chitinases in asthma. Int. Arch. Allergy Immunol. 2009;149(4):369–377. doi: 10.1159/000205583. [DOI] [PubMed] [Google Scholar]
- 93.Coffman F.D. Chitinase 3-Like-1 (CHI3L1): A putative disease marker at the interface of proteomics and glycomics. Crit. Rev. Clin. Lab. Sci. 2008;45(6):531–562. doi: 10.1080/10408360802334743. [DOI] [PubMed] [Google Scholar]
- 94.Kzhyshkowska J., Yin S., Liu T., Riabov V., Mitrofanova I. Role of chitinase-like proteins in cancer. Biol. Chem. 2016;397(3):231–247. doi: 10.1515/hsz-2015-0269. [DOI] [PubMed] [Google Scholar]
- 95.Patel S., Goyal A. Chitin and chitinase: Role in pathogenicity, allergenicity and health. Int. J. Biol. Macromol. 2017;97:331–338. doi: 10.1016/j.ijbiomac.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 96.Moreira M.F., dos Santos A.S., Marotta H.R., Mansur J.F., Ramos I.B., Machado E.A., et al. A chitin-like component in Aedes aegypti eggshells, eggs and ovaries. Insect Biochem. Mol. Biol. 2007;37(12):1249–1261. doi: 10.1016/j.ibmb.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 97.Blokesch M. Chitin colonization, chitin degradation and chitin-induced natural competence of Vibrio cholerae are subject to catabolite repression. Environ. Microbiol. 2012;14(8):1898–1912. doi: 10.1111/j.1462-2920.2011.02689.x. [DOI] [PubMed] [Google Scholar]
- 98.Iqbal R.K., Anwar F.N. Chitinases potential as biocontrol. Biomed J Sci Tech Res. 2019;14(5):10994–11001. [Google Scholar]
- 99.Gomma E.Z. Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: Their potential in antifungal biocontrol. J. Microbiol. 2012;50:103–111. doi: 10.1007/s12275-012-1343-y. [DOI] [PubMed] [Google Scholar]
- 100.Sinha M., Singh R.P., Kushwaha G.S., Iqbal N., Singh A., Kaushik S., et al. Current overview of allergens of plant pathogenesis related protein families. ScientificWorldJournal. 2014;2014: 543195. doi: 10.1155/2014/543195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aranda A., Campo P., Palacin A., Do˜na I., Gomez-Casado C., Galindo L., et al. Antigenic proteins involved in occupational rhinitis and asthma caused by obeche wood (Triplochiton scleroxylon). PLoS One. 2013;8(1): e53926. doi: 10.1371/journal.pone.0053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jeebhay M.F., Robins T.G., Lehrer S.B., Lopata A.L. Occupational seafood allergy: A review. Occup. Environ. Med. 2001;58(9):553–562. doi: 10.1136/oem.58.9.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duru S., Yüceege M., Ardıç S. Chitinases and lung diseases. Tuberk. Toraks. 2013;61(1):71–75. doi: 10.5578/tt.3773. [DOI] [PubMed] [Google Scholar]
- 104.Resch Y., Blatt K., Malkus U., Fercher C., Swoboda I., Lupinek C., et al. molecular, structural and immunological characterization of Derp18, a chitinase-like house dust mite allergen. PLoS One. 2016;11(8): e0160641. doi: 10.1371/journal.pone.0160641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reese T.A., Liang H.E., Tager A.M., Luster A.D., Van Rooijen N., Voehringer D., et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447(7140):92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poddighe D., Mathias C.B., Freyschmidt E.J., Kombe D., Caplan B., Marseglia G.L., et al. Basophils are rapidly mobilized following initial aeroallergen encounter in naïve mice and provide a priming source of IL-4 in adaptive immune responses. J. Biol. Regul. Homeost. Agents. 2014;28(1):91–103. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.