Abstract
The present study determined the levels of plasma biomarkers in patients with gastric carcinoma (GC) and investigated their clinical significance and diagnostic value. Between April 2014 and December 2018, 90 patients with GC, 90 patients with precancerous lesions (Pre) and 45 healthy controls (NC) were recruited from the Affiliated Liutie Central Hospital of Guangxi Medical University. Five markers were measured: microRNA-650 (miRNA-650; using reverse transcription-quantitative polymerase chain reaction), and carcinoembryonic antigen (CEA), carbohydrate antigen (CA)125, CA211 and CA50 using electrochemiluminescence. Circulating markers were all upregulated in patients with GC (P<0.05), and CA211 and CA50 were significantly increased in patients with Pre. The miRNA-650 and CA211 had an area under the curve (AUC) of 0.700 (moderate) and 0.866 (high), respectively, in the diagnosis of GC. Differentiation of GC from Pre yielded an AUC of 0.665 (low) and 0.708 (moderate), respectively. The combination model of miRNA-650 and CA211 showed an appropriate value of AUC (0.887) to discriminate the GC patients from the healthy subjects with a sensitivity and specificity of 82.5 and 97.7%. Additionally, differentiating GC from Pre yielded an AUC of 0.767 with a sensitivity of 57.1% and a specificity of 95%, respectively. In terms of clinicopathological features, the expression of miRNA-650 and CA211 in plasma was not associated with the patients' age, sex, Tumor-Node-Metastasis stage, or histological type. In conclusion, plasma miRNA-650 and CA211 is a promising and powerful non-invasive marker for the detection of GC.
Keywords: miRNA-650, carcinoembryonic antigen, carbohydrate antigen 125, carbohydrate antigen 211, carbohydrate antigen 50, gastric carcinoma
Introduction
Gastric carcinoma (GC) is the fourth most common cancer type worldwide and the third leading cause of cancer-associated mortality (1). According to an annual report on the status of cancer in China, GC was recorded as the second most common cancer in terms of incidence and mortality (2). As the majority of patients are diagnosed at advanced stages with complications, poorer prognoses, and limited treatment options, GC remains a major clinical challenge (3). At present, gastroscopy and biopsy remain the standard diagnostic methods in populations at a high risk of GC. However, gastroscopy is difficult to use as a first-line examination method due to its invasiveness and cost, and limited medical resources, which limit its utility in a large number of people. Therefore, a novel diagnostic strategy to solve the aforementioned problems is urgently required. Plasma tumor markers have become a common clinical screening method due to their easy detection in recent years. Tumor markers, including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen (CA)125, and CA19-9 have been extensively used as routine examination items in the diagnosis of GC (4,5), but they have certain limitations under certain conditions. When these markers are used alone in the diagnosis of GC, they tend to have very low sensitivity and specificity. In recent years (6–8), microRNAs (miRNAs) have worked as a DNA transcription regulator for gene expression and have opened up a new use of tumor biomarkers for early cancer diagnosis. For GC, miRNA-21, miRNA-218, miRNA-223, miRNA-378 and miRNA-421 have been reported to function as tumor biomarkers (9–11). Previously, our group reported that miRNA-650 is significantly upregulated in GC plasma (unpublished data). However, as an independent tumor marker, its diagnostic efficacy may not be satisfactory.
A recent study reported that the use of AFP in combination with CEA, CA125, and CAl9-9 may improve sensitivity for the diagnosis of GC (12). Therefore, we hypothesized that the combination of two types of tumor markers may avoid inconsistencies and improve the sensitivity of diagnostic rates. Therefore, in the present follow-up study, the plasma levels of tumor markers CEA, CA125, CA211, CA50, and miRNA-650 were detected in 90 patients with GC, 90 patients with precancerous lesions (Pre) and 45 healthy controls. The aim of the present study was to investigate the expression profiles of tumor markers, CEA, CA125, CA211, CA50, and miRNA-650, and their contribution to the diagnosis of gastric cancer.
Materials and methods
Study design
The present study consisted of three phases: The screening phase, the candidate phase, and the validation phase (Fig. S1). In the screening phase, plasma samples were collected from 90 patients with GC, 90 patients with Pre and 45 healthy controls, and the differential expression levels of miRNA-650, CEA, CA125, CA211, and CA50 in the plasma samples were statistically analyzed. In the candidate phase, multiple logistic regression analysis was conducted to identify the potential biomarkers. The results demonstrated that miRNA-650 and CA211 were the markers for the prediction of the presence of GC. Their diagnostic efficacy in GC and Pre was subsequently determined using receiver operating characteristic (ROC) curves. To further evaluate the diagnostic accuracy of the targeted biomarkers, receiver-operating characteristic (ROC) curves were used to confirm the diagnostic efficacy of the two markers in combination.
Patients
The present study consisted of 90 patients with GC with a mean age of 65 (range, 36–89 years) years, including 68 males and 22 females, 90 Pre patients with a mean age of 61.5 years (range, 29–88 years), including 48 males and 42 females, and 45 healthy controls with a mean age of 59 years (range, 39–80 years), including 21 males and 24 females. All participants were recruited from the Affiliated Liutie Central Hospital of Guangxi Medical University between April 2014 and December 2018. Diagnoses of gastric cancer and Pre were confirmed by histopathology. None of the patients had undergone preoperative therapies, including chemotherapy and radiotherapy. The tumor type and stage were identified for patients with GC based on the Union of International Cancer Control (UICC) Tumor-Node-Metastasis (TNM) system, 7th edition (13). The histology of all patients was evaluated according to World Health Organization (WHO) criteria (14). Among the 90 patients with Pre, 80 had intestinal metaplasia, 6 had severe atypical hyperplasia and 4 had chronic atrophic gastritis. A total of 45 healthy subjects with normal biochemical indexes without a previous history of tumors were selected as normal controls (NCs), and their age, sex, and area of residence were matched with those of the patients with GC or Pre. All participants or their guardians provided written informed consent prior to participation in the study. The Ethics Committee of the Affiliated Liutie Central Hospital of Guangxi Medical University approved the present study.
Sample collection and storage
Approximately 5 ml venous blood samples were collected from the study participants in EDTA-anticoagulant tubes (BD Biosciences) and centrifuged at 1,520 × g for 5 min at 4°C. The plasma samples were transferred into RNase/DNase-free tubes and frozen at −80°C for miRNA extraction. For conventional tumor marker determination, the plasma samples were separated and kept at −20°C until assayed.
Extraction of plasma total RNAs, microRNA validation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 200 µl plasma using a Blood (serum/plasma) MicroRNA Extraction and Purification kit (spin column; LN-0114B; Novland Co., Ltd.), according to the manufacturer's protocol. The concentration and quality of RNA were measured using the NanoQ Micro-Volume Spectrophotometer (CapitalBio, Beijing, China). miR-16 was used as an internal reference in the present study. The expression of the selected plasma miRNA with an initial 2 µl template was determined using a one-step Stemaim-it miR-RT-qPCR kit Quantitation (TaqMan Probes; LK-0106B; Novland Co., Ltd.). The reaction was incubated in a 96-well plate under the following conditions: 45°C for 30 min for reverse transcription, 94°C for 2 min for degeneration, 40 cycles of 94°C for 15 sec, 55°C for 45 sec, and 72°C for 60 sec. The primer sequences for PCR were as follows: miR-650 forward, 5′-AGAGGAGGCAGCGCTCT-3′ and reverse, 5′-CAGTGCGTGTCGTGGAGT-3′ (mature sequence of hsa-miR-650, 5′-AGGAGGCAGCGCUCUCAGGAC-3′). Reference miRNA (hsa-miR-16): Forward, 5′-GTCGTATCCAGTGCAGGGTCCGAGTCGCACTGGATACGACCGCCAA-3′ and reverse, 5′-GTATCCAGTGCAGGGTCCGAGGT-3′. The expression levels of miR-650 were performed in the ABI-7500 PCR system and calculated by cycle threshold (Ct) value with SDS 2.0 software (Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative expression of plasma miRNA-650 was calculated using the 2−ΔΔCq method (15), where ΔCq = Cq (miR-650)-Cq (miR-16).
Conventional tumor markers
Conventional tumor markers were tested by electrochemiluminescence immunoassay, according to the standard procedure of Roche Company's kit, using the Roche E170 automatic immunity analyzer (both Roche Diagnostic GmbH).
Statistical analysis
All statistical analyses and graphics were performed using MedCalc statistical software v18.2.1(MedCalc Software Ltd.) or GraphPad Prism 8.0 (GraphPad Software). Mean values of quantitative variables were evaluated using Student's t-test or the Mann-Whitney U test when the Student's t-test was not satisfied. The diagnostic efficacy was assessed using ROC curve analysis. All statistical tests were two-tailed, and P<0.05 was considered to indicate a statistically significant difference.
Results
Differential expression levels of potential biomarkers in the plasma of patients with GC or Pre and health controls
A previous study demonstrated that oncogenic miRNA-650 expression levels are significantly increased in GC tissues compared with paired normal tissues (16). To assess whether miRNA-650 is a potential circulating tumor marker for the early detection of GC, RT-qPCR was performed on 90 patients with GC, 90 patients with Pre, and 45 healthy controls. As shown in Table I, the expression levels of miRNA-650, CEA, CA125, CA211, and CA50 were significantly increased in patients with GC compared with patients with Pre and normal controls (P<0.05), while no difference in miRNA-650, CEA and CA125 expression were detected between the patients with Pre and normal controls (P>0.05).
Table I.
Differential expression levels of biomarkers in plasma from patients with GC or Pre, and health controls.
| Marker | n | GC, mean ± SD | n | Pre, mean ± SD | n | NC, mean ± SD | GC vs. Pre, P-value | GC vs. NC, P-value | Pre vs. NC, P-value |
|---|---|---|---|---|---|---|---|---|---|
| CEA | 75 | 36.43±135.61 | 62 | 1.92±1.35 | 44 | 2.11±1.18 | <0.0001 | <0.0001 | 0.4706a |
| CA125 | 68 | 58.63±89.08 | 53 | 12.48±7.65 | 45 | 11.06±4.0 | 0.0075 | 0.0091 | 0.9363 |
| CA211 | 40 | 17.52±46.11 | 57 | 2.96±1.38 | 45 | 2.08±0.47 | 0.0011 | 0.0002 | <0.0001 |
| CA50 | 47 | 37.95±94.87 | 77 | 8.16±5.96 | 34 | 4.91±4.39 | 0.0456 | <0.0001 | 0.0101a |
| miR-650(ΔCt) | 90 | 1.17±2.91 | 90 | 2.94±3.02 | 45 | 2.87±0.77 | 0.0001a | <0.001 | 0.7186 |
P-values were calculated using Student's t-test. Mann-Whitney U test was used to determine other categorical variables with statistical significance at the level of P<0.05. The ΔCt values denote the normalized Ct value obtained by subtracting the Ct value of miR-16 from that of miR-650. The lower ΔCt value indicates a higher level of miR-650 expression. GC, gastric carcinoma; Pre, precancerous lesions; SD, standard deviation; NC, normal control; miR, miRNA; CEA, carcinoembryonic antigen; CA, carbohydrate antigen.
Identification of candidate diagnostic biomarkers for predicting GC
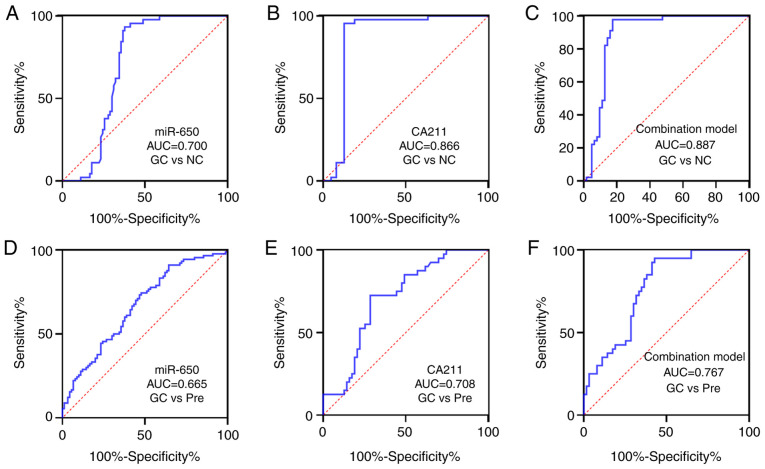
Next, whether the candidate biomarkers were able to predict the presence of GC was assessed using multiple logistic regression analysis. The results indicated that the increase in miRNA-650 and CA211 levels were significantly associated with the presence of GC (P<0.05; Table II). The P-value of Hosmer-Lemeshow test was 0.979, indicating that the model was a good fitted. Based on the ROC analysis, the sensitivity, specificity, area under the ROC curve (AUC), Youden index, accuracy, negative predictive value (NPV), positive predictive value (PPV) and the cut-off values for detecting GC are summarized in Table III. At a cut-off 1.98, the AUC of miRNA-650 was 0.700 (moderate) with 93.3% specificity and 62.2% sensitivity. CA211 had a greater AUC compared with miRNA-650 for discriminating patients developing GC from healthy controls (Fig. 1).
Table II.
Candidate plasma markers independently associated with the presence of GC.
| Markers | Beta | S.E | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| miR-650 (ΔCt) | −0.460 | 0.218 | 0.632 | 0.412–0.968 | 0.035 |
| CEA | 0.208 | 0.160 | 1.232 | 0.901–1.685 | 0.192 |
| CA211 | 1.326 | 0.450 | 3.767 | 1.558–9.106 | 0.003 |
| CA125 | 0.074 | 0.046 | 1.076 | 0.984–1.178 | 0.109 |
| CA50 | 0.155 | 0.088 | 1.168 | 0.982–1.389 | 0.079 |
GC, gastric carcinoma; OR, odds ratio; CI, confidence interval; miR, microRNA; CEA, carcinoembryonic antigen; CA, carbohydrate antigen.
Table III.
Performance of candidate metabolites and blood markers for predicting GC.
| Variable | AUC (95%CI) | Youden index | Cut-off | Sensitivity, % | Specificity, % | Accuracy, % | PPV, % | NPV, % | P-value |
|---|---|---|---|---|---|---|---|---|---|
| GC vs. Pre | |||||||||
| miR-650 | 0.665 (0.586–0.744) | 0.2267 | 1.06 | 52.2 | 74.4 | 63.3 | 24.04 | 68.51 | 0.0001 |
| CA211 | 0.708 (0.608–0.807) | 0.4393 | 3.06 | 71.4 | 72.5 | 71.8 | 12 | 30.60 | 0.0004 |
| Model | 0.767 (0.678–0.857) | 0.5214 | 0.671 | 57.1 | 95 | 71.8 | 3 | 39.40 | <0.0001 |
| GC vs. NC | |||||||||
| miR-650 | 0.700 (0.613–0.788) | 0.5556 | 1.98 | 62.2 | 93.3 | 72.5 | 4.01 | 43.21 | 0.0001 |
| CA211 | 0.866 (0.785–0.947) | 0.8286 | 2.20 | 87.3 | 95.6 | 90.7 | 2.98 | 48.39 | <0.0001 |
| Model | 0.887 (0.818–0.956) | 0.8032 | 0.49 | 82.5 | 97.7 | 88.8 | 1.99 | 47.99 | <0.0001 |
GC, gastric carcinoma; AUC, area under the curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; Pre, precancerous lesions; NC, normal control; miR, microRNA; CA211, carbohydrate antigen 211.
Figure 1.
AUC of markers, (A) miR-650, (B) CA211 and (C) combination model (miR-650 and CA211), to discriminate GC from NC. AUC of markers, (D) miR-650, (E) CA211 and (F) combination model (miR-650 and CA211), to discriminate GC from Pre. AUC, area under the receiver operating characteristic curve; GC, gastric carcinoma; NC, normal control; miR, microRNA; CA211, carbohydrate antigen 211; Pre, precancerous lesions.
Diagnostic model using candidate markers
To evaluate whether the combined application of tumor markers may improve the diagnostic efficiency of GC, the significant variables in univariate analysis were inserted into a stepwise logistic regression analysis with consequent development of a novel model that combined the most discriminatory factors (miRNA-650 and CA211) for predicting GC. The model is illustrated as follows: −0.277–0.588 × miR-650 (ΔCq) + 1.643×CA211 (ng/ml). The AUC (95% CI) of the combination model was 0.887 (0.818–0.956) for distinguishing patients with GC from healthy controls (sensitivity, 82.5% and specificity, 97.7%) and 0.767 (0.678–0.857) for gastric precancerous lesions (sensitivity, 57.1% and specificity, 95%), respectively (Table III; Fig. 1). When the two markers were combined, there was a much stronger diagnostic value for GC (AUC=0.887; P<0.0001) compared with when either was used separately. Compared with the diagnostic efficiency of miRNA-650 alone, combined detection improved the diagnostic efficiency of GC to a certain extent (Table IV).
Table IV.
Diagnostic efficacy of tumor markers.
| CA211 | miR-650 | |||
|---|---|---|---|---|
| ROC curve comparison | Z | P-value | Z | P-value |
| Combination | 0.719 | 0.4722 | 2.868 | 0.0041 |
| miR-650 | 2.035 | 0.0418 | ‒ | ‒ |
ROC curve comparisons were analyzed using MedCalc statistical software with statistical significance at the level of P<0.05. ROC, receiver operating characteristic; CA211, carbohydrate antigen 211; miR, mircoRNA.
Associations between miRNA-650 and CA211 expression levels, and clinicopathological factors in patients with GC
The associations between the expression of the miRNA-650 and CA211, and clinicopathological parameters of patients with GC were further elaborated. As shown in Table V, miRNA-650 expression levels were not associated with the following characteristics of patients with GC; age (P=0.1489), sex (P=0.7122), TNM stage (P=0.4769), or histological type (P=0.2679). Similarly, the same result was observed for CA211. The plasma levels of CA211 did not correlate with age (P=0.0537), sex (P=0.9856), TNM stage (P=0.1064) or histological type (P=0.7942).
Table V.
The associations between the expression levels of miR-650 (2−ΔCt) and CA211 in plasma and clinicopathological factors of patients with gastric cancer.
| Parameter | na | miR-650a, average fold-change ± SD | P-valuea | nb | CA211b | P-valueb |
|---|---|---|---|---|---|---|
| Age, median years | 0.1489 | 0.0537 | ||||
| <60 | 30 | 23.23±48.69 | 11 | 2.795±1.25 | ||
| ≥60 | 60 | 6.83±10.09 | 29 | 3.89±40.95 | ||
| Sex | 0.7122 | 0.9856 | ||||
| Male | 68 | 15.04±31.14 | 31 | 4.63±44.67 | ||
| Female | 22 | 26.15±62.19 | 9 | 3.09±57.66 | ||
| TNM stage | 0.4769 | 0.1064 | ||||
| I | 22 | 15.72±25.86 | 6 | 0.67±1.86 | ||
| II | 8 | 13.21±26.40 | 5 | 3.09±0.78 | ||
| III | 13 | 4.00±6.32 | 9 | 4.48±22.67 | ||
| IV | 47 | 14.94±33.96 | 20 | 3.89±58.55 | ||
| Histological type | 0.6947 | 0.7942 | ||||
| A | 63 | 3.42±47.52 | 28 | 3.415±45.56 | ||
| Other | 27 | 4.01±10.43 | 12 | 3.26±1.33 |
miR, microRNA; CA211, carbohydrate antigen 211; SD, standard deviation; TNM, Tumor-Node-Metastasis; A, adenocarcinoma. ‘Other’ refers to mucinous carcinoma, Signet-ring cell carcinoma and adenocarcinoma with Signet-ring cell carcinoma.
The 90 patients with GC included in the present study
The 40 patients with GC who participated in CA211 test.
Discussion
GC is one of the leading causes of cancer-associated mortality due to late diagnosis and limited therapeutic strategies. Circulating miRNAs are promising, noninvasive biomarkers for cancer screening (17). The present study reported an investigation on miR-650 expression in human GC. The present study revealed that circulating miRNA-650, CEA, CA125, CA211 and CA50 were differentially expressed in GC, compared with healthy individuals. Furthermore, the increased expression levels of miRNA-650 and CA211 were significantly associated with the presence of GC. AUC analysis showed that plasma miRNA-650 association with CA211 improved the diagnostic efficiency of GC.
The aberrant expression of miRNA-650 is associated with the progression of glioma, breast cancer, colorectal cancer, gastric cancer, osteosarcoma, and lung adenocarcinoma (18,19). Previous studies have demonstrated that the positive expression of miRNA-650 is a poor prognostic indicator in glioma (18). It has been reported that miRNA-650 may target ING4 to promote the development of GC (16). Lango-Chavarría et al (19) indicated that overexpression of miRNA-650 is associated with the downregulated expression of tumor suppressors, ING4 and NDRG2, in breast cancer (20). A clinical investigation on osteosarcoma indicated that miRNA-650 serves an important role in the synthesis of IL6, which is regulated by ING4 expression and NF-κB signaling pathways (21). Furthermore, a research study reported that upregulation of miRNA-650 was correlated with enhanced malignant potential and poor prognosis of patients with lung adenocarcinoma (22).
Different methods have been used to study the expression profile of miRNAs in GC. Certain miRNAs, including miR-21, miR-223, miR-218, miR-106 and miR-421 (19,23,24) have been reported to exhibit significant upregulation or downregulation. Notably, miR-106, miR-21 and miR-221 have been reported as potential biomarkers for tumor diagnosis and prognosis (25). The present study reported that circulating miR-650 was highly expressed in patients with GC, compared with those with Pre and healthy controls. However, whether or not miRNA-650 is a potential biomarker for the diagnosis of GC is yet to be reported. In order to address this, multiple logistic regression and ROC curve analysis were performed. The results indicated that the increase in miRNA-650 and CA211 expression levels were significantly associated with the presence of GC. The present study demonstrated that circulating miRNA-650 and CA211 levels were significantly associated with the presence of GC, and the AUC of miR-650 alone was 0.70 for diagnosing GC, suggesting that this miRNA may be a useful screening biomarker for GC.
As a classification of RNA, miRNAs are also unstable and prone to degradation in the presence of RNA enzymes; therefore, as an independent tumor marker, the diagnostic efficacy of miR-650 may not be satisfactory. Previous studies have demonstrated that biomarker combinations may improve the diagnostic performance of a model for various cancer types (26–28). To further investigate this, a predictive logistic regression analysis model called the Cancer Screening Model for diagnosing GC was developed. This screening model had an AUC of 0.887 and indicated that a combination of plasma miR-650 and CA211 was an effective and novel diagnostic biomarker panel in the diagnosis of GC.
Prior to the present study, very little was known regarding circulating miR-650 expression in GC, and its correlation with the clinicopathological features of these patients. To address these questions, miR-650 expression levels and the clinicopathological characteristics of 90 patients with GC were examined, but there was no association between miR-650 expression and sex, histological type, differentiation grade or TNM stage. However, whether miR-650 is abnormally expressed in the early stages of GC and has significant value in the early diagnosis of GC requires further study.
Although the results are novel, the present study has certain limitations. To begin with, the TNM stages were not divided into T1a and T1b, so there was no data relating to T1b. Therefore, the significance of miR-650 in the early diagnosis of GC requires further study. Another potential limitation of the present study was that no specific genotyping was performed in the patients with GC, and the miRNA expression may differ between gene subtypes. Furthermore, the results of the present study may reflect biases inherent in the acquisition of such clinical data; therefore, using more external bioinformatics data for an external validation may be a future direction.
In conclusion, miRNA-650, which is upregulated in GC, may be a novel early diagnostic marker for GC. Furthermore, a new screening model was developed in the present study, which patients may be more willing to accept for detecting GC.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81760501); The Natural Science Foundation of the Guangxi Zhuang Autonomous Region (grant nos. 2017GXNSFAA198041 and 2018GXNSFAA294055); Liuzhou Scientific Research and Technological Development Programs (grant no. 2014JC010); The Self-Funded Research Project of Guangxi Zhuang Autonomous Region health and Family Planning Commission (grant no. Z2014613).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
JC conceived, designed and performed the experiments. JC, LW, YFS, CL and XC analyzed the data. LW, JD, GP, CH and ZW contributed toward the collection of clinical samples, and performed experiments and data analysis. JC wrote the manuscript. YQS analysed and interpreted the data, was involved in drafting and revision of the manuscript and gave final approval of the version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by The Ethical Review Committee of Affiliated Liutie Central Hospital of Guangxi Medical University (Liuzhou, China). All patients provided written informed consent.
Patient consent for publication
All patients provided written informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18:534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, Guo M, Lian X, Fan D, Zhang H. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17:737. doi: 10.1186/s12885-017-3738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YK, Shen L, Zhang XT. The prognosis and clinicopathological characteristics of 70 gastric cancer patients with elevated serum AFP. Zhonghua Zhong Liu Za Zhi. 2017;39:514–517. doi: 10.3760/cma.j.issn.0253-3766.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Han Z, Li Y, Zhang J, Guo C, Li Q, Zhang X, Lan Y, Gu W, Xing Z, Liang L, et al. Tumor-derived circulating exosomal miR-342-5p and miR-574-5p as promising diagnostic biomarkers for early-stage lung adenocarcinoma. Int J Med Sci. 2020;17:1428–1438. doi: 10.7150/ijms.43500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satapathy S, Kumar C, Singh RK. MicroRNAs as key regulators of ovarian cancers. Cell Med. 2019;11:2155179019873849. doi: 10.1177/2155179019873849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu M, Xiong S, Chen Q, Zhu S, Zhou X. Novel role of microRNA-126 in digestive system cancers: From bench to bedside. Oncol Lett. 2019;17:31–41. doi: 10.3892/ol.2018.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo X, Mao XH, Zou QM, Yu PW, Zuo QF, et al. Plasma microRNAs, miR-223, miR-21 and miR-218, as novel potential biomarkers for gastric cancer detection. PLoS One. 2012;7:e41629. doi: 10.1371/journal.pone.0041629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Zhu L, Liu B, Yang L, Meng X, Zhang W, Ma Y, Xiao H. Genome-wide microRNA profiles identify miR-378 as a serum biomarker for early detection of gastric cancer. Cancer Lett. 2012;316:196–203. doi: 10.1016/j.canlet.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Wu L, Sun Y, Yin Q, Chen X, Liang S, Meng Q, Long H, Li F, Luo C, Xiao X. Mir-421 in plasma as a potential diagnostic biomarker for precancerous gastric lesions and early gastric cancer. PeerJ. 2019;7:e7002. doi: 10.7717/peerj.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He CZ, Zhang KH, Li Q, Liu XH, Hong Y, Lv NH. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol. 2013;13:87. doi: 10.1186/1471-230X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catalano V, Sisti V, Spada D, Graziano F, Giordani P, Alessandroni P, Baldelli A, Casadei V, Rossi D, D'Emidio S, et al. The 7th edition of the tnm classification for gastric cancer and a proposal of a new classification for D2 gastrectomy. Ann Oncol. 2012;23 doi: 10.1016/S0923-7534(20)30275-1. [DOI] [Google Scholar]
- 14.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. 4th. Vol. 3. World Health Organization Classification of Tumours; Lyon: 2010. WHO Cassification of Tumours of the Digestive System. [Google Scholar]
- 15.Pan J, Zhou C, Zhao X, He J, Tian H, Shen W, Han Y, Chen J, Fang S, Meng X, et al. A two-miRNA signature (miR-33a-5p and miR-128-3p) in whole blood as potential biomarker for early diagnosis of lung cancer. Sci Rep. 2018;8:16699. doi: 10.1038/s41598-018-35139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Zhu W, Zhang J, Huo S, Zhou L, Gu Z, Zhang M. MicroRNA-650 targets ING4 to promote gastric cancer tumorigenicity. Biochem Biophys Res Commun. 2010;395:275–280. doi: 10.1016/j.bbrc.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Santangelo A, Tamanini A, Cabrini G, Dechecchi MC. Circulating microRNAs as emerging non-invasive biomarkers for gliomas. Ann Transl Med. 2017;5:277. doi: 10.21037/atm.2017.06.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun B, Pu B, Chu D, Chu X, Li W, Wei D. MicroRNA-650 expression in glioma is associated with prognosis of patients. J Neurooncol. 2013;115:375–380. doi: 10.1007/s11060-013-1243-y. [DOI] [PubMed] [Google Scholar]
- 19.Lango-Chavarría M, Chimal-Ramírez GK, Ruiz-Tachiquín ME, Espinoza-Sánchez NA, Suárez-Arriaga MC, Fuentes-Pananá EM. A 22q11.2 amplification in the region encoding microRNA-650 correlates with the epithelial to mesenchymal transition in breast cancer primary cultures of Mexican patients. Int J Oncol. 2017;50:432–440. doi: 10.3892/ijo.2017.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng L, Xie Y, Zhang H, Wu Y. Down-regulation of NDRG2 gene expression in human colorectal cancer involves promoter methylation and microRNA-650. Biochem Biophys Res Commun. 2011;406:534–538. doi: 10.1016/j.bbrc.2011.02.081. [DOI] [PubMed] [Google Scholar]
- 21.Yun JH, Moon S, Lee HS, Hwang MY, Kim YJ, Yu HY, Kim Y, Han BG, Kim BJ, Kim JM. MicroRNA-650 in a copy number-variable region regulates the production of interleukin 6 in human osteosarcoma cells. Oncol Lett. 2015;10:2603–2609. doi: 10.3892/ol.2015.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang JY, Cui SY, Chen YT, Song HZ, Huang GC, Feng B, Sun M, De W, Wang R, Chen LB. MicroRNA-650 was a prognostic factor in human lung adenocarcinoma and confers the docetaxel chemoresistance of lung adenocarcinoma cells via regulating Bcl-2/Bax expression. PLoS One. 2013;8:e72615. doi: 10.1371/journal.pone.0072615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W, Xiao S, Lu H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest. 2008;88:1358–1366. doi: 10.1038/labinvest.2008.94. [DOI] [PubMed] [Google Scholar]
- 24.Peng Q, Shen Y, Lin K, Zou L, Shen Y, Zhu Y. Comprehensive and integrative analysis identifies microRNA-106 as a novel non-invasive biomarker for detection of gastric cancer. J Transl Med. 2018;16:127. doi: 10.1186/s12967-018-1510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishiguro H, Kimura M, Takeyama H. Role of microRNAs in gastric cancer. World J Gastroenterol. 2014;20:5694–5699. doi: 10.3748/wjg.v20.i19.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Tu H, Chen T, Yuan Q, Liu J, Dong N, Yuan Y. Three-dimensional combined biomarkers assay could improve diagnostic accuracy for gastric cancer. Sci Rep. 2017;7:11621. doi: 10.1038/s41598-017-12022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng J, Yang A, Cheng S, Feng L, Wu X, Lu X, Zu M, Cui J, Yu H, Zou L. Circulating miR-19a-3p and miR-483-5p as novel diagnostic biomarkers for the early diagnosis of gastric cancer. Med Sci Monit. 2020;26:e923444. doi: 10.12659/MSM.923444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alemar B, Izetti P, Gregório C, Macedo GS, Castro MA, Osvaldt AB, Matte U, Ashton-Prolla P. miRNA-21 and miRNA-34a Are potential minimally invasive biomarkers for the diagnosis of pancreatic ductal adenocarcinoma. Pancreas. 2016;45:84–92. doi: 10.1097/MPA.0000000000000383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.