Abstract
Background: Sorafenib is the first oral therapeutic agent to show the activity against human hepatocellular carcinoma. Sorafenib leads to severe toxicity due to the multiple-dose regimen. Reducing the overall dose of sorafenib through injectable dosage form to release sustainably is of therapeutically more important to combat drug-induced toxicity.
Objective: The purpose of this study was to formulate and evaluate the physical parameters of sorafenib-loaded Sodium Selenite Nanoparticles (SSSNP).
Methods: Two different methods: chemical crosslinking and solvent evaporation were applied for the formulation of nanoparticles using various crosslinkers such as formaldehyde, magnesium sulfate, tripolyphosphate, dextran sulfate, and aluminum hydroxide. Physical characterization was performed with zeta potential analysis, polydispersity index, particle size and scanning electron microscopic studies for morphological analysis for all the formulated nanoparticles developed using the chemical crosslinking technique based ionic interaction.
Results: Tripolyphosphate was selected as an ideal crosslinker and used for nanoparticle formulation with the solvent evaporation technique. Based on the physical characterization, SSSNP was formulated successfully with the solvent evaporation technique using tripolyphosphate as a cross-linker. The zeta potential of SSSNP was -37.5 mV, PDI was approximately 0.3 to 0.4, and the observed size (diameter) was in the range of 208 nm to 0.2 µm. Furthermore, the particles were smooth in morphology and appeared as crystals.
Conclusion: The novel injectable sorafenib loaded sodium selenite nanoparticle dosage form will serve better than conventional oral dosage form to elicit a safe therapeutic effect.
Keywords: Hepatoma, sorafenib, sodium selenite, nanoparticles, formulation and development, physical characterization
1. INTRODUCTION
Hepatocellular Carcinoma (HCC) is caused by chronic hepatitis B & C virus infections, non-alcoholic fatty liver disease, alcohol addiction, and dietary toxins such as aflatoxins and aristolochic acid [1]. It is the 4th most common cause of cancer mortality worldwide, and it is a disease that is growing in prevalence due to an increased global incidence of hepatitis. According to the most recent Saudi Cancer Registry (2015), liver cancer accounts for 3.1% of all newly diagnosed cancers [2]. The statistical data for liver cancer and the importance of searching for new anti-liver cancer agents is increasing day by day [3, 4]. However, sorafenib is the only promising drug that is significantly used as the first-line treatment of hepatoma [5].
Sorafenib is an orally administered drug that acts by inhibiting multiple kinase pathways. It is the only drug approved by the United States Food and Drug Administration (USFDA) for the treatment of HCC. Sorafenib is the first accepted and the only available drug therapy for HCC in the past 10 years [2, 6, 7]. It inhibits the serine-threonine kinase RAF, which is the part of RAS/MEK/ERK signaling pathway, by suppressing several receptor tyrosine kinases involved in tumor progression and tumor angiogenesis, including vascular endothelial growth receptors 2 and 3, platelet-derived growth factor receptor, FLT3 and C-KIT [8]. Although Sorafenib is a successful molecular target, multiple-dose administrations lead to many adverse effects such as anorexia, gastrointestinal bleeding, and severe skin toxicity. Therefore, it is of primary importance to reduce the overall dose while sustaining the drug effect. The main objective of anticancer drug development is to deliver therapeutic agents in a targeted and selected fashion to their sites of action, decrease adverse effects, and enhance efficacy. Thus, drug targeting to the actual site using nanoparticle delivery systems improves the efficacy of therapy, stability, and may reduce side effects associated with drugs [9].
The concept of nanotechnology has attracted remarkable attention in the pharmaceutical industry as regards the development of therapeutic innovation. In recent years, the development of nanoparticulate carrier systems for targeting cancer has aroused increasing interest. These targeted nanosystems can deliver actively through covalent conjugation of targeting molecules on the nanoparticle surface. Sodium selenite nanoparticles have a unique advantage to target cancer cells since sodium selenite is a versatile inorganic compound that exerts anticancer effect by inducing apoptosis [10]. The present work aimed to develop a safe delivery system for sorafenib by encapsulating it in sodium selenite nanoparticles for sustained release of the drug to elicit synergetic action. The physical characteristics of sorafenib-loaded sodium selenite nanoparticles were established as a prime step in the formulation and development of nano injectables.
2. EXPERIMENTAL DESIGN
2.1. Materials
Sodium selenite, sorafenib tosylate, polyvinyl alcohol (PVA, 87-89% hydrolyzed), formaldehyde, dextran sulfate, tripolyphosphate, and magnesium sulphate was purchased from Sigma Aldrich, USA. The other organic solvents were purchased from Scharlau, Spain.
2.2. Methods
A range of manufacturing methods for the preparation of sodium selenite nanoparticles was investigated in this study. Sorafenib-loaded sodium selenite nanoparticles were formulated using the chemical cross-linking method with various cross-linkers and solvent evaporation technique.
2.2.1. Preparation of Drug Analyte
Sorafenib tosylate was mixed in 1 ml of 95% (v/v) ethanol and aspirated 5 times with micropipette for uniform dissolution to achieve a concentration of 10 mg/ml. The solution was diluted with 3 ml of Milli Q water to a concentration of 2.5 mg/ml and immediately transferred to an amber-colored vial. It was used as a stock solution.
2.2.2. Chemical cross-linking method
Sodium selenite solution (5% w/v) was prepared in Milli Q water and 1% (v/v) of each chemical cross-linker i.e. formaldehyde, dextran sulfate, tripolyphosphate, and magnesium sulfate was prepared in Milli Q water. The sodium selenite solution was kept stirred on a hot plate with a magnetic bead and the speed was regulated at 2000 rpm for about 3 hours. Initially after running for about 30 minutes, 1 ml of 0.5% (w/ v) sorafenib was added dropwise to the solution and run for 15 minutes. Thereafter, the predetermined concentration of 0.5 ml of 1% (v/v) formaldehyde and 1% (w/v) dextran sulfate, tripolyphosphate and magnesium sulfate were separately added as crosslinkers, every 30 minutes, to develop separate nanoparticles. During the formulation of nanoparticles, sonication was done for 3 minutes twice at pre-determined time intervals. Finally, the mixture was filtered through a 0.2µm PVDF membrane, and the filtrate was subjected to various physical characterizations such as zeta potential analysis and scanning electron microscopy.
2.2.3. Solvent Evaporation Technique
The sodium selenite solution was kept stirred on a hot plate with a magnetic bead and the speed was regulated at 2000 rpm for 30 minutes. Then, the solution was transferred to a beaker containing 25 ml of 10% (w: v) polyvinyl alcohol and kept stirred on a hot plate with magnetic beads at a speed of 2000 rpm. After 30 minutes, 1 ml of 0.5% (w/ v) sorafenib was added, and stirring was continued for 1 h. Thereafter, 1% (w:v) tripolyphosphate was added twice at pre-determined time intervals during the mixing process, and the mixture was subjected to solvent evaporation overnight with constant stirring at 1000 rpm, resulting in complete drying. A very thin film was produced, and this was scrapped off from the beaker. The nanoparticles were obtained in the form of fine powder which was then subjected to various physical characterizations such as zeta potential and scanning electron microscopic analysis.
2.2.4. Determination of Morphological Features
The morphological features and the particle size of nanoparticles were studied using a high-resolution scanning electron microscope (SEM) with FEI Quanta 200 F (FEI Company, USA). One drop of the sample was placed at the center of a clean glass slide 3 × 3 cm in size and air-dried in a laminar hood. Then, the sample was coated with gold and its image was observed at various magnifications.
2.2.5. Determination of Zeta Potential, Size, and PDI
Zeta potential is an important technique used to determine the surface electrical charge through electrophoretic mobility of the injectable nanoparticle formulation. The zeta potential, size, and Polydispersity Index (PDI) of sorafenib-loaded sodium selenite nanoparticles were measured using Nano-ZS zeta sizer (Malvern Instruments, UK). The sorafenib-loaded sodium selenite nanoparticles (1% w: v) prepared in Milli Q water was placed in the capillary cells and its zeta potential, size and PDI were determined.
3. RESULTS
Sodium selenite nanoparticles were successfully formulated using chemical cross-linking and solvent evaporation methods. Fig. (1A-F) shows the scanning electron micrographs of the morphological features of sodium selenite nanoparticles prepared using various chemical cross-linkers. Based on the ionic interactions, the crosslinkers were linked with sodium selenite to form nanoparticles. Table 1 explains the influence of various chemical crosslinkers and the method of formulation of sodium selenite nanoparticles. From the study, it was revealed that the nanoparticles developed using formaldehyde as crosslinker exhibited a zeta potential of 1.43 mV, and the PDI of the formulated injectable nanoparticles was 0.732, indicating an unstable formulation. The nanoparticles developed with magnesium sulfate as crosslinkers were spherical, with a smooth surface and particle size of 400-550 nm (Fig. 1B). The electrophoretic charge was -19.4 mV, indicating moderately stable formulation with PDI of 0.5, and the particles were uniform in morphological character. However, many particles were clubbed during the formulation, indicating that the nanoparticles were not uniform in size. Therefore, the magnesium sulfate formulation was not successful.
Fig. (1).
The Scanning electron micrographs of sorafenib entrapped sodium selenite nanoparticles using various techniques (A) Nanoparticles formulated by using 0.5% v/v of formaldehyde solution through chemical cross linking method, the figure shows the nanoparticle at 60,000 × magnification; (B) Nanoparticles formulated by using 0.5% w/v of magnesium sulfate through chemical cross linking method, the figure shows the nanoparticle at 30,000 × magnification; (C) Nanoparticles formulated by using 0.5% w/v of tripolyphosphate through chemical cross linking method, the figure shows the nanoparticle at 60,000 × magnification; (D) Nanoparticles formulated by using 0.5% w/v of dextran sulfate through chemical cross linking method, the figure shows the nanoparticle at 12,000 × magnification; (E) Nanoparticles formulated by using 0.5% w/v of Aluminium hydroxide through chemical cross linking method, the figure shows the nanoparticle at 60,000 × magnification; (F) Nanoparticles formulated by using 0.5% w/v of tripolyphosphate through solvent evaporation method, the figure shows the nanoparticle at 15,000 × magnification.
Table 1.
Physical characterization of sorafenib entrapped sodium selenite nanoparticles formulated by using various techniques.
|
Chemical Cross
Linking Agents |
Strength
(% v/v or w/v) |
Zeta Potential (mV) | PDI |
% Poly
Dispersity |
Zeta Average Size (d.nm) | SEM Studies Size (nm/µm) |
Mobility
mS/cm |
%Mass (r.nm) |
|---|---|---|---|---|---|---|---|---|
| Cross linking method | ||||||||
| Formaldehyde | 0.5 | 1.43 | 0.732 | 85.5 | 202.4 | 280 | 5.55 | 1.9 |
| Magnesium sulfate | 0.5 | -19.4 | 0.513 | 71.6 | 456.4 | 520 | 23.5 | 33 |
| Tripolyphosphate | 0.5 | -20.1 | 0.342 | 88.7 | 189 | 210 | 23.8 | 46.5 |
| Dextran Sulfate | 0.5 | -8.7 | 0.565 | 75.2 | 796.4 | 930 | 14.1 | 5.2 |
| Aluminium hydroxide | 0.5 | 2.72 | 0.731 | 85.5 | 209.5 | 270 | 8.51 | 3.3 |
| Solvent evaporation method (Ideal batch) | ||||||||
| Tripolyphosphate | 0.5 | –37.5 | 0.414 | 66.4 | 391.9 | 456 | 33.5 | 44.6 |
Dextran sulfate is an anionic polymer that was utilized as a chemical crosslinker to formulate sodium nanoparticles. As shown in Fig. (1D), the particles were crystalline in morphology but did not exhibit promising physical characteristics (Table 1). This might be due to the lack of electrostatic attraction between sodium selenite and dextran sulfate.Fig. (1E) shows the morphological characteristics of sodium selenite nanoparticles developed with aluminum hydroxide as a crosslinker. The nanoparticles were clumped together and aggregated, and failed to develop as promising nano-formulation, that was reflected in their physical characterization.
Sodium tripolyphosphate was the most successful among all of the crosslinkers screened in this study. The scanning electron micrograph (Fig. 1C) showed that smooth particles were formulated with uniform distribution using the chemical cross-linking technique. Sodium tripolyphosphate was also successful as a crosslinker in the development of nanoparticles using solvent evaporation technique. Fig. (2A-C) illustrates the zeta potential characterization of SSSNP, a unique peak demonstrates the zeta potential quality of an ideal batch, which was about 85%. The size distribution of the SSSNP was observed about 88 – 89% either by percent intensity or percent mass. The zeta potential of the developed nanoparticles was within the satisfactory range i.e. -20.1 to - 37.5 (Table 1), indicating the stability of the formulation.
Fig. (2).
Zeta potential report of SSSNP formulated by solvent evaporation technique. (A) The peak shows the Surface charge of the nanoparticles. (B) The peak shows the size distribution analysis of SSSNP by percent intensity. (C) The peak shows the size distribution analysis of SSSNP by mass, percent volume.
In our study, the % mass of nanoparticles was observed high when tripolyphosphate was used as a crosslinker followed by magnesium sulfate (Table 1). However, the rest of the cross-linkers such as formaldehyde, dextran sulfate, and aluminum hydroxide exhibited very less % mass which showed aggregation of particles and failed to comply with the injectable dosage form. The % r.nm of the ideal batch formulated by the solvent evaporation method was 44.6%. Table 1 indicates that the uniform particle size range was about 392 nm.
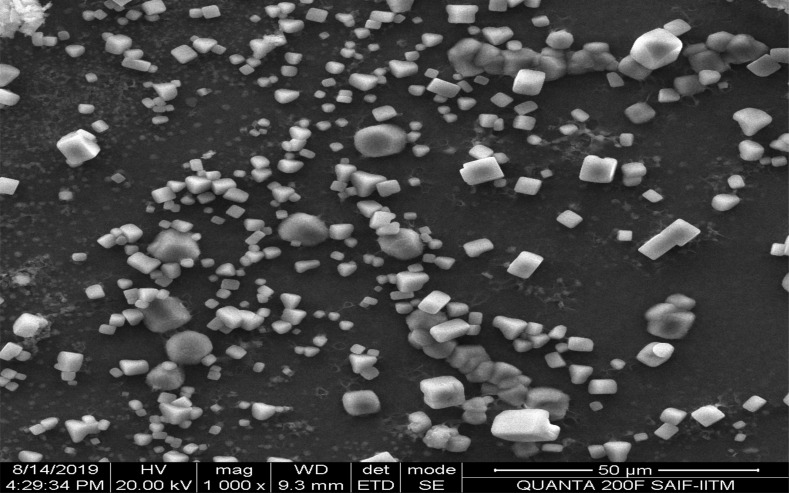
Based on the results of physical characterization, the solvent evaporation method was a better method for the formulation of sodium selenite nanoparticles. Therefore, sorafenib was entrapped in sodium selenite nanoparticles using 0.5% (w/v) tripolyphosphate as a crosslinker. Fig. (1F) shows the morphological features of nanoparticles formulated with the solvent evaporation technique before loading the drug. The particles were discrete and smooth in morphological features. However, particle aggregations were also observed, probably due to improper homogenization. To overcome this negative characteristic, the sonication process was increased for about 3 minutes to improve the quality of the particles. Fig. (3) shows the morphological features of the final drug-entrapped nanoparticles. The particles appear as crystals with smooth morphology, with most of them having sizes in the micron range (about 2 to 3 µm; Fig. 3). Physical characterization revealed that stable injectable formulation (Table 1) was achieved with the solvent evaporation technique. The mobility of particles is an important factor in particle dispersion in injectable dosage forms. In this study, the mobility was high when nanoparticles were prepared using the solvent evaporation technique (Table 1).
Fig. (3).
The scanning electron micrographs of sorafenib entrapped sodium selenite nanoparticles. The nanoparticles were formulated by using 0.5% w/v of tripolyphosphate through solvent evaporation method. The figure is self-exemplary of particles, which are discrete, crystal like structure with few are nano sized particles and most of the particles are micron sized particles.
4. DISCUSSION
Sorafenib is a lipophilic molecule with poor solubility and mean half-life of 20 to 48 hours. Due to the delayed pharmacokinetic profile, the USFDA has accepted the oral dosage form for treating hepatocellular carcinoma. The present oral dosage formulation leads to severe adverse effects [11]. Awareness of the pathological consequences of HCC has continued to increase tremendously day by day. However, the treatment aspect of HCC is still in the under-development phase. Although sorafenib is a successful drug molecule, its application has been limited due to toxicity and poor water solubility. To overcome this problem, many researchers have recently developed some formulations such as bovine serum albumin nanoparticles, dextran-poly (dl-lactide-co-glycolide) nanoparticles and suspension nanoparticles for oral administration [12-14].
Nanoparticle technology is the most unique technology that can provide suitable tools for effective targeted delivery of drugs into specific cells, especially in cases where the targets are localized intracellularly. It improves Cmax and reduces drug toxicity. Sorafenib-loaded sodium selenite nanoparticles (SSSNP) were formulated successfully by screening the suitability of various chemical crosslinkers in the formulation of nanoparticles through chemical cross-linking techniques. In this study, the most successful crosslinker for nanoparticle formulation was tripolyphosphate when compared to the rest. SSSNP was formulated successfully by the solvent evaporation technique using tripolyphosphate as a cross-linker. However, it is important to note the percentage polydispersity of the formulation to ascertain the monodisperse system. Generally, to get monodisperse formulations, the percentage of polydispersity should be less than 20%.The present study demonstrated that in all the formulations, the percentage of polydispersity was above 60%, which indicates non-linear monodisperse formulations.
The potential promise of the nanoparticle characteristics was seen in zeta potential analysis as well as scanning electron microscopy studies. The particle size of the formulation was in the range of 392 nm to 2.9 µm. The zeta potential was about -37.5 mV, and the PDI was about 0.414, indicating the stability of the formulation. The surface charge and the size of the particles dictate the pharmacokinetic profile of the nanoparticles for biodistribution [15]. According to an earlier report, the nanoparticles with a high surface charge and/or large particle size are efficiently phagocytosed by murine macrophage [16].
In a colloidal system, the shift of zeta potential and PDI were highly influenced by the nanoparticle concentration. However, the mobility of the particle in a colloidal system is also an important factor that influences conductivity [17]. Particle sizes less than <20 nm have high mobilities that influence particle surface charge. In this study, sodium selenite nanoparticles with fewer mobilities when formaldehyde was used as a crosslinker failed to develop good zeta potential values, indicating unstable formulation. However, tripolyphosphate was the best stabilizing agent that exhibited high mobility of 33.5 mS/cm when the sorafenib loaded sodium selenite nanoparticles were formulated using the solvent evaporation method. Sorafenib encapsulated Sodium selenite nanoparticles showed high mobilities, with particle sizes in the range of 200-320 nm. Enhanced Permeability and Retention (EPR) effect is a very important factor that is influenced by the size of the drug entrapped-nanoparticles [18, 19]. Therefore, passive targeting cancer cells can be channelized through optimum size formulation that enables the release of drugs into the cancer cell through enhanced permeability and retention (EPR) effect [20]. Although the sizes of some of the particles were in the micron range, the majority of particles were below 400 nm in size. This demonstrates a successful formulation for passive targeting of hepatocytes. The therapeutic use of nanoparticle is achieved through the cellular uptake process, and the size of nanoparticles dictates the efficacy of their cellular uptake. Recently, Nazanin Hoshyar et al. (2016) [21] reported that nanoparticles of size 50 to 200 nm are ideal for cellular uptake. Earlier studies suggesting that nanoparticle size between 30-200 nm were considered as optimum size to target tumor cells. The particles could easily pass through the cell membrane by passive targeting and overcome the EPR effect [22, 23]. Conversely, particle size also influences the clearance of nanoparticles from the human body. Reported that the nanoparticle size 20-150nm will significantly clear from the liver. In the case of the spleen, the rapid clearance of particle should be larger than 200 nm and in unrainy excretion, the size should be less than 8 nm. Online with these reports, in the present study, the majority of particle size varied from 200-320 nm and some are in 6 µm in size. Therefore, the biodistribution of SSSNP will be abstemious since some particles were observed in the micron level indicating that the particles can easily form depot at the site of injection and slowly distributing throughout the system. The majority of particle size of SSSNP ranged from 200-320 nm, indicating that their cellular uptake is likely to be moderate. However, based on the zeta potential studies, the formulation of an ideal batch was highly stable and can be used as injectable.
CONCLUSION
The present study revealed that sorafenib-loaded sodium selenite nanoparticles in an injectable dosage form can be successfully formulated using the solvent evaporation technique. Physical characteristics such as zeta potential, PDI and particle size of the nanoparticles determine the stability and quality of injectable dosage form. From this study, it is obvious that the sorafenib injectable dosage form can be formulated successfully. Based on the present study further work has been accomplished to develop a new formulation and to standardize the uniform particle size with unique zeta potential to target liver cancer cells. Thus, the development of a better therapeutic delivery system for sorafenib using sodium selenite nanoparticles will serve the betterment of human health care.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for this study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This research work was supported by the Deanship of Scientific Research, Jazan University, Jazan, Kingdom of Saudi Arabia, Project Reference No. 38/08/398.
ACKNOWLEDGEMENTS
The authors acknowledge the Deanship of Scientific Research, Jazan University for funding the project (Reference No. 38/08/398). The investigators also thank Ms. Kalpana, Project Associate, HR – SEM, SAIF, IIT, Chennai, India for performing SEM analysis of sodium selenite nanoparticles.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Dong, Ju;, Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sulieman A., Ali A., Shouki B., Hani A., Hind A., Khaled S. Cancer Incidence Report Saudi Arabia.Kingdom of Saudi Arabia Saudi Health Council National Health Information Center. Saudi Cancer Registry. 2015. pp. 1–84. [Google Scholar]
- 3.Pejin B., Jovanović K.K., Mojović M., Savić A.G. New and highly potent antitumor natural products from marine-derived fungi: Covering the period from 2003 to 2012. Curr. Top. Med. Chem. 2013;13(21):2745–2766. doi: 10.2174/15680266113136660197. [DOI] [PubMed] [Google Scholar]
- 4.Pejin B., Glumac M. New cytotoxic natural products from the mangrove biome: Covering the period 2007-2015. Nat. Prod. Res. 2019;33(11):1624–1628. doi: 10.1080/14786419.2018.1425854. [DOI] [PubMed] [Google Scholar]
- 5.Medavaram S., Zhang Y. Emerging therapies in advanced hepatocellular carcinoma. Exp. Hematol. Oncol. 2018;7(17) doi: 10.1186/s40164-018-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leathers J.S., Balderramo D., Prieto J., Diehl F., Gonzalez-Ballerga E., Ferreiro M.R., Carrera E., Barreyro F., Diaz-Ferrer J., Singh D., Mattos A.Z., Carrilho F., Debes J.D. Sorafenib for treatment of hepatocellular carcinoma: A survival analysis from the South American liver research network. J. Clin. Gastroenterol. 2019;53(6):464–469. doi: 10.1097/MCG.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 7.Bosch F.X., Ribes J., Díaz M., Cléries R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology, 2004; 127(5) doi: 10.1053/j.gastro.2004.09.011. ,(Suppl. 1), S5-S16. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm S.M., Carter C., Tang L., Wilkie D., McNabola A., Rong H., Chen C., Zhang X., Vincent P., McHugh M., Cao Y., Shujath J., Gawlak S., Eveleigh D., Rowley B., Liu L., Adnane L., Lynch M., Auclair D., Taylor I., Gedrich R., Voznesensky A., Riedl B., Post L.E., Bollag G., Trail P.A. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 9.Torchilin V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007;9(2):E128–E147. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liping F., Qiong Liu., Liming S., Liming S. Proteomic study on sodium selenite-induced apoptosis of human cervical cancer HeLa cells. J. Trace Elem. Med. Biol. 2011;25(3):130–137. doi: 10.1016/j.jtemb.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Minami H., Kawada K., Ebi H., Kitagawa K., Kim Y.I., Araki K., Mukai H., Tahara M., Nakajima H., Nakajima K. Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors. Cancer Sci. 2008;99(7):1492–1498. doi: 10.1111/j.1349-7006.2008.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H., Sun S., Zhang Y., Wang J., Zhang S., Yao X., Chen L., Gao Z., Xie B. Improved drug targeting to liver tumor by sorafenib-loaded folate-decorated bovine serum albumin nanoparticles. Drug Deliv. 2019;26(1):89–97. doi: 10.1080/10717544.2018.1561766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X.Q., Fan J.M., Liu Y.O., Zhao B., Jia Z.R., Zhang Q. Bioavailability and pharmacokinetics of sorafenib suspension, nanoparticles and nanomatrix for oral administration to rat. Int. J. Pharm. 2011;419(1-2):339–346. doi: 10.1016/j.ijpharm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Kim D.H., Kim M.D., Choi C.W., Chung C.W., Ha S.H., Kim C.H. Anti-tumor activity of sorafenib incorporated nanoparticles of dextran / poly(dl-lactide-co-glycolide) block copolymer. Nanoscale Res. Lett. 2012;7(1):1–6. doi: 10.1186/1556-276X-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abouelhag H.A., Sivakumar S.M., Bagul U.S., Mohamed E., Safhi M.M. Preparation and physical characterization of cisplatin chitosan nanoparticles by zeta nano sizer “prime step for formulation and development”. IJPSR. 2017;8(10):4245–4249. [Google Scholar]
- 16.Clift M.J., Rothen-Rutishauser B., Brown D.M., Duffin R., Donaldson K., Proudfoot L., Guy K., Stone V. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicol. Appl. Pharmacol. 2008;232(3):418–427. doi: 10.1016/j.taap.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Ito T., Sun L., Bevan M.A., Crooks R.M. Comparison of nanoparticle size and electrophoretic mobility measurements using a carbon-nanotube-based coulter counter, dynamic light scattering, transmission electron microscopy, and phase analysis light scattering. Langmuir. 2004;20(16):6940–6945. doi: 10.1021/la049524t. [DOI] [PubMed] [Google Scholar]
- 18.Hare J.I., Lammers T., Ashford M.B., Puri S., Storm G., Barry S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018;3(7):7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aftab S., Shah A., Nadhman A., Kurbanoglu S., Aysıl Ozkan S., Dionysiou D.D., Shukla S.S., Aminabhavi T.M. Nanomedicine: An effective tool in cancer therapy. Int. J. Pharm. 2018;540(1-2):132–149. doi: 10.1016/j.ijpharm.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Hoshyar N., Gray S., Han H., Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond.) 2016;11(6):673–692. doi: 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun T., Zhang Y.S., Pang B., Hyun D.C., Yang M., Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 2014;53(46):12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 23.Jain R.K., Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.