Highlights
-
•
A case of paraneoplastic cerebellar degeneration developed with progressive ovarian cancer during postoperative chemotherapy.
-
•
Intravenous immunoglobulin should be attempted when paraneoplastic cerebellar degeneration is diagnosed.
-
•
Severe neurological symptoms were dramatically improved without sequelae by intravenous immunoglobulin.
-
•
Intravenous immunoglobulin can be effective in improving neuropathy, whether it is severe neuropathy or advanced cancer.
-
•
Intravenous immunoglobulin could have beneficial effects on not only neuropathy but also on the tumor itself.
Keywords: Immunoglobulin, Ovarian neoplasms, Paraneoplastic cerebellar degeneration, Paraneoplastic syndromes
Abstract
We report the effect of intravenous immunoglobulin (IVIG) against paraneoplastic cerebellar degeneration (PCD) in a case of progressive ovarian cancer. Our patient developed PCD soon after postoperative chemotherapy and became bedridden. After undergoing IVIG, however, symptoms dramatically improved, and unexpectedly the remaining tumors were confirmed to have completely disappeared. When PCD is diagnosed, IVIG treatment should be attempted to reduce neuropathy regardless of its degree and the state of the cancer. It may also have further potential benefits that should be considered.
1. Introduction
Paraneoplastic cerebellar degeneration (PCD) is a type of paraneoplastic neurological syndrome (PNS) where neuropathy develops in patients with malignant tumors. Patients with gynecological cancers have been reported to have PCD in several case reports, mainly of ovarian cancer. Two-thirds of these patients are thought to develop neuropathy prior to cancer diagnosis, and some develop it under or after antineoplastic treatment (Braik et al., 2010, Vedeler et al., 2006, Rojas et al., 2000). Treatment for PCD has not yet been established, though some therapies have been attempted, such as with steroids, by plasmapheresis and with intravenous immunoglobulin (IVIG) (Widdess-Walsh et al., 2003, Phuphanich and Brock, 2006, Cui et al., 2017, David et al., 1996). Among these therapies, IVIG has been reported to have an anti-neuropathic effect (Braik et al., 2010, Vedeler et al., 2006, Widdess-Walsh et al., 2003, Phuphanich and Brock, 2006, Cui et al., 2017, David et al., 1996). Early IVIG treatment has been reported to be more effective than later treatment, but overall prognosis remains poor (Widdess-Walsh et al., 2003).
Regarding PCD associated with progressive ovarian cancer, there are no reports in which there was disappearance of tumors as well as neuropathy after IVIG treatment.
Our patient with PCD developed severe neuropathy with progressive ovarian cancer during postoperative chemotherapy. We began treatment using IVIG to reduce her neuropathy. Unexpectedly, not only her neuropathy, but the remaining tumors also completely disappeared after the IVIG treatment. The mechanism behind IVIG for cancer regression is unclear, but we show the potential effect of IVIG from this experience.
2. Case history/examination
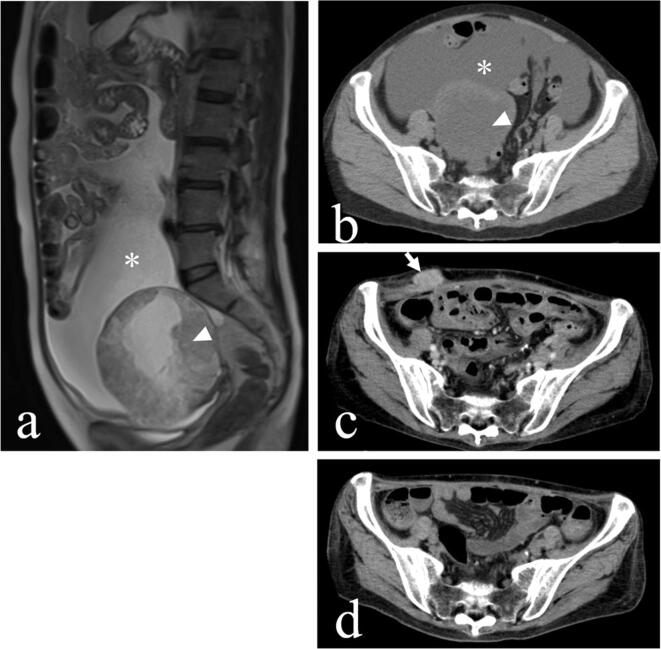
A 65-year-old multiparous woman without notable medical history presented with abdominal swelling. Pelvic computed tomography (CT) showed a 15 cm ovarian tumor and massive ascites (Fig. 1a, b). Serum tumor marker tests revealed CA-125 380.8 IU/mL.
Fig. 1.
(a, b) Computed tomography showing a 15 cm ovarian tumor (arrowhead) and massive ascites (asterisk) before the operation. (c) Tumor that was enlarged and invading the abdominal wall (arrow) one month after the operation. Other than this, minute carcinomatosis were observed on the liver surface and peritoneum. (d) One month after IVIG, the tumor had completely disappeared.
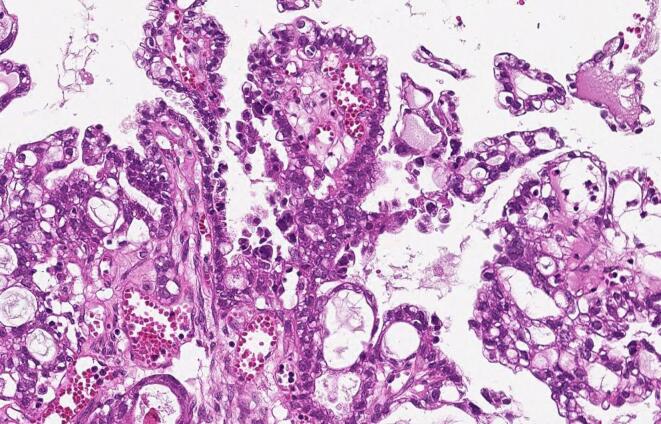
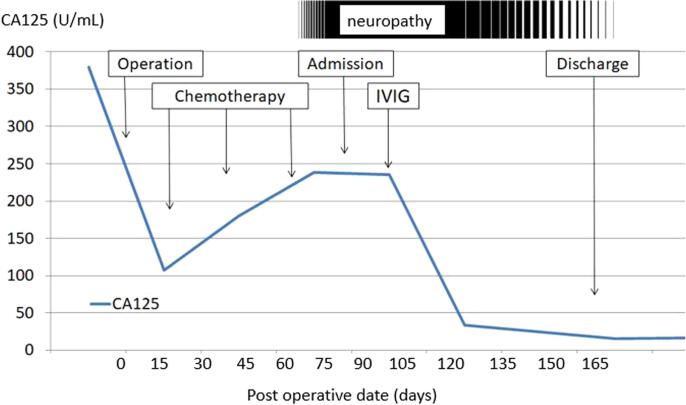
She underwent abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and debulking surgery. The tumor had already ruptured and extensive carcinomatosis was observed in the mesentery and vesicouterine excavation. We removed almost all of the clearly visible tumor tissue, but some minute carcinomatosis remained after surgery. The patient was pathologically diagnosed with stage IIIC (FIGO 2014) ovarian clear cell carcinoma (Fig. 2). She began receiving combination chemotherapy with paclitaxel and carboplatin (paclitaxel 180 mg/m2, carboplatin [AUC = 5]) from the 15th postoperative day. Just after the third cycle of the chemotherapy, the patient presented with weakness and diplopia, but she assumed these were complications of the chemotherapy. A few weeks later, however, she gradually developed ptosis, difficulty in walking, dysphagia and dysarthria. Finally, she could not move her limbs and she became bedridden. When further CT and blood tests confirmed growth of the remaining tumor (Fig. 1c) and the increase of CA125 (Fig. 3), we considered changing her treatment to palliative therapy. We discontinued chemotherapy because of her poor performance status.
Fig. 2.
Pathological findings showing clear cell carcinoma (hematoxylin-eosin stain; original magnification, 400 x).
Fig. 3.
CA125 levels throughout the clinical course.
3. Differential diagnosis, investigations and treatments
We initially suspected brain metastasis and cerebrovascular disease. According to CT and magnetic resonance imaging (MRI), however, there were no abnormal findings inside her brain. Miller Fisher syndrome, Lambert Eaton myasthenic syndrome (LEMS), and PCD were listed as differential diagnoses connected with developed symptoms such as weakness and subacute ataxia, diplopia, and ptosis.
There was no abnormality in electrophysiological tests. Cerebrospinal tests showed a mild elevation of protein (129 mg/dL), a characteristic of PNS (pleocytosis was not confirmed). There was no detection of antibodies against GQ1b for anti-Fisher syndrome or anti-P/Q-type voltage-gated calcium channel (VGCC) antibody for LEMS.
Auto-antibodies (anti-Yo, anti-Hu, anti-Ri, anti-M2 antibodies), which are characteristic of PCD, were also not detected.
These auto-antibodies are not always detected in all patients with PCD, so their detection is not necessary for PCD diagnosis (Saeed and Gupta, 2014). Three processes have been suggested as clinical diagnoses of PCD; cancer detected within five years of onset of neuropathy, exclusion of other diagnoses, and neurological symptoms indicative of PCD (Braik et al., 2010). Clinical presentation has been reported to begin with gait disorders and worsen subacutely, this is followed by development of trunk/limb weakness, dysarthria, and diplopia. Within three months from onset, many patients become bedridden with severe disabilities (Vedeler et al., 2006). Our patient’s symptoms met the diagnostic criteria for PCD, (Braik et al., 2010, Graus, 2004) so 27 days after the onset of neuropathy, we administered a five-day course of 400 mg/kg per day of IVIG.
4. Outcome and follow-up
The patient’s symptoms began to improve gradually but clearly. First, dysphagia disappeared and within a week she became able to move her knees. Two weeks after that, she could move her upper limbs and stand up independently. Her diplopia and ptosis disappeared, and from five weeks after administration she gradually became able to eat by herself. She was able to walk without assistance ten weeks after the start of IVIG treatment. She has not received any other immunosuppressive treatments including corticosteroids, plasmapheresis or further courses of IVIG. At the time of writing, five years have passed. Neuropathy has completely disappeared and she is living independently without sequelae.
Unexpectedly, according to CT, the remaining tumors had all completely disappeared one month after the start of IVIG treatment (Fig. 1d). Chemotherapy has not been resumed, but over the last five years there has been no recurrence of tumors.
5. Discussion
[Improvement of neurological symptoms]
Dramatic improvement of neurological symptoms by the treatment of IVIG in cases of PCD with development of ovarian cancer has not been widely reported (Widdess-Walsh et al., 2003, Cui et al., 2017, David et al., 1996).
The current case confirmed that IVIG can be effective in improving neuropathy, whether it is by severe neurological symptoms or advanced cancer.
Neurological symptoms of PCD have been reported to be improved by cancer treatment (Braik et al., 2010, Vedeler et al., 2006). Similar effects of chemotherapy against the cancer may have led to the improvement of neurological symptoms in the currently reported case. We speculate, however, that because of the following clinical characteristics, the improvement of neurological symptoms was actually an effect of IVIG. The neurological symptoms were worsening, despite a month having passed since the last chemotherapy treatment. Conversely, the patient’s dramatic recovery began soon after administration of IVIG. The effects of IVIG in our case appeared with similar timing to in previous reports (Widdess-Walsh et al., 2003).
[Disappearance of tumor]
The cause of the tumor disappearance in this case would generally be attributed to the effect of surgery and chemotherapy performed prior to IVIG treatment. In our case, however, various factors do not appear to fit this pattern.
Firstly, the tumor showed a tendency toward further growth, even after surgery and chemotherapy. Moreover, the chemotherapy was stopped approximately halfway through the scheduled course, after which clear cell carcinoma, known to be less responsive to chemotherapy, disappeared within a short period of just one month. Chemotherapy was not resumed, but there has been no recurrence of the carcinoma in the five years since then.
We can only make suggestions based on a single case, but other reports have also indicated similar cancer regression, although not ovarian cancer or clear cell carcinoma (Sapir and Shoenfeld, 2005). We suggest that the effect of IVIG on the tumor cannot be proven, but it cannot be completely ruled out.
6. Conclusion
Our experience showed that administration of IVIG may be beneficial, even for patients with severe symptoms of PCD developed from progressive cancer. When PCD is diagnosed, IVIG treatment may reduce neuropathy and have further potential benefits that should be considered, including possible reduction or eradication of tumors, regardless of the state of the cancer and the degree of neuropathy
7. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images
Author contribution
Takashi SHIBATA: designed the work, and wrote the initial draft of the manuscript.
Tetsuya OISHI: contributed to the conception of the work, and assisted in preparation of the manuscript.
Yasunori FUKUOKA, Shigeki NISHIKAWA, Noriaki IIZUKA, Hiroki KATO: critically revised the manuscript for important intellectual content.
All the authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge proofreading and editing by Benjamin Phillis at the Clinical Study Support Center, Wakayama Medical University.
References
- Braik Tareq, Evans Arthur T., Telfer Margaret, McDunn Susan. Paraneoplastic neurological syndromes: unusual presentations of cancer. A practical review. Am. J. Med. Sci. 2010;340(4):301–308. doi: 10.1097/MAJ.0b013e3181d9bb3b. [DOI] [PubMed] [Google Scholar]
- Cui Dan, Xu Li, Li Wen-Yi, Qian Wei-Dong. Anti-Yo positive and late-onset paraneoplastic cerebellar degeneration associated with ovarian carcinoma: A case report. Medicine. 2017;96(32):e7362. doi: 10.1097/MD.0000000000007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David Y.B., Warner E., Levitan M., Sutton D.M., Malkin M.G., Dalmau J.O. Autoimmune paraneoplastic cerebellar degeneration in ovarian carcinoma patients treated with plasmapheresis and immunoglobulin. A case report. Cancer. 1996;78:2153–2156. [PubMed] [Google Scholar]
- Graus F. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry. 2004;75(8):1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuphanich Surasak, Brock Charles. Neurologic improvement after high-dose intravenous immunoglobulin therapy in patients with paraneoplastic cerebellar degeneration associated with anti-purkinje cell antibody. J. Neurooncol. 2006;81(1):67–69. doi: 10.1007/s11060-006-9198-x. [DOI] [PubMed] [Google Scholar]
- Rojas I., Graus F., Keime-Guibert F., Rene R., Delattre J.Y., Ramon J.M., Dalmau J., Posner J.B. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology. 2000;55(5):713–715. doi: 10.1212/WNL.55.5.713. [DOI] [PubMed] [Google Scholar]
- Saeed D.B., Gupta L. Paraneoplastic cerebellar degeneration associated with serous adenocarcinoma of the ovary. BMJ Case Rep. 2014;2014(nov 28) doi: 10.1136/bcr-2014-206377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir T., Shoenfeld Y. Uncovering the hidden potential of intravenous immunoglobulin as an anticancer therapy. Clin. Rev. Allergy Immunol. 2005;29(3):307–310. doi: 10.1385/CRIAI:29:3:307. [DOI] [PubMed] [Google Scholar]
- Vedeler C.A., Antoine J.C., Giometto B., Graus F., Grisold W., Hart I.K., Honnorat J., Sillevis Smitt P.A.E., Verschuuren J.J.G.M., Voltz R. Management of paraneoplastic neurological syndromes: report of an EFNS Task Force. Eur. J. Neurol. 2006;13(7):682–690. doi: 10.1111/ene.2006.13.issue-710.1111/j.1468-1331.2006.01266.x. [DOI] [PubMed] [Google Scholar]
- Widdess-Walsh P., Tavee J.O., Schuele S., Stevens G.H. Response to intravenous immunoglobulin in anti-Yo associated paraneoplastic cerebellar degeneration: case report and review of the literature. J. Neurooncol. 2003;63(2):187–190. doi: 10.1023/a:1023931501503. [DOI] [PubMed] [Google Scholar]