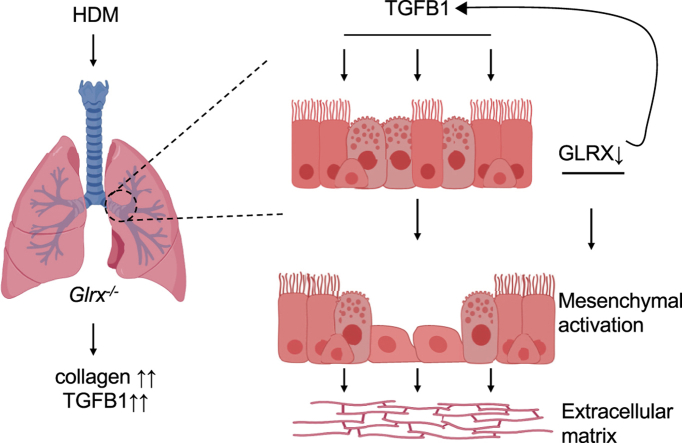
Abstract
S-glutathionylation of reactive protein cysteines is a post-translational event that plays a critical role in transducing signals from oxidants into biological responses. S-glutathionylation can be reversed by the deglutathionylating enzyme glutaredoxin (GLRX). We have previously demonstrated that ablation of Glrx sensitizes mice to the development of parenchymal lung fibrosis(1). It remains unclear whether GLRX also controls airway fibrosis, a clinical feature relevant to asthma and chronic obstructive pulmonary disease, and whether GLRX controls the biology of airway epithelial cells, which have been implicated in the pathophysiology of these diseases. In the present study we utilized a house dust mite (HDM) model of allergic airway disease in wild type (WT) and Glrx-/- mice on a C57BL/6 background prone to develop airway fibrosis, and tracheal basal stem cells derived from WT mice, global Glrx-/- mice, or bi-transgenic mice allowing conditional ablation of the Glrx gene. Herein we show that absence of Glrx led to enhanced HDM-induced collagen deposition, elevated levels of transforming growth factor beta 1 (TGFB1) in the bronchoalveolar lavage, and resulted in increases in airway hyperresponsiveness. Airway epithelial cells isolated from Glrx-/- mice or following conditional ablation of Glrx showed spontaneous increases in secretion of TGFB1. Glrx-/- basal cells also showed spontaneous TGFB pathway activation, in association with increased expression of mesenchymal genes, including collagen 1a1 and fibronectin. Overall, these findings suggest that GLRX regulates airway fibrosis via a mechanism(s) that involve the plasticity of basal cells, the stem cells of the airways.
Keywords: S-glutathionylation, Fibrosis, Asthma, COPD, Basal cells
Graphical abstract
List of abbreviations
- ACTA2
alpha smooth muscle actin
- ACTB
beta actin
- AHR
airway hyperresponsiveness
- ALI
air-liquid interface
- ALK
activin receptor-like kinase
- BALF
bronchoalveolar lavage fluid
- BMP
bone morphogenic protein
- CDH1
E-cadherin
- Col1a1
collagen 1A1
- COPD
chronic obstructive pulmonary disease
- DUOX1
dual specific oxidase-1
- EGFR
epidermal growth factor receptor
- EtOH
ethanol
- Fn1
fibronectin
- G
tissue resistance
- GLRX
glutaredoxin-1
- GSH
glutathione
- H
elastance
- H2O2
hydrogen peroxide
- HDM
house dust mite
- IL
interleukin
- IPF
idiopathic pulmonary fibrosis
- KRT5
keratin 5
- MTB cells
mouse tracheal basal cells
- MUC5AC
mucin 5AC
- PAS
Periodic acid-Schiff
- POSTN
periostin
- PSSG
protein S-glutathionylation
- Rn
Newtonian resistance
- TGFB
transforming growth factor beta
- Th2
T help 2
- TMX
4-hydroxytamoxifen
- TSLP
thymic stromal lymphopoietin
- WT
wild type
1. Introduction
Asthma is a complex disease characterized by chronic inflammation, reversible airflow obstruction and hyperresponsiveness. Goblet cell hyperplasia, mucus hypersecretion, and increases in airway smooth muscle mass are changes in the airways that can occur in asthmatics [2,3]. Fibrotic remodeling, accompanied by increases in collagens type 1 and 3 [4], fibronectin [5], fibulin 1 [6,7] and periostin [8,9], and increased expression of various growth factors and cytokines including interleukin-11 (IL11), IL13, IL17, transforming growth factor-beta (TGFB), amongst others, also is observed in airways of patients with chronic asthma [4,10,11]. Fibrotic airway remodeling has been linked to increased disease severity and insensitivity to treatment with corticosteroids [4,12]. Numerous cell types have been implicated in the pathogenesis of asthma, and among these, airway epithelial cells are well accepted to play a critical role through the secretion of a range of mediators, such as IL25, IL33 and thymic stromal lymphopoietin (TSLP) important in the orchestration of innate and adaptive immune responses [3]. IL25, TSLP, IL33 and TGFB1 derived from airway epithelial cells promote the development of airway fibrosis in models of asthma [11,13,14], lung fibrosis [15,16], or the development of fibrosis in other organs [14,17]. The epithelium also provides a physical barrier between the host and the environment, and previous studies showed that epithelial cells from asthmatics have altered expression of junctional proteins, decreased barrier function [18,19], along with a loss of structural cell communication [20].
Changes in the redox environment have been linked to asthma, including increased oxidant production [21], oxidation of glutathione [22], and inactivation of antioxidants [23]. Previous work from our laboratories has shown that allergen-mediated activation of the hydrogen peroxide (H2O2)-producing enzyme, dual specific oxidase-1 (DUOX1) is a key step in the release of IL33 from airway epithelial cells [24] and that this requires oxidative activation of epidermal growth factor receptor (EGFR) [25]. Despite these observations, the mechanisms whereby signals from oxidants are transduced to regulate allergic airway inflammation and remodeling remain to be unraveled.
Oxidation of cysteine thiol groups within proteins serves as a sensor that converts an oxidant signal into a biological response. Protein S-glutathionylation (PSSG) represents the conjugation of glutathione to reactive protein cysteines, and the glutathionylation/deglutathionylation cycle has emerged as a key mechanism where oxidants regulate biological functions [[26], [27], [28], [29]]. The addition of glutathione (GSH) to a protein cysteine changes the size and charge of the protein, owing to the addition of 3 amino acids and a glutamic acid moiety, respectively, thereby imparting potential structural and/or functional changes. In addition, S-glutathionylation also protects proteins from irreversible overoxidation. Following an initial oxidative insult, the subsequent S-glutathionylation reaction can occur spontaneously or be catalyzed by glutathione S-transferases, notably glutathione S-transferase P [30]. Conversely, glutaredoxins (GLRX), under physiological conditions, catalyze the deglutathionylation process, restoring the sulfhydryl groups to their native state [28,29].
Changes in GLRX and protein S-glutathionylation have been observed in the airways of patients with asthma [31]. Our laboratory has previously demonstrated that global ablation of Glrx attenuated airways hyperresponsiveness in mice on the BALB/c background using the ovalbumin model of allergic airways disease [32]. Glrx-/- mice also displayed attenuated expression of IL6, IL13, as well as mucin 5AC (MUC5AC) in a house dust mite (HDM) model of allergic airways disease [33]. In models of parenchymal pulmonary fibrosis, we recently established that absence of Glrx promotes fibrogenesis [1]. The goal of the present study was to determine whether GLRX affects airway fibrosis following exposure to HDM. Given the aforementioned role of epithelial cells in airway remodeling, we also investigated whether GLRX status regulated the homeostasis of airway epithelial cells and their responses to HDM or TGFB1.
2. Materials and methods
HDM-induced allergic airways disease and assessment of airways hyperresponsiveness: For all experiments, 8–12 week old mice were used. Glrx-/- mice described previously [34] were backcrossed more than 10 generations onto a C57BL/6/NJ background. Wild type (WT) littermates served as controls. All studies were approved by the Institutional Animal Care and Use Committee at the University of Vermont. Experiments were performed at least twice, and animal numbers in each group are noted in each Figure Legend. Mice were subjected to intranasal instillations with 10 μg of HDM protein extract (GREER®, cat: XPB70D3A2.5, Lot: 259585, Lenoir, NC, USA) resuspended in saline, or with saline alone as a vehicle control, once a week for two weeks, and 5 consecutive challenges in the third week. Mice were euthanized 24 h following the fifth challenge in the third week. Following completion of the HDM protocol, mice were anesthetized with intraperitoneal pentobarbital sodium (90 mg/kg), tracheotomized, and mechanically ventilated at 200 breaths/min. Mice were subjected to increasing doses of methacholine (0, 3 mg, 13 mg, 25 mg and 50 mg) and respiratory mechanics were assessed using a forced oscillation technique on a computer-controlled small animal ventilator (SCIREQ, QC, Canada), as previously described [35,36]. Parameters of Newtonian resistance (Rn), tissue resistance (G) and elastance (H) were calculated and quantified by averaging the three highest measurements obtained at each incremental methacholine dose for each mouse.
Creation of transgenic mice expressing Glrx with LoxP-flanked alleles (GlrxLoxP) and conditional ablation of Glrx in airway basal cells:
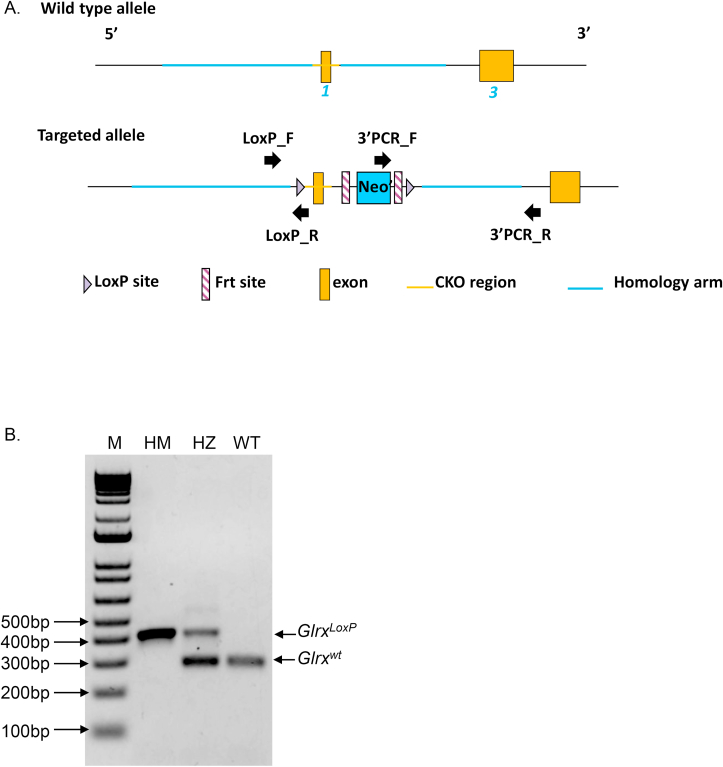
Mice with Glrx LoxP-flanked alleles were created by Cyagen Biosciences Inc. (Santa Clara, CA). Exon 1 of Glrx was targeted by the incorporation of flanking LoxP sites and a neomycin resistance cassette flanked by Frt sites. The targeting construct was electroporated into C57BL/6 embryonic stem cells, followed by selection of G418 resistant clones. Recombinant ES cell line clones were verified by PCR and Southern blot analysis for presence of the neomycin (neo) targeting cassette prior to injection into blastocysts. Chimeric founder mice were bred with C57BL/6 mice to confirm germline transmission, and resulting heterozygous mice were bred to the Flp-deleter strain to remove the neo cassette (Cyagen Biosciences). GlrxLoxP mice were subsequently bred for 10+ generations onto the C56BL/6/NJ background. Primers to genotype the GlrxLoxP transgenic mice are Forward: GCTGGTTGGTGTGTGGATAGTG Reverse: GGTGGGGCTAGGTAAGAATGGACA resulting in a product size of 344 bp for the WT Glrx allele and 414 bp for the GlrxLoxP allele. Homozygous GlrxLoxP/LoxP mice were bred with Krt5CreERT2 mice [37] (Jackson Laboratories, Stock #029155). The Cre-ERT2 fusion protein consists of Cre recombinase fused to a mutant form of the human estrogen receptor which binds the synthetic estrogen receptor ligand, 4-hydroxytamoxifen (TMX). This strategy allows for the selective ablation of the Glrx gene from Krt5-expressing basal cells to permit investigation of acute ablation of Glrx in airway basal cells. Single transgenics were used as controls.
2.1. Bronchoalveolar lavage fluid
After mice were euthanized, bronchoalveolar lavage fluid (BALF) was performed using 1 ml PBS. BALF was collected and total cell counts were determined by staining cells with trypan blue and counted with a hemocytometer. BALF was spun down at 1200g for 5 min. Cells were transferred to slides using a cytospin, fixed in methanol and stained using the Hema3 kit (Fisher Scientific, Kalamazoo, MI) and analyzed by counting a minimum of 300 cells per mouse, as described elsewhere [36]. Supernatants were flash frozen in liquid nitrogen and stored at -80 °C until analysis.
2.2. Measurement of airway remodeling
The right lung was fixed in 10% formalin and embedded in paraffin. Lung sections (5 μm) were stained using Masson's Trichrome to visualize collagen. Images were acquired using an Olympus BX50 Light Microscope with QImaging Retiga 2000R digital camera. Collagen content was determined by isolating the superior lobe of the right lung and measuring the total content of hydroxyproline as previously described [38]. Periodic acid-Schiff (PAS) staining was performed and the positive staining areas were quantified by MetaMorph imaging software (Molecular Devices). Alpha-smooth muscle actin staining (ACTA2, 1:4000; A2547; Sigma) was used to evaluate airway smooth muscle via immunohistochemistry.
2.3. Cell culture and stimulation with HDM or TGFB1
Primary mouse tracheal basal (MTB) cells were isolated from WT C57BL/6 mice, C57BL/6 mice lacking glutaredoxin-1 globally (Glrx-/-) or conditionally (Krt5CreERT2:GlrxLoxP/LoxP) and cultured as previously described [39,40]. A minimum of three tracheas were pooled for each basal cell culture, and cultures were initiated at least three times, unless otherwise noted. MTB cells were incubated in serum-free medium. After reaching confluence, MTB cells were stimulated with 5 ng/ml TGFB1 (240-B-002/CF; R&D systems, Minneapolis, MN, USA) or 50 μg/ml HDM protein extract (Lot: 259585), and at the indicated times, cells were harvested for expression of mesenchymal genes, proteins and TGFB pathway activation.
For air-liquid interface (ALI) cultures, MTB cells were seeded onto 0.4-μm transwell membranes pre-coated with collagen with a density of 6000 cells/mm2. Medium was replaced with complete Pneumacult-ALI medium (05001; StemCell Technologies, Vancouver, Canada) on day 2 in both the upper and lower chambers. ALI medium was added to only the lower chamber starting day 3, with the media replaced every other day for 21 days. In experiments involving airway basal cells isolated either from Krt5CreERT2:GlrxLoxP/LoxP bi-transgenic mice or single transgenic mice, cells were treated with TMX (500 nM, Sigma, cat. T176) or 0.004% EtOH (v/v) as a vehicle control on days 2 and 3, prior to the differentiation of basal cells into fully differentiated epithelia. On day 24, fully differentiated epithelial cultures were starved for 24 h with Pneumacult-ALI maintenance medium or serum-free media. Cells were stimulated on the apical side with 100 μl of Pneumacult-ALI maintenance medium or serum-free media with 50 μg/ml of HDM protein extract (Lot: 259585 or 348718). 24 h after HDM stimulation, supernatants from apical and basolateral sides were collected for the assessment of TGFB1.
2.4. Enzyme-linked immunosorbent assay (ELISA)
TGFB1 was detected by ELISA kit (DY1679; R&D systems, Minneapolis, MN, USA) in BALF or supernatants from cell cultures according to manufacturer's instructions. This assay kit measures total TGFB1, consisting of latent plus active growth factor.
2.5. Western Blotting
Lung tissues or cell lysates were lysed in buffer containing 20 mM Tris, 150 mM NaCl, 1% (v/v) Nonidet P-40, 1% (v/v) protease and phosphatase inhibitor cocktail (78444; Thermo Scientific, Waltham, MA, USA). Total protein was assessed by the Bio-Rad DC Protein Assay kit (Bio-Rad, Hercules, CA, USA), according to manufacturer's protocol. Beta actin (ACTB) or glyceraldehyde-3 phosphate dehydrogenase (GAPDH) (2118; Cell Signaling Technology, Danvers, MA, USA) antibodies were used as a loading control. An anti-GSH antibody (101-A; ViroGen, Watertown, MA, USA) was used to evaluate protein S-glutathionylation. The inclusion of dithiothreitol to decompose S-glutathionylated proteins was used to validate the antibody (data not shown).
2.6. Immunofluorescence and confocal imaging
Cells were fixed in 4% PFA and blocked in 5% BSA before staining. The following primary antibodies were used: rabbit anti-pSmad2 (1:1000, 3108, Cell Signaling Technology), rabbit anti-cytokeratin 5 (1:1000, ab52635, Abcam), rabbit anti-Ecadherin (1:1000, 3195, Cell Signaling Technology). Secondary antibodies used were conjugated to Alexa Fluor 488, 647 (Life Technologies, Carlsbad, CA, USA). 4′,6-diamidino-2-phenylindole (DAPI) (1:4000, D1306, ThermoFisher) was used for nuclear counter stain.
Fluorescent images were collected on a confocal microscope (Zeiss LSM 510 META laser scanning confocal microscope). Scale bars were added and images were processed using Photoshop (Adobe). As a control, primary antibodies were omitted to establish background fluorescence. Signal intensities were quantified using Metamorph.
2.7. Gene expression
Total RNA was isolated from lung tissues using the RNeasy mini-kit (74106; Qiagen, Valencia, CA, USA), subjected to reverse transcription and DNase treatment to produce cDNA for Taqman gene analysis using SYBR green and CFX96 Real-Time System (50001111; Biorad, Hercules, CA, USA). The relative expression was normalized to Actb using the expression Primer sequences provided in Table 1. Sequences were taken from Genbank. All accession numbers are denoted.
Table 1.
Primer sequences used in this study.
| Genes | Forward | Reverse |
|---|---|---|
| Col1a1 | CACCCTCAAGAGCCTGAGTC | AGACGGCTGAGTAGGGAACA |
| Fn1 | AATGGAAAAGGGGAATGGAC | CTCGGTTGTCCTTCTTGCTC |
| Actb | CCCTCCCAGGGAGACCAA | CTGAATGGCCCAGGTCTGA |
2.8. Statistical analyses
All analyses were performed using GraphPad Prism software by ANOVA. All experiments were repeated at least twice and data from combined experiments are represented as mean ± S.E.M. Scoring of histological staining was analyzed by Kruskal-Wallis test and Dunn's post hoc test. For all analyses, P values ≤ 0.05 were considered significant.
3. Results
Glrx-/- mice display similar increases in airway inflammation and elevated mucus hyperplasia in response to HDM exposure.
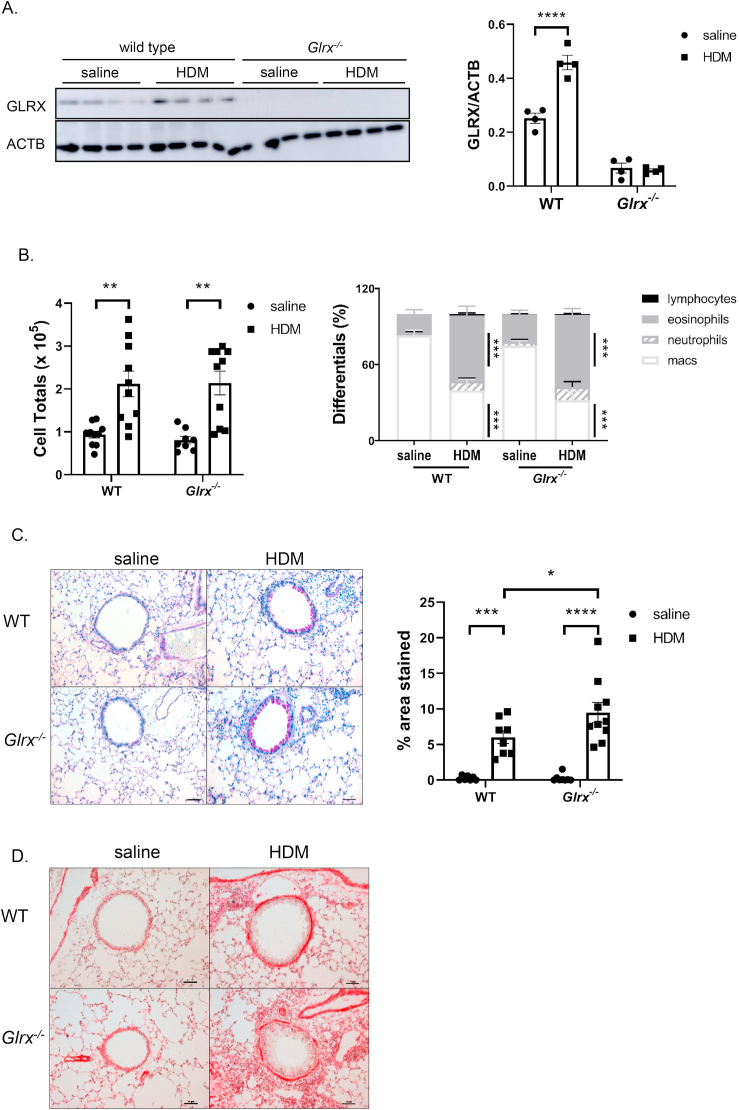
We recently described that mice lacking Glrx were more prone to the development of parenchymal pulmonary fibrosis [1]. Airway fibrosis accompanies numerous chronic lung diseases, including asthma and chronic obstructive pulmonary disease (COPD) [4,41]. To address whether GLRX regulates airway fibrosis, herein we use a model of HDM-induced airway fibrosis, by using WT mice or mice globally lacking Glrx (Glrx-/-), bred onto a C57BL/6 background, as this strain is prone to development of fibrosis, in contrast to BALB/c mice traditionally used to study allergic disease. In line with prior observations in BALB/c mice [33], HDM sensitization and challenge of C57BL/6 mice led to increases in GLRX expression in lung tissue (Fig. 1A). In mice subjected to HDM, the increases in cellular influx into the airways measured by cell counts in the in bronchoalveolar lavage (BAL) fluid were comparable between WT and Glrx-/- mice (Fig. 1B, left panel). Statistically significant increases in eosinophil percentages, and decreases in macrophage percentages occurred in WT or Glrx-/- mice exposed to HDM, compared to respective saline controls, while the proportion of neutrophils trended toward increases but did not reach statistical significance and lymphocyte numbers accounted for low percentages (<1%) not visible in the schematic (Fig. 1B, right panel). No significant differences in the proportion of inflammatory cell types was observed in Glrx-/- mice compared to their wild type counterparts following HDM exposure (Fig. 1B, right panel).
Fig. 1.
Assessment of inflammation, mucus metaplasia and smooth muscle cells in WT or Glrx-/- mice following repeated exposure to house dust mite. A: Western Blot analysis for GLRX in homogenized lung tissue of WT or Glrx-/- mice exposed to saline or house dust mite (HDM). ACTB (β-actin): loading control. B: Total (left) and differential cell counts (right) in BAL fluid from WT or Glrx-/- mice after exposure to saline or HDM. C: Periodic acid-Schiff (PAS) staining in WT or Glrx-/- mice exposed to HDM or saline (scale bar = 50 μm). Quantification of airway mucus staining intensity was determined by positive staining areas using Metamorph. D: Alpha-smooth muscle actin (ACTA2) immune-reactivity in WT or Glrx-/- mice exposed to saline or HDM (scale bar = 50 μm). WT PBS n = 8, WT HDM n = 10, Glrx-/- PBS n = 8, Glrx-/- HDM n = 10 mice. ***p < 0.001, ANOVA.
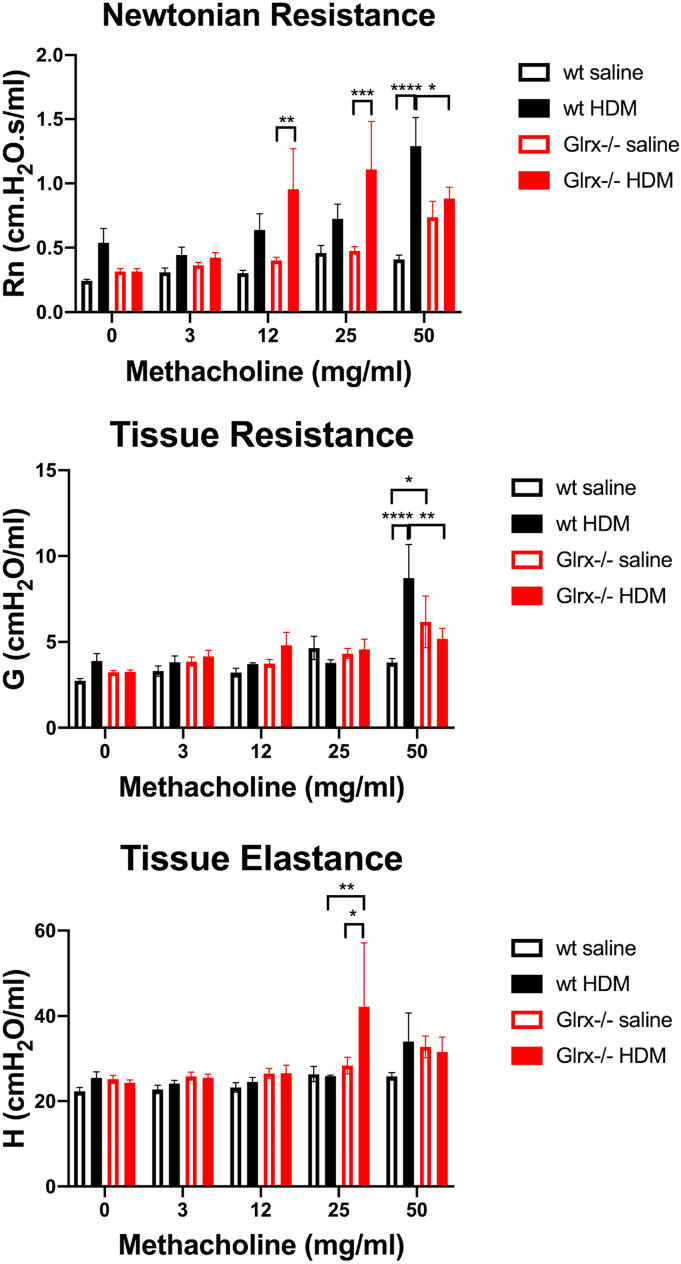
We next investigated the impact of Glrx ablation on HDM-induced airways remodeling. Manifestations of airways remodeling include mucus metaplasia and increased alpha smooth muscle actin content (ACTA2). As expected, an increase in Periodic acid-Schiff (PAS) staining, indicative of mucus metaplasia occurred in response to HDM sensitization and challenge, and this was significantly enhanced in Glrx-/- mice as compared to respective WT animals (Fig. 1C). ACTA2 immunoreactivity was qualitatively increased to a similar extent in WT or Glrx-/- mice exposed to HDM (Fig. 1D). Assessment of airway hyperresponsiveness (AHR) in WT mice exposed to HDM showed increases in in central airways resistance (Rn) and tissue resistance (G) in response to 50 mg/ml methacholine, while tissue elastance (H) was not affected (Fig. 2). In contrast, Glrx-/- mice exposed to HDM showed increases in central airways resistance and tissue elastance at lower doses of methacholine, although these patterns reversed at the highest dose (50 mg/ml) of methacholine (Fig. 2, top and bottom panels). In the absence of exposure to HDM, mice lacking Glrx also showed statistically significant increases in tissue resistance in response to 50 mg/ml methacholine, compared to the respective WT groups (Fig. 2, middle panel), and similar trends towards increases in central airway resistance also were observed in Glrx-/- mice, although these did not reach statistical significance. These results suggest intrinsic differences in respiratory mechanics in mice lacking Glrx. The lack of changes in tissue elastance (H) in WT animals suggests that in C57BL/6 mice, HDM-mediated changes in respiratory mechanics are largely restricted to the central airways, in contrast to observations in the BALB/c strain [33].
Fig. 2.
Assessment of airway hyperresponsiveness (AHR) in WT or Glrx-/- mice exposed to saline or HDM.
Mice were tracheostomized and ventilated, and respiratory mechanics evaluated at baseline, or in response to ascending doses of the bronchoconstricting agent, methacholine. Shown are the Newtonian resistance (Rn, a marker of airway stiffness or central airway resistance, top), tissue resistance (G, middle), and tissue elastance (H, bottom) parameters. (*p < 0.05; **p < 0.01; ***p < 0.001). WT PBS n = 8, WT HDM n = 10, Glrx-/- PBS n = 8, Glrx-/- HDM n = 10 mice.
Glrx-/- mice display elevated subepithelial collagen deposition in response to HDM exposure.
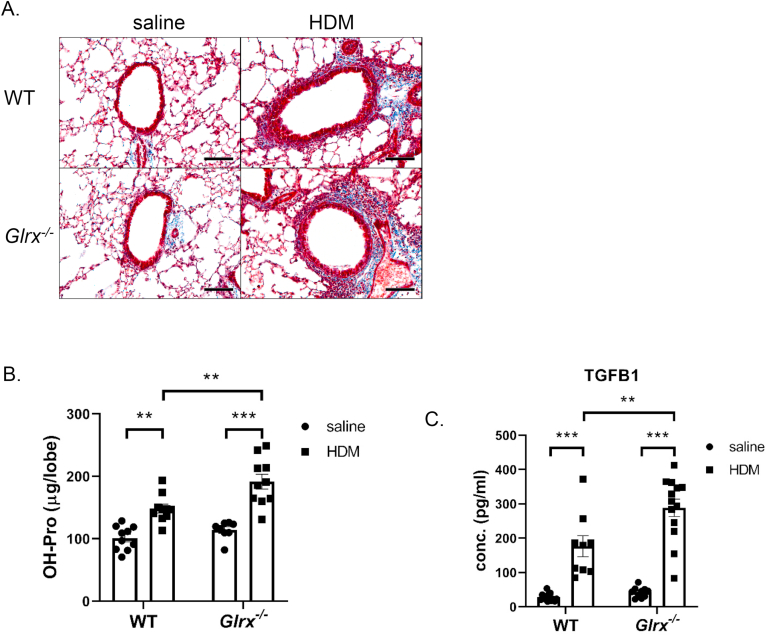
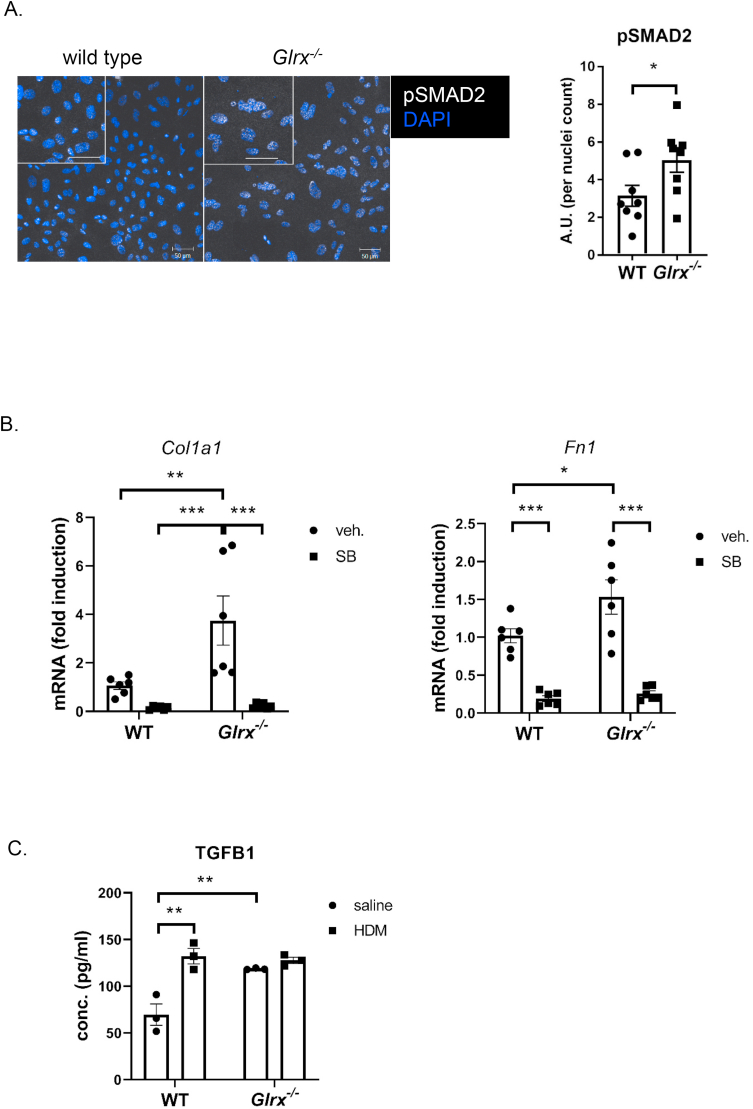
We next evaluated whether GLRX regulated the extent of airway collagen deposition. Qualitative Masson's Trichrome staining demonstrated an increased subepithelial collagen content in WT and Glrx-/- mice exposed to HDM (Fig. 3A). Quantitative assessment of hydroxyproline content, indicative of total lung collagen, showed significant increases in WT mice exposed to HDM, and that HDM-induced increases in hydroxyproline were more pronounced in Glrx-/- mice compared to WT mice (Fig. 3B). Consistent with these observations, levels of the pro-fibrogenic growth factor, TGFB1, were elevated in the BALF of WT mice exposed to HDM, with further elevations occurring in Glrx-/- mice exposed to HDM (Fig. 3C).
Fig. 3.
Evaluation of airway fibrosis in WT or Glrx-/- mice exposed to saline or HDM.
A. Masson's Trichrome staining in WT or Glrx-/- mice exposed to HDM or saline (scale bar = 50 μm). B: Assessment of hydroxyproline content in the right superior lobe 24 h after the final challenge. C: Evaluation of TGFB1 level in the BAL fluid by enzyme-linked immunosorbent assay (ELISA). WT PBS n = 13, WT HDM n = 9, Glrx-/- PBS n = 10, Glrx-/- HDM n = 13 mice.
Airway basal cells (MTB) isolated from Glrx-/- mouse tracheas have increased expression of the basal cell marker keratin 5, but decreased expression of E-cadherin.
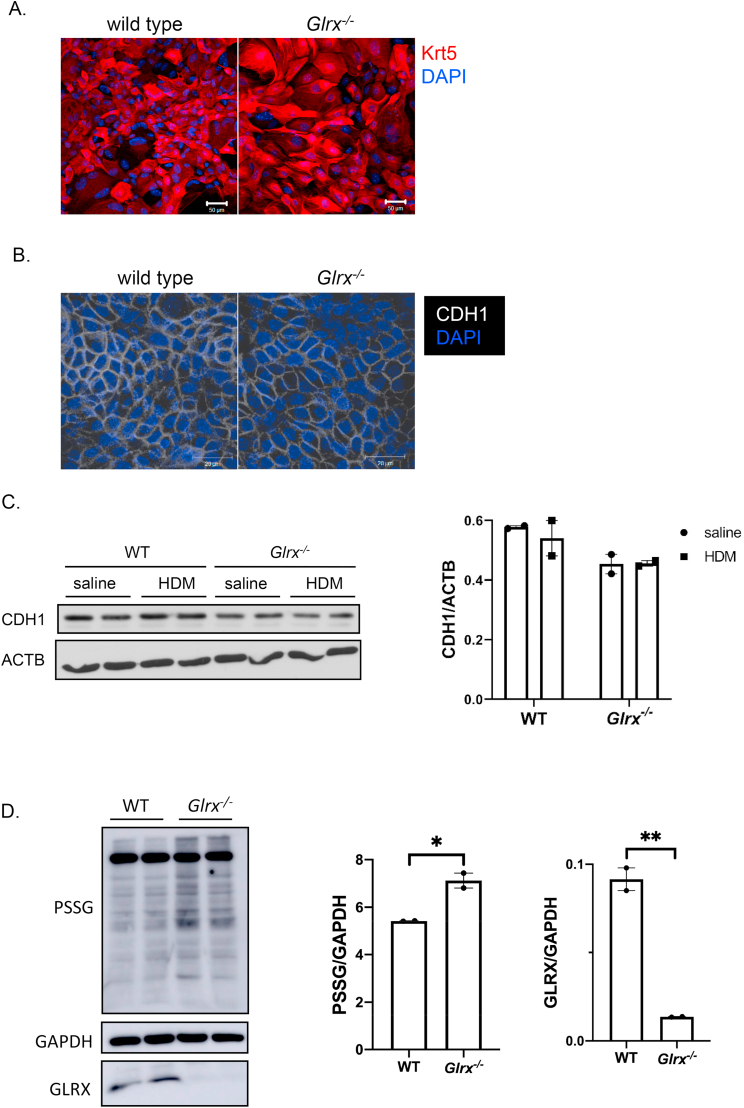
Airway basal cells line the basement membrane and are the stem cells of the airways that give rise to ciliated, secretory and other terminally differentiated cells [42,43]. Shifts in the characteristics of basal cells and resultant reduced epithelial diversity have been linked to type 2 immune-mediated barrier tissue dysfunction [44]. We have shown that in response to TGFB1, basal cells deposit a provisional extracellular matrix that in turn induces mesenchymal activation in subsequently plated control basal cells [45], suggesting that basal cells are linked to fibrotic airway remodeling. Given the increases in airway fibrosis in Glrx-/- mice exposed to HDM, compared to WT mice, we therefore sought to further characterize airway basal cells (MTB) isolated from WT or Glrx-/- mice. As expected, WT or Glrx-/- MTB cells both expressed the basal cell marker, keratin 5 (KRT5), although KRT5 expression was higher in Glrx-/- basal cells, compared to WT counterparts (Fig. 4A). Interestingly, expression of the epithelial marker, E-cadherin, tended to be lower in Glrx-/- cells, compared to WT counterparts (Fig. 4B and C). These findings suggest that basal cells lacking Glrx are intrinsically distinct from WT cells. Western blot analysis using an anti-GSH antibody showed that Glrx-/- basal cells intrinsically showed increases in protein S-glutathionylation, compared to WT counterparts (Fig. 4D).
Fig. 4.
Characteristics of primary airway basal cells isolated from WT or Glrx-/- mouse tracheas. A: Expression of the basal cell marker, cytokeratin 5 (KRT5) in WT and Glrx-/- basal cells via immunofluorescence analysis (scale bar = 50 μm). B: Expression of the epithelial marker, E-cadherin (CDH1) in WT or Glrx-/- basal cells evaluated via immunofluorescence (scale bar = 20 μm). C: Assessment of the epithelial marker, E-cadherin (CDH1) in WT or Glrx-/- basal cells exposed to HDM (50 μg/ml, 7 days) evaluated via Western blot analysis. ACTB (β-actin): loading control. D: Assessment of protein S-glutathionylation in WT or Glrx-/- basal cells evaluated via Western blot analysis. GAPDH (glyceraldehyde-3-phosphate dehydrogenase): loading control. GLRX blot confirms the absence of GLRX proteins in Glrx-/- airway basal cells. Shown is a representative blot out of 3 independent experiments.
Airway basal cells isolated from Glrx-/- mouse tracheas have elevated expression of mesenchymal proteins and upon differentiation in air liquid interface conditions spontaneously secrete TGFB1.
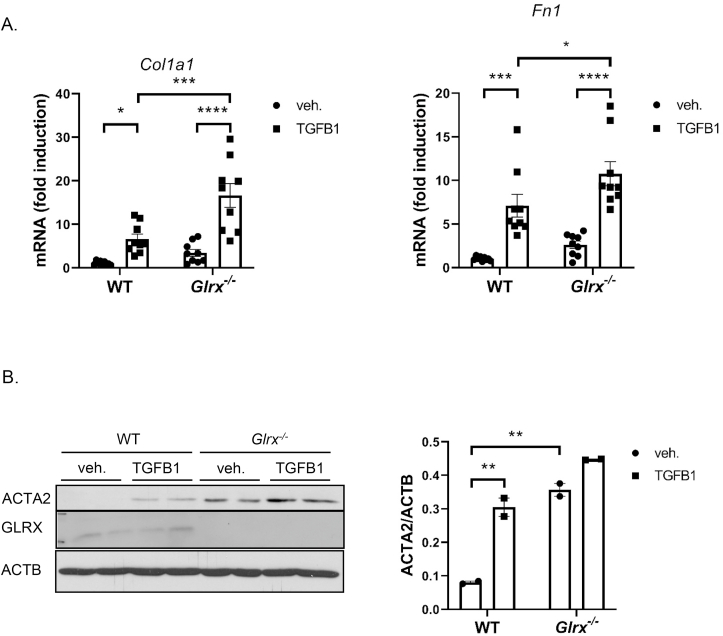
The observed increased subepithelial fibrosis and TGFB1 in the BALF, and decreases in E-cadherin prompted us to address whether Glrx-/- MTB cells responded differently to HDM or TGFB1 compared to WT epithelial cells. In addition, Glrx-/- basal cells showed intrinsic increases in expression of collagen 1a1 (Col1a1) and fibronectin (Fn1) (Fig. 5A), genes implicated in airways remodeling. In response to stimulation with TGFB1, expression of these genes increased further in WT and Glrx-/- MTB cells (Fig. 5B). Similarly, constitutive expression of alpha smooth muscle actin (ACTA2) was observed in Glrx-/- MTB cells, even in the absence of stimulation with TGFB1, and further increases occurred in response to TGFB1 (Fig. 5B). These findings demonstrate an increased mesenchymal character of Glrx-/- MTB cells, characteristic of activation of the TGFB pathway. In order to further address this, we examined phosphorylation of SMAD2 (pSMAD2) as an indicator of TGFB pathway activation and showed that pSMAD2 was increased in Glrx-/- MTB cells compared to WT counterparts, even in the absence of TGFB stimulation (Fig. 6A). To assess whether TGFB pathway activation contributed to the increases in Col1a1 and Fn1 expression in Glrx-/- basal cells, we inhibited the type I receptor for TGFB, activin receptor-like kinase (ALK4, 5 and 7), using SB431542. Exposure of WT or Glrx-/- MTB cells to SB431542 decreased Col1a1 and Fn1 expression to comparative levels (Fig. 6B). Basal cells are the stem cells of the airways that give rise to terminally differentiated epithelial cells when cultured under air liquid interface conditions (ALI). We therefore addressed whether the mesenchymal character of Glrx-/- epithelial cells was maintained following their culture on ALI. In this setting, WT epithelial cells exposed to HDM secreted TGFB1 into the medium (Fig. 6C). Interestingly, Glrx-/- epithelial cells, spontaneously released more TGFB1 than WT cells, even in the absence of HDM, while no further increases were observed in response to HDM (Fig. 6C).
Fig. 5.
Primary Glrx-/- basal cells spontaneously secrete TGFB1 and express mesenchymal proteins. A: mRNA expression levels of Col1a1 and Fn1 in WT vs. Glrx-/- MTB cells in response to TGFB1 (5 ng/ml, 48 h). B: Western Blot analysis of ACTA2 in WT vs. Glrx-/- MTB cells stimulated with TGFB1 (5 ng/ml, 3 days). ACTB (β-actin): loading control.
Fig. 6.
Constitutive activation of the TGFβ pathway in primary airway basal cells lacking Glrx. A: Assessment of phosphorylation(s) of SMAD2 in WT vs. Glrx-/- basal cells (scale bar = 50 μm). B: mRNA expression levels of Col1a1 and Fn1 in WT vs. Glrx-/- MTB cells in response to the ALK5 inhibitor, SB431542 (10 μM, 48 h) (*p < 0.05; **p < 0.01; ***p < 0.001). C: Assessment of TGFB1 levels in supernatants of WT vs. Glrx-/- epithelial cells differentiated under air liquid interface conditions (ALI), in the absence of stimuli, or following exposure to HDM (50 μg/ml, 24 h).
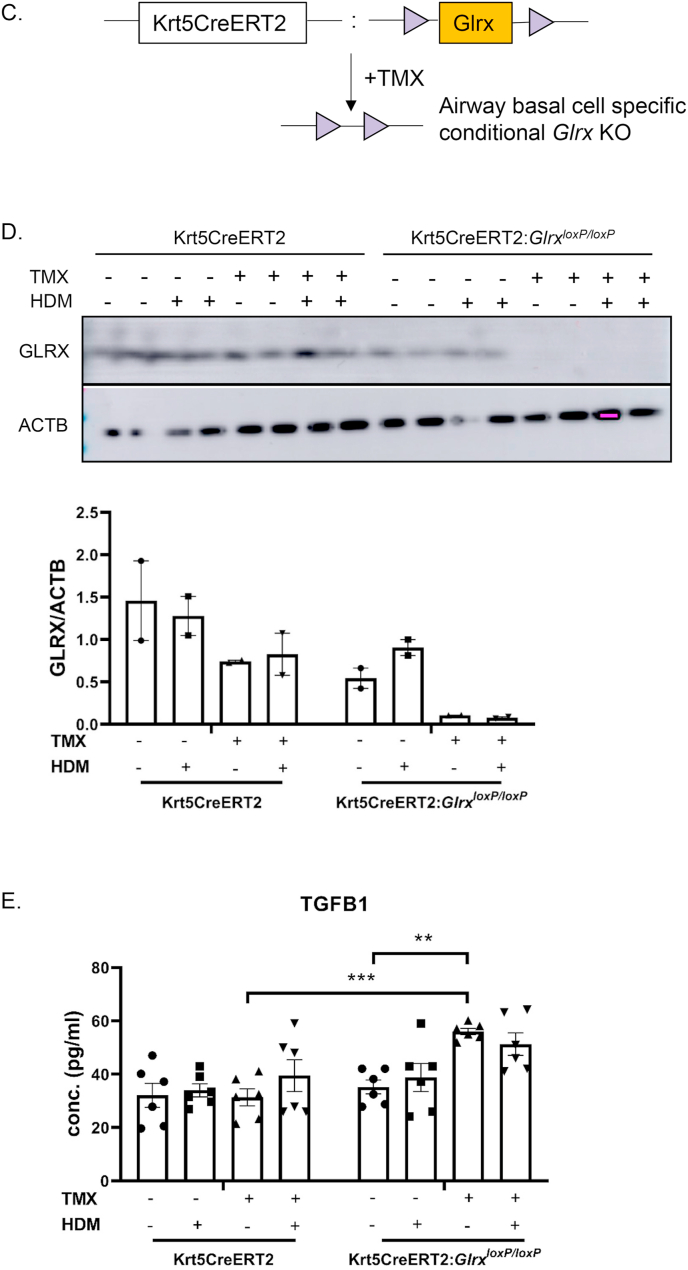
It is plausible that the mesenchymal character of Glrx-/- airway basal cells is the result of prolonged absence of the Glrx gene. In order to corroborate our observations, we created transgenic mice containing a LoxP-flanked Glrx gene (Fig. 7A and B) and bred these mice with animals expressing a Cre-driver controlled by the keratin-5 promoter in a 4-hydroxytamoxifen (TMX)-dependent manner (Krt5CreERT2). Administration of TMX to airway basal cells from GlrxLoxP/LoxP:Krt5CreERT2 bi-transgenic mice during days 2 and 3 of the ALI protocol, a time prior to their differentiation to terminally differentiated cells resulted in ablation of the GLRX protein. TMX did not impact GLRX in airway basal cells isolated from Krt5Cre-ERT2 single transgenic mice (expressing WT Glrx) (Fig. 7D). In contrast to observations in Fig. 6C, we did not observe increases in secretion of TGFB1 in response to HDM (Fig. 7E), possibly due to minor variations in culture conditions to accommodate for treatment of TMX, the presence of ethanol, used as a vehicle control, or differences between HDM lots. However, administration of TMX to airway basal cells from GlrxLoxP/LoxP:Krt5CreERT2 bi-transgenic mice resulted in enhanced secretion of TGFB1, while in the control groups secretion of TGFB1 was not affected (Fig. 7E). Overall, these results suggest that increased TGFB pathway activation may contribute to the intrinsically enhanced mesenchymal responses in airway epithelial cells lacking Glrx.
Fig. 7.
Acute ablation of Glrx from airway basal cells isolated from Krt5Cre-ERT2:GlrxLoxP/LoxP bi-transgenic mice results in secretion of TGFB1. A: Design of mice with a LoxP-flanked Glrx allele. B: PCR screening of GlrxLoxP/LoxP transgenic mice. C: Schematic of TMX-mediated ablation of Glrx from airway basal cells. D: Deletion of GLRX protein in airway basal cells from Krt5Cre-ERT2:GlrxLoxP/LoxP bi-transgenic mice following administration of 500 nM 4hydroxytamoxifen (TMX) for 2 days. ACTB (β-actin): loading control. E: TMX-mediated ablation of GLRX from airway basal cells prior to their differentiation under ALI results in spontaneous TGFB1 secretion when assessed at day 25 of ALI culture. Results are obtained from 2 separate primary cell cultures, each initiated by pooling 3 tracheas from either Krt5CreERT2 single transgene or Krt5CreERT2:GlrxloxP bitransgenic mice. **p < 0.01 ANOVA.
4. Discussion
Airway fibrosis is a feature of asthma that has been linked to increased severity and insensitivity to treatment with corticosteroids [4,12]. The mechanistic details that promote the development of fibrosis in the airways of asthmatic patients remain not fully known, although various growth factors and cytokines have been implicated, including IL11, IL13, IL17, IL33 and TGFB [4,10,11,15] as stated earlier. Changes in the redox environment also have been implicated in the pathogenesis of asthma, and development of interstitial pulmonary fibrosis [1,46]. In the present manuscript, we confirm that mice exposed to HDM, a major allergen in asthma, bred onto the C57BL/6 background develop airway fibrosis. Herein, we also show that mice that globally lack the deglutathionylating enzyme, Glrx, are more prone to the development of airway fibrosis in response to HDM, and show increases in airway responsiveness, as compared to WT counterparts. We further show that these changes occurred in the absence of overall alterations of inflammatory cell profiles or ACTA2 immunoreactivity between WT or Glrx-/- mice exposed to HDM. However, HDM-exposed Glrx-/- mice showed increases in mucus metaplasia, compared to the respective WT groups. Overall, these results show that the pro-fibrotic phenotype previously reported in Glrx-/- mice using the bleomycin or adenovirus expressing active TGFB1 models of pulmonary fibrosis is also observed in settings of airway fibrosis induced by an asthma-relevant allergen. We speculate that increases in airway fibrosis could account for the observed increases in central airways resistance (Rn) in Glrx-/- mice compared to respective WT groups, as increased fibrosis and subsequent narrowing of larger airways could lead to an accentuated response for every given to degree of airway smooth muscle shortening [47].
Results herein also demonstrate the spontaneous release of TGFB from airway epithelial cells isolated from either Glrx-/- mice (Fig. 6C) or GlrxLoxP/LoxP:Krt5CreERT2 mice (Fig. 7E). Glrx-/- airway basal cells have increases in the mesenchymal protein ACTA2 and increased expression of Fn1 and Col1a1, in association with activation of the TGFB pathway, based upon our findings that phosphorylation of SMAD2 was increased, and that a TGFBR inhibitor attenuated expression of Fn1 and Col1a1 in cells lacking Glrx. Future studies will be necessary to unravel how the TGFB pathway becomes activated in the absence of Glrx, aided by the identification of S-glutathionylated target proteins that have the potential to control activation of the TGFB pathway.
Airway basal cells are the stem cells within the airways that give rise to KRT8-positive early progenitor cells and subsequent secretory (club or goblet), ciliated, and other terminally differentiated cells, with new cell lineages only recently being discovered through single cell RNA sequencing [20,42]. Secretory cells or ciliated cells can give rise to mucin producing cells that synthesize MUC5AC and MUC5B, among others, important in host defense and clearance of pathogens [43,48]. Glrx-/- mice exposed to HDM developed mucus metaplasia to a greater extent compared to the respective WT group. The mechanistic details that link GLRX to MUC5AC will require additional studies. It is possible that Glrx-/- basal cells are intrinsically more prone to give rise to mucin-producing secretory cells. Alternatively, it is plausible that the cytokine environment of the Glrx-/- mice promotes mucus metaplasia more readily compared to WT mice [49]. Notably the cytokines IL33 and IL13 promote mucus metaplasia in mice with HDM-induced allergic airway disease [24,25]. However, assessment of levels of IL13 or IL33 in the lungs of Glrx-/- mice exposed to HDM revealed no statistically significant differences between WT or Glrx-/- mice (data not shown). Finally, the possibility also exists that GLRX directly regulates oxidation state and/or function of airway mucins, based upon a prior study showing that mucin disulfide cross-links stiffens mucus gels [49]. Future studies that incorporate recently developed GlrxloxP mice in combination with lineage tagging will elucidate the role of Glrx in airway basal cells in dictating the generation of MUC5AC-producing cells and their properties.
Our laboratories have previously described the importance of the hydrogen peroxide-producing enzyme DUOX1 in the oxidative activation of EGFR and subsequent release of IL33 from airway basal cells [24,25]. IL33 in turn induces increases of IL13 and subsequent mucus metaplasia [50]. It remains unclear whether GLRX regulates responsiveness to IL33 in settings of allergic airway disease. Intriguingly, in macrophages, Glrx expression is critical of lipopolysaccharide-mediated deglutathionylation of tumor necrosis factor associating factor 6, subsequent activation of nuclear factor kappa B and release of IL33. Furthermore, exposure of macrophages to recombinant IL33 also promotes subsequent release of IL33 in a Glrx-dependent manner [51] demonstrating that GLRX controls responsiveness to IL33. A role for EGFR and IL33 signaling in airway fibrosis also has been reported. Notably, it was shown that IL33 enhanced production of the EGFR ligand, amphiregulin by memory T helper 2 (Th2) cells which in turn reprogrammed eosinophils to produce osteopontin, a pro-fibrotic immunomodulatory protein. In cooperation with IL-5-producing memory Th2 cells, these pathways were found to cooperate to establish lung fibrosis [52]. It remains unclear whether GLRX or S-glutathionylation control these pathways or the expression of osteopontin. Additional studies will be required to elucidate whether GLRX in basal cells controls the activation and/or release of IL33. Such studies are particularly germane, given that basal cells are reservoirs of IL33 sequestered in the nucleus [53,54].
Previous studies have shown that TGFB controls the proliferation and differentiation of basal cells. Notably, SMAD signaling was shown to promote mucociliary differentiation, while conversely, dual inhibition of SMAD and bone morphogenic protein (BMP) signaling promoted proliferation of basal cells [55]. Findings that airway basal cells that lack Glrx have increased SMAD2 activation suggest that their progenitor potential and/or differentiation function is compromised. The importance of basal cells in fibrosis also is emerging. Single cell RNA sequencing of epithelial cells isolated from distal lung from patients with idiopathic pulmonary fibrosis (IPF) showed the emergence of cell population(s) with basal cell characteristics that are of an “indeterminate” phenotype in contrast to the expected alveolar type 2 epithelial signature observed in the healthy lung [56]. Analysis of the transcriptome of BAL samples of three independent cohorts of patients with IPF revealed that genes associated with mortality were significantly enriched for genes expressed in airway basal cells [57]. A recent study showed that expression of the keratin 8 (KRT8) was increased via activation of the TGFB pathway, and prevented the differentiation of alveolar type 2 epithelial cells into type 1 alveolar cells which cover the majority of the alveolar surface in the healthy lung [56]. These studies, corroborated by histopathological findings in fibrotic lungs [58] collectively support a link between altered TGFB signaling, basal cell homeostasis and parenchymal fibrosis.
Single cell RNA sequencing analyses have also revealed that basal cell homeostasis is affected in asthma. Notably, a population of activated basal cells emerged in epithelial cell populations isolated from patients with asthma, which expressed the periostin (POSTN) gene [20] implicated in lung fibrosis and airway remodeling [8]. Small airway fibrosis is also a feature of chronic obstructive pulmonary disease (COPD) [41], and similar to asthma, the airway epithelium of cigarette smokers is markedly altered, displaying basal cell hyperplasia, mucus and squamous metaplasia, altered ciliated cell differentiation and decreased barrier function [59]. Exhaustion of basal cell progenitor function has been implicated in COPD pathogenesis [60]. Interestingly, metabolome analysis in primary airway basal cells cultured from smokers compared to healthy nonsmokers revealed decreases in nicotinamide adenine dinucleotide, nicotinamide and glutathione and increases in flavin adenine dinucleotide, coenzyme A and 3-nitrotyrosine among other changes [61]. These observations implicate potential changes in redox homeostasis in altered basal cell function in patients with cigarette smoke induced COPD. A previous study demonstrated that dynamic changes in redox homeostasis are important in basal cell homeostasis. Notably the authors demonstrated that the ability of basal cells to form tracheospheres was linked to their oxidation state, based upon cell sorting using redox-sensitive probes. They demonstrated that lowering “ROS levels” with N-acetyl cysteine or glutathione, or inhibition of NOX/DUOX or mitochondrial oxidants lowered tracheosphere formation, while conversely low concentrations of hydrogen peroxide increased tracheosphere formation. The same study demonstrated that the activation of nuclear factor erythroid-2 related factor-2 (Nrf2) controlled basal cell proliferation and tracheosphere formation [62].
Our current findings suggest that the GLRX redox system controls basal cell homeostasis. The potential implications of these findings will be illuminated in future studies involving conditional ablation of the Glrx gene in basal cells in vivo, modeling of basal cell expansion and differentiation using tracheosphere cultures and discovery of the S-glutathionylated proteins that regulate the function of this important progenitor cell population.
Declaration of competing interest
Yvonne Janssen-Heininger and Vikas Anathy hold patents: United States Patent No. 8,679,811, “Treatments Involving Glutaredoxins and Similar Agents” (YJ-H, VA), United States Patent No. 8,877,447, “Detection of Glutathionylated Proteins” (YJ-H), United States Patent, 9,907,828, “Treatments of oxidative stress conditions” (YJ-H, VA).
Yvonne Janssen-Heininger and Vikas Anathy have received consulting fees from Celdara Medical LLC for their contributions to the proposed commercialization of glutaredoxin for the treatment of pulmonary fibrosis.
Acknowledgements
This work was supported by grants NIH R01 HL060014, R35HL135828 (Y-JH), NIH R01HL137268, R01HL085646, R01HL138708, R21AG055325 (AvdV) and NIH R01HL122383, R01HL141364 and RO1HL136917 (VA). We acknowledge the technical expertise of Nirav Daphtary, Minara Aliyeva and Nicole Bouffard.
References
- 1.Anathy V., Lahue K.G., Chapman D.G., Chia S.B., Casey D.T., Aboushousha R., van der Velden J.L.J., Elko E., Hoffman S.M., McMillan D.H., Jones J.T., Nolin J.D., Abdalla S., Schneider R., Seward D.J., Roberson E.C., Liptak M.D., Cousins M.E., Butnor K.J., Taatjes D.J., Budd R.C., Irvin C.G., Ho Y.S., Hakem R., Brown K.K., Matsui R., Bachschmid M.M., Gomez J.L., Kaminski N., van der Vliet A., Janssen-Heininger Y.M.W. Reducing protein oxidation reverses lung fibrosis. Nat. Med. 2018;24(8):1128–1135. doi: 10.1038/s41591-018-0090-y. Epub 2018/07/11, PubMed PMID: 29988126; PMCID: PMC6204256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahy J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. PubMed PMID: 25534623; PMCID: PMC4390063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambrecht B.N., Hammad H., Fahy J.V. The cytokines of asthma. Immunity. 2019;50(4):975–991. doi: 10.1016/j.immuni.2019.03.018. Epub 2019/04/18, PubMed PMID: 30995510. [DOI] [PubMed] [Google Scholar]
- 4.Chakir J., Shannon J., Molet S., Fukakusa M., Elias J., Laviolette M., Boulet L.P., Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 2003;111(6):1293–1298. doi: 10.1067/mai.2003.1557. Epub 2003/06/06, PubMed PMID: 12789232. [DOI] [PubMed] [Google Scholar]
- 5.Kohan M., Muro A.F., Bader R., Berkman N. The extra domain A of fibronectin is essential for allergen-induced airway fibrosis and hyperresponsiveness in mice. J. Allergy Clin. Immunol. 2011;127(2):439–446. doi: 10.1016/j.jaci.2010.10.021. e1-5. Epub 2010/12/21, PubMed PMID: 21167578. [DOI] [PubMed] [Google Scholar]
- 6.Liu G., Cooley M.A., Jarnicki A.G., Hsu A.C., Nair P.M., Haw T.J., Fricker M., Gellatly S.L., Kim R.Y., Inman M.D., Tjin G., Wark P.A., Walker M.M., Horvat J.C., Oliver B.G., Argraves W.S., Knight D.A., Burgess J.K., Hansbro P.M. Fibulin-1 regulates the pathogenesis of tissue remodeling in respiratory diseases. JCI Insight. 2016;1(9) doi: 10.1172/jci.insight.86380. Epub 2016/07/12, PubMed PMID: 27398409; PMCID: PMC4936823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G., Cooley M.A., Nair P.M., Donovan C., Hsu A.C., Jarnicki A.G., Haw T.J., Hansbro N.G., Ge Q., Brown A.C., Tay H., Foster P.S., Wark P.A., Horvat J.C., Bourke J.E., Grainge C.L., Argraves W.S., Oliver B.G., Knight D.A., Burgess J.K., Hansbro P.M. Airway remodelling and inflammation in asthma are dependent on the extracellular matrix protein fibulin-1c. J. Pathol. 2017;243(4):510–523. doi: 10.1002/path.4979. Epub 2017/09/02, PubMed PMID: 28862768. [DOI] [PubMed] [Google Scholar]
- 8.O'Dwyer D.N., Moore B.B. The role of periostin in lung fibrosis and airway remodeling. Cell. Mol. Life Sci. : CMLS. 2017;74(23):4305–4314. doi: 10.1007/s00018-017-2649-z. Epub 2017/09/18, PubMed PMID: 28918442; PMCID: PMC5659879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takayama G., Arima K., Kanaji T., Toda S., Tanaka H., Shoji S., McKenzie A.N., Nagai H., Hotokebuchi T., Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 2006;118(1):98–104. doi: 10.1016/j.jaci.2006.02.046. Epub 2006/07/04, PubMed PMID: 16815144. [DOI] [PubMed] [Google Scholar]
- 10.Hur G.Y., Broide D.H. Genes and pathways regulating decline in lung function and airway remodeling in asthma. Allergy, Asthma Immunol. Res. 2019;11(5):604–621. doi: 10.4168/aair.2019.11.5.604. Epub 2019/07/25, PubMed PMID: 31332973; PMCID: PMC6658410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou B., Comeau M.R., De Smedt T., Liggitt H.D., Dahl M.E., Lewis D.B., Gyarmati D., Aye T., Campbell D.J., Ziegler S.F. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6(10):1047–1053. doi: 10.1038/ni1247. Epub 2005/09/06, PubMed PMID: 16142237. [DOI] [PubMed] [Google Scholar]
- 12.Tomkowicz A., Kraus-Filarska M., Bar J., Rabczynski J., Jelen M., Piesiak P., Fal A., Panaszek B. Bronchial hyper-responsiveness, subepithelial fibrosis, and transforming growth factor-beta(1) expression in patients with long-standing and recently diagnosed asthma. Arch. Immunol. Ther. Exp. 2008;56(6):401–408. doi: 10.1007/s00005-008-0044-z. Epub 2008/12/02, PubMed PMID: 19043669; PMCID: PMC2805797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar R.K., Herbert C., Foster P.S. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin. Exp. Allergy : J. Br. Soc. Allergy Clin. Immunol. 2004;34(4):567–575. doi: 10.1111/j.1365-2222.2004.1917.x. Epub 2004/04/15, PubMed PMID: 15080809. [DOI] [PubMed] [Google Scholar]
- 14.Vannella K.M., Ramalingam T.R., Borthwick L.A., Barron L., Hart K.M., Thompson R.W., Kindrachuk K.N., Cheever A.W., White S., Budelsky A.L., Comeau M.R., Smith D.E., Wynn T.A. Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci. Transl. Med. 2016;8(337) doi: 10.1126/scitranslmed.aaf1938. 337ra65. Epub 2016/05/06, PubMed PMID: 27147589. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.U., Chang H.S., Lee H.J., Jung C.A., Bae D.J., Song H.J., Park J.S., Uh S.T., Kim Y.H., Seo K.H., Park C.S. Upregulation of interleukin-33 and thymic stromal lymphopoietin levels in the lungs of idiopathic pulmonary fibrosis. BMC Pulm. Med. 2017;17(1):39. doi: 10.1186/s12890-017-0380-z. Epub 2017/02/17, PubMed PMID: 28202030; PMCID: PMC5312598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D., Guabiraba R., Besnard A.G., Komai-Koma M., Jabir M.S., Zhang L., Graham G.J., Kurowska-Stolarska M., Liew F.Y., McSharry C., Xu D. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 2014;134(6):1422–1432. doi: 10.1016/j.jaci.2014.05.011. e11. Epub 2014/07/06, PubMed PMID: 24985397; PMCID: PMC4258609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Z., Liu Q., Jiang R., Lv L., Shoto S.S., Maillet I., Quesniaux V., Tang J., Zhang W., Sun B., Ryffel B. Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell. Mol. Immunol. 2018;15(4):388–398. doi: 10.1038/cmi.2016.63. Epub 2017/02/15, PubMed PMID: 28194023; PMCID: PMC6052839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Boer W.I., Sharma H.S., Baelemans S.M., Hoogsteden H.C., Lambrecht B.N., Braunstahl G.J. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can. J. Physiol. Pharmacol. 2008;86(3):105–112. doi: 10.1139/y08-004. PubMed PMID: 18418437. [DOI] [PubMed] [Google Scholar]
- 19.Xiao C., Puddicombe S.M., Field S., Haywood J., Broughton-Head V., Puxeddu I., Haitchi H.M., Vernon-Wilson E., Sammut D., Bedke N., Cremin C., Sones J., Djukanovic R., Howarth P.H., Collins J.E., Holgate S.T., Monk P., Davies D.E. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011;128(3):549–556. doi: 10.1016/j.jaci.2011.05.038. e1-12. Epub 2011/07/15, PubMed PMID: 21752437. [DOI] [PubMed] [Google Scholar]
- 20.Vieira Braga F.A., Kar G., Berg M., Carpaij O.A., Polanski K., Simon L.M., Brouwer S., Gomes T., Hesse L., Jiang J., Fasouli E.S., Efremova M., Vento-Tormo R., Talavera-Lopez C., Jonker M.R., Affleck K., Palit S., Strzelecka P.M., Firth H.V., Mahbubani K.T., Cvejic A., Meyer K.B., Saeb-Parsy K., Luinge M., Brandsma C.A., Timens W., Angelidis I., Strunz M., Koppelman G.H., van Oosterhout A.J., Schiller H.B., Theis F.J., van den Berge M., Nawijn M.C., Teichmann S.A. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 2019;25(7):1153–1163. doi: 10.1038/s41591-019-0468-5. Epub 2019/06/19, PubMed PMID: 31209336. [DOI] [PubMed] [Google Scholar]
- 21.Antczak A., Nowak D., Shariati B., Krol M., Piasecka G., Kurmanowska Z. Increased hydrogen peroxide and thiobarbituric acid-reactive products in expired breath condensate of asthmatic patients. Eur. Respir. J. 1997;10(6):1235–1241. doi: 10.1183/09031936.97.10061235. PubMed PMID: 9192922. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick A.M., Teague W.G., Holguin F., Yeh M., Brown L.A. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J. Allergy Clin. Immunol. 2009;123(1):146–152. doi: 10.1016/j.jaci.2008.10.047. e8. Epub 2009/01/10, PubMed PMID: 19130935; PMCID: PMC2649685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comhair S.A., Ricci K.S., Arroliga M., Lara A.R., Dweik R.A., Song W., Hazen S.L., Bleecker E.R., Busse W.W., Chung K.F., Gaston B., Hastie A., Hew M., Jarjour N., Moore W., Peters S., Teague W.G., Wenzel S.E., Erzurum S.C. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am. J. Respir. Crit. Care Med. 2005;172(3):306–313. doi: 10.1164/rccm.200502-180OC. Epub 2005/05/11, PubMed PMID: 15883124; PMCID: PMC2718470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hristova M., Habibovic A., Veith C., Janssen-Heininger Y.M., Dixon A.E., Geiszt M., van der Vliet A. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J. Allergy Clin. Immunol. 2016;137(5):1545–1556. doi: 10.1016/j.jaci.2015.10.003. e11. Epub 2015/11/26, PubMed PMID: 26597162; PMCID: PMC4860024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habibovic A., Hristova M., Heppner D.E., Danyal K., Ather J.L., Janssen-Heininger Y.M., Irvin C.G., Poynter M.E., Lundblad L.K., Dixon A.E., Geiszt M., van der Vliet A. DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma. JCI Insight. 2016;1(18) doi: 10.1172/jci.insight.88811. Epub 2016/11/05, PubMed PMID: 27812543; PMCID: PMC5085603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anathy V., Roberson E.C., Guala A.S., Godburn K.E., Budd R.C., Janssen-Heininger Y.M. Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death. Antioxidants Redox Signal. 2012;16(6):496–505. doi: 10.1089/ars.2011.4281. PubMed PMID: 21929356; PMCID: PMC3304251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui R., Ferran B., Oh A., Croteau D., Shao D., Han J., Pimentel D.R., Bachschmid M.M. Redox regulation via glutaredoxin-1 and protein S-glutathionylation. Antioxidants Redox Signal. 2020;32(10):677–700. doi: 10.1089/ars.2019.7963. Epub 2019/12/10, PubMed PMID: 31813265; PMCID: PMC7047114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mieyal J.J., Gallogly M.M., Qanungo S., Sabens E.A., Shelton M.D. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxidants Redox Signal. 2008;10(11):1941–1988. doi: 10.1089/ars.2008.2089. Epub 2008/09/09, PubMed PMID: 18774901; PMCID: PMC2774718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chia S.B., Elko E.A., Aboushousha R., Manuel A.M., van de Wetering C., Druso J.E., van der Velden J., Seward D.J., Anathy V., Irvin C.G., Lam Y.W., van der Vliet A., Janssen-Heininger Y.M.W. Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases. Am. J. Physiol. Cell Physiol. 2020;318(2) doi: 10.1152/ajpcell.00410.2019. C304-c27. Epub 2019/11/07, PubMed PMID: 31693398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J., Grek C., Ye Z.W., Manevich Y., Tew K.D., Townsend D.M. Pleiotropic functions of glutathione S-transferase P. Adv. Canc. Res. 2014;122:143–175. doi: 10.1016/B978-0-12-420117-0.00004-9. Epub 2014/06/30, PubMed PMID: 24974181; PMCID: PMC5079281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuipers I., Louis R., Manise M., Dentener M.A., Irvin C.G., Janssen-Heininger Y.M., Brightling C.E., Wouters E.F., Reynaert N.L. Increased glutaredoxin-1 and decreased protein S-glutathionylation in sputum of asthmatics. Eur. Respir. J. 2013;41(2):469–472. doi: 10.1183/09031936.00115212. PubMed PMID: 23370801; PMCID: PMC3855363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman S.M., Tully J.E., Lahue K.G., Anathy V., Nolin J.D., Guala A.S., van der Velden J.L., Ho Y.S., Aliyeva M., Daphtary N., Lundblad L.K., Irvin C.G., Janssen-Heininger Y.M. Genetic ablation of glutaredoxin-1 causes enhanced resolution of airways hyperresponsiveness and mucus metaplasia in mice with allergic airways disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;303(6):L528–L538. doi: 10.1152/ajplung.00167.2012. PubMed PMID: 22752969; PMCID: PMC3468477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman S.M., Qian X., Nolin J.D., Chapman D.G., Chia S.B., Lahue K.G., Schneider R., Ather J.L., Randall M.J., McMillan D.H., Jones J.T., Taatjes D.J., Aliyeva M., Daphtary N., Abdalla S., Lundblad L.K., Ho Y.S., Anathy V., Irvin C.G., Wouters E.F., Reynaert N.L., Dixon A.E., van der Vliet A., Poynter M.E., Janssen-Heininger Y.M. Ablation of glutaredoxin-1 modulates house dust mite-induced allergic airways disease in mice. Am. J. Respir. Cell Mol. Biol. 2016;55(3):377–386. doi: 10.1165/rcmb.2015-0401OC. PubMed PMID: 27035878; PMCID: PMC5023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho Y.S., Xiong Y., Ho D.S., Gao J., Chua B.H., Pai H., Mieyal J.J. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic. Biol. Med. 2007;43(9):1299–1312. doi: 10.1016/j.freeradbiomed.2007.07.025. PubMed PMID: 17893043; PMCID: PMC2196211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riesenfeld E., Allen G.B., Bates J.H., Poynter M.E., Wu M., Aimiand S., Lundblad L.K. The temporal evolution of airways hyperresponsiveness and inflammation. J. Allergy Ther. 2012;1(5):1–7. doi: 10.4172/2155-6121.S1-005. Epub 2012/01/25, PubMed PMID: 23565340; PMCID: PMC3615437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pantano C., Ather J.L., Alcorn J.F., Poynter M.E., Brown A.L., Guala A.S., Beuschel S.L., Allen G.B., Whittaker L.A., Bevelander M., Irvin C.G., Janssen-Heininger Y.M. Nuclear factor-kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2008;177(9):959–969. doi: 10.1164/rccm.200707-1096OC. PubMed PMID: 18263801; PMCID: PMC2361423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y., Randell S.H., Hogan B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. U. S. A. 2009;106(31):12771–12775. doi: 10.1073/pnas.0906850106. Epub 2009/07/25, PubMed PMID: 19625615; PMCID: PMC2714281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woessner J.F., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. PubMed PMID: 13786180. [DOI] [PubMed] [Google Scholar]
- 39.Wu R., Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro. 1982;18(9):800–812. doi: 10.1007/bf02796504. Epub 1982/09/01, PubMed PMID: 6757109. [DOI] [PubMed] [Google Scholar]
- 40.Alcorn J.F., Guala A.S., van der Velden J., McElhinney B., Irvin C.G., Davis R.J., Janssen-Heininger Y.M., Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J. Cell Sci. 2008;121(Pt 7):1036–1045. doi: 10.1242/jcs.019455. Epub 2008/03/13, PubMed PMID: 18334556; PMCID: PMC2876720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnes P.J. Small airway fibrosis in COPD. Int. J. Biochem. Cell Biol. 2019;116:105598. doi: 10.1016/j.biocel.2019.105598. Epub 2019/09/10, PubMed PMID: 31499176. [DOI] [PubMed] [Google Scholar]
- 42.Montoro D.T., Haber A.L., Biton M., Vinarsky V., Lin B., Birket S.E., Yuan F., Chen S., Leung H.M., Villoria J., Rogel N., Burgin G., Tsankov A.M., Waghray A., Slyper M., Waldman J., Nguyen L., Dionne D., Rozenblatt-Rosen O., Tata P.R., Mou H., Shivaraju M., Bihler H., Mense M., Tearney G.J., Rowe S.M., Engelhardt J.F., Regev A., Rajagopal J. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560(7718):319–324. doi: 10.1038/s41586-018-0393-7. Epub 2018/08/03, PubMed PMID: 30069044; PMCID: PMC6295155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barkauskas C.E., Chung M.I., Fioret B., Gao X., Katsura H., Hogan B.L. Lung organoids: current uses and future promise. Development (Camb., Engd.) 2017;144(6):986–997. doi: 10.1242/dev.140103. Epub 2017/03/16, PubMed PMID: 28292845; PMCID: PMC5358104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ordovas-Montanes J., Dwyer D.F., Nyquist S.K., Buchheit K.M., Vukovic M., Deb C., Wadsworth M.H., 2nd, Hughes T.K., Kazer S.W., Yoshimoto E., Cahill K.N., Bhattacharyya N., Katz H.R., Berger B., Laidlaw T.M., Boyce J.A., Barrett N.A., Shalek A.K. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649–654. doi: 10.1038/s41586-018-0449-8. Epub 2018/08/24, PubMed PMID: 30135581; PMCID: PMC6133715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Velden J.L., Wagner D.E., Lahue K.G., Abdalla S.T., Lam Y.W., Weiss D.J., Janssen-Heininger Y.M.W. TGF-beta1-induced deposition of provisional extracellular matrix by tracheal basal cells promotes epithelial-to-mesenchymal transition in a c-Jun NH2-terminal kinase-1-dependent manner. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314(6):L984–L997. doi: 10.1152/ajplung.00053.2017. Epub 2018/02/23, PubMed PMID: 29469614; PMCID: PMC6032072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erzurum S.C. New insights in oxidant biology in asthma. Ann. Am. Thoracic Soc. 2016;13(Suppl 1):S35–S39. doi: 10.1513/AnnalsATS.201506-385MG. Epub 2016/03/31, PubMed PMID: 27027950; PMCID: PMC5015728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosse Y., Riesenfeld E.P., Pare P.D., Irvin C.G. It's not all smooth muscle: non-smooth-muscle elements in control of resistance to airflow. Annu. Rev. Physiol. 2010;72:437–462. doi: 10.1146/annurev-physiol-021909-135851. Epub 2010/02/13, PubMed PMID: 20148684. [DOI] [PubMed] [Google Scholar]
- 48.Hogan B.L., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C., Niklason L., Calle E., Le A., Randell S.H., Rock J., Snitow M., Krummel M., Stripp B.R., Vu T., White E.S., Whitsett J.A., Morrisey E.E. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15(2):123–138. doi: 10.1016/j.stem.2014.07.012. Epub 2014/08/12, PubMed PMID: 25105578; PMCID: PMC4212493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan S., Hollinger M., Lachowicz-Scroggins M.E., Kerr S.C., Dunican E.M., Daniel B.M., Ghosh S., Erzurum S.C., Willard B., Hazen S.L., Huang X., Carrington S.D., Oscarson S., Fahy J.V. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci. Transl. Med. 2015;7(276) doi: 10.1126/scitranslmed.3010525. 276ra27. Epub 2015/02/27, PubMed PMID: 25717100; PMCID: PMC4403633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickinson J.D., Alevy Y., Malvin N.P., Patel K.K., Gunsten S.P., Holtzman M.J., Stappenbeck T.S., Brody S.L. IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy. 2016;12(2):397–409. doi: 10.1080/15548627.2015.1056967. Epub 2015/06/11, PubMed PMID: 26062017; PMCID: PMC4835964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberg E.O., Ferran B., Tsukahara Y., Hatch M.M.S., Han J., Murdoch C.E., Matsui R. IL-33 induction and signaling are controlled by glutaredoxin-1 in mouse macrophages. PloS One. 2019;14(1) doi: 10.1371/journal.pone.0210827. Epub 2019/01/27, PubMed PMID: 30682073; PMCID: PMC6347181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morimoto Y., Hirahara K., Kiuchi M., Wada T., Ichikawa T., Kanno T., Okano M., Kokubo K., Onodera A., Sakurai D., Okamoto Y., Nakayama T. Amphiregulin-producing pathogenic memory T helper 2 cells instruct eosinophils to secrete osteopontin and facilitate airway fibrosis. Immunity. 2018;49(1):134–150. doi: 10.1016/j.immuni.2018.04.023. e6. Epub 2018/07/01, PubMed PMID: 29958800. [DOI] [PubMed] [Google Scholar]
- 53.Byers D.E., Alexander-Brett J., Patel A.C., Agapov E., Dang-Vu G., Jin X., Wu K., You Y., Alevy Y., Girard J.P., Stappenbeck T.S., Patterson G.A., Pierce R.A., Brody S.L., Holtzman M.J. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Invest. 2013;123(9):3967–3982. doi: 10.1172/jci65570. Epub 2013/08/16, PubMed PMID: 23945235; PMCID: PMC3754239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pichery M., Mirey E., Mercier P., Lefrancais E., Dujardin A., Ortega N., Girard J.P. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 2012;188(7):3488–3495. doi: 10.4049/jimmunol.1101977. Epub 2012/03/01, PubMed PMID: 22371395. [DOI] [PubMed] [Google Scholar]
- 55.Mou H., Vinarsky V., Tata P.R., Brazauskas K., Choi S.H., Crooke A.K., Zhang B., Solomon G.M., Turner B., Bihler H., Harrington J., Lapey A., Channick C., Keyes C., Freund A., Artandi S., Mense M., Rowe S., Engelhardt J.F., Hsu Y.C., Rajagopal J. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell. 2016;19(2):217–231. doi: 10.1016/j.stem.2016.05.012. Epub 2016/06/21, PubMed PMID: 27320041; PMCID: PMC4975684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang P., Gil de Rubio R., Hrycaj S.M., Gurczynski S.J., Riemondy K.A., Moore B.B., Omary M.B., Ridge K.M., Zemans R.L. Ineffectual AEC2-to-AEC1 differentiation in IPF: persistence of KRT8(hi) transitional state. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.201909-1726LE. Epub 2020/02/20, PubMed PMID: 32073903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prasse A., Binder H., Schupp J.C., Kayser G., Bargagli E., Jaeger B., Hess M., Rittinghausen S., Vuga L., Lynn H., Violette S., Jung B., Quast K., Vanaudenaerde B., Xu Y., Hohlfeld J.M., Krug N., Herazo-Maya J.D., Rottoli P., Wuyts W.A., Kaminski N. BAL cell gene expression is indicative of outcome and airway basal cell involvement in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019;199(5):622–630. doi: 10.1164/rccm.201712-2551OC. Epub 2018/08/25, PubMed PMID: 30141961; PMCID: PMC6396865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jonsdottir H.R., Arason A.J., Palsson R., Franzdottir S.R., Gudbjartsson T., Isaksson H.J., Gudmundsson G., Gudjonsson T., Magnusson M.K. Basal cells of the human airways acquire mesenchymal traits in idiopathic pulmonary fibrosis and in culture. Labor. Invest.; A J. Techn. Meth. Pathol. 2015;95(12):1418–1428. doi: 10.1038/labinvest.2015.114. Epub 2015/09/22, PubMed PMID: 26390052. [DOI] [PubMed] [Google Scholar]
- 59.Zuo W.L., Yang J., Gomi K., Chao I., Crystal R.G., Shaykhiev R. EGF-amphiregulin interplay in airway stem/progenitor cells links the pathogenesis of smoking-induced lesions in the human airway epithelium. Stem Cells (Dayton, Ohio) 2017;35(3):824–837. doi: 10.1002/stem.2512. Epub 2016/10/07, PubMed PMID: 27709733; PMCID: PMC5330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh M., Miller Y.E., Nakachi I., Kwon J.B., Baron A.E., Brantley A.E., Merrick D.T., Franklin W.A., Keith R.L., Vandivier R.W. Exhaustion of airway basal progenitor cells in early and established chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018;197(7):885–896. doi: 10.1164/rccm.201704-0667OC. Epub 2017/12/07, PubMed PMID: 29211494; PMCID: PMC6020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deeb R.S., Walters M.S., Strulovici-Barel Y., Chen Q., Gross S.S., Crystal R.G. Smoking-associated disordering of the airway basal stem/progenitor cell metabotype. Am. J. Respir. Cell Mol. Biol. 2016;54(2):231–240. doi: 10.1165/rcmb.2015-0055OC. Epub 2015/07/15, PubMed PMID: 26161876; PMCID: PMC4821042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paul M.K., Bisht B., Darmawan D.O., Chiou R., Ha V.L., Wallace W.D., Chon A.T., Hegab A.E., Grogan T., Elashoff D.A., Alva-Ornelas J.A., Gomperts B.N. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell. 2014;15(2):199–214. doi: 10.1016/j.stem.2014.05.009. Epub 2014/06/24, PubMed PMID: 24953182; PMCID: PMC4127166. [DOI] [PMC free article] [PubMed] [Google Scholar]