Abstract
Purpose
Müller glia are important in retinal health and disease and are a major source of retinal VEGF-A. Of the different VEGF family members, the role of VEGF-A in retinal health and disease has been studied extensively. The potential contribution of other VEGF family members to retinal pathophysiology, however, remains poorly defined. This study aimed to understand the role of VEGF-B in Müller cell pathophysiology.
Methods
The expression of different VEGFs and their receptors in human MIO-M1 and mouse QMMuC-1 Müller cell lines and primary murine Müller cells was examined by RT-PCR, ELISA, and Western blot. The effect of recombinant VEGF-B or VEGF-B neutralization on Müller cell viability and survival under normal, hypoxic, and oxidative (4-hydroxynonenal [4-HNE]) conditions was evaluated by Alamar Blue, Yo-Pro uptake, and immunocytochemistry. The expression of glial fibrillary acidic protein, aquaporin-4, inward rectifying K+ channel subtype 4.1, glutamine synthetase, and transient receptor potential vanilloid 4 under different treatment conditions was examined by RT-PCR, immunocytochemistry, and Western blot. Transient receptor potential vanilloid 4 channel activity was assessed using a Fura-2–based calcium assay.
Results
VEGF-B was expressed in Müller cells at the highest levels compared with other members of the VEGF family. VEGF-B neutralization did not affect Müller cell viability or functionality under normal conditions, but enhanced hypoxia– or 4-HNE–induced Müller cell death and decreased inward rectifying K+ channel subtype 4.1 and aquaporin-4 expression. Recombinant VEGF-B restored Müller cell glutamine synthetase expression under hypoxic conditions and protected Müller cells from 4-HNE–induced damage by normalizing transient receptor potential vanilloid 4 channel expression and activity.
Conclusions
Autocrine production of VEGF-B protects Müller cells under pathologic conditions.
Keywords: Mueller cell, VEGF-B, hypoxia, retina, oxidative stress
Müller cells are the principal macroglia in the retina.1 Their unique position, extending from the ganglion cell layer to the photoreceptor inner segments, facilitates their physical contact with many cell types in the retina. Müller cells have a pivotal role in the maintenance of retinal homeostasis, architecture, and responses to stress conditions.1–3 Müller cell functions include blood–retinal barrier regulation, release of trophic factors, neurotransmitter recycling (e.g., glutamate, γ-aminobutyric acid), control of water and ion (mainly K+) homeostasis and functional hyperemia,4 as well as regulation of retinal innate immunity and the visual cycle. In pathologic conditions such as diabetic retinopathy, Müller cells contribute to neurovascular dysfunction by producing inflammatory and angiogenic factors.1,2 In particular, Müller cells are a major source of VEGF-A, in the retina under pathophysiologic conditions.5,6
The VEGF family consists of five members (VEGF-A, B, C, D and placental growth factor (PlGF)), which are all structurally related.7,8 VEGF ligands bind three different tyrosine kinase receptors (VEGFR1, VEGFR2, and VEGFR3), and two neuropilin coreceptors (NRP1 and NRP2) that enhance the affinity of the ligands to VEGFR receptors, thereby enhancing their physiologic actions.9 The VEGF family members are distinguished by their receptor-binding pattern and unique functions; for example, human VEGF-C and -D bind to VEGFR2 and VEGFR3, although their function lymphangiogenesis role seems to be mediated predominately by VEGFR3.10–12 Mouse VEGF-D only binds to VEGFR3 but not VEGFR2.12 Similarly, placental growth factor has been described to participate only in pathologic angiogenesis by binding to VEGFR1, whereas VEGF-A, the best understood member of this family, binds mainly to VEGFR2 to elicit angiogenic activity. VEGF-B, the most elusive member of the VEGF family, binds exclusively to VEGFR1, although its functions remain to be fully elucidated.13–15
Recently, the neuroprotective role of different VEGF family members has received increasing attention.16–19 The use of VEGF-A as a neuroprotective therapy, however, has been hindered by its proangiogenic effects.17,20 There is some preclinical evidence that VEGF-B is protective in pathologies in which oxidative stress is a key pathologic factor, including amylotrophic lateral sclerosis, Parkinson's disease,18 retinitis pigmentosa15 and Alzheimer's disease.21 An association between VEGF-B and advanced stages of diabetic retinopathy has also been described, although the cellular source of retinal VEGF-B and exactly how VEGF-B contributes to retinal disease remain elusive.15,22
The current study investigated the role of VEGF family members in Müller glia pathophysiology. We found that, among the different VEGF family members, Müller cells produce more VEGF-B than other VEGF members, and that VEGF-B protects Müller cells under oxidative and hypoxic conditions.
Methods
Cell Culture and Treatments
QMMuC-1, an immortalized murine Müller cell line23 and the human Müller cell line Moorfields Institute of Ophthalmology-Müller 1 (MIO-M1) (courtesy of Dr. G.A. Limb, UCL Institute of Ophthalmology, London, UK)24 were cultured in Dulbecco's Modified Eagle Media (Thermo Fisher Scientific, Waltham, MA; Catalog# 41965039) supplemented with 10% fetal calf serum. Primary Müller cells (PMC) were isolated from C57BL/6J neonatal mice (P7) and cultured using a protocol described previously.23 Passage number 3-5 PMCs were used in the study.
To induce different stress conditions and investigate the effect of VEGF-A or VEGF-B and their receptors, Müller cells were treated with the compounds listed in Table 1.
Table 1.
Concentration of Compounds Used for Functionality Assays and to Induce Different Stress Conditions
| Compound | Reference | Concentration/Length | Source | Catalog No. |
|---|---|---|---|---|
| VEGF-A murine recombinant | 34 , 38 | 100 ng/mL | Peprotech | 450-32 |
| VEGF-B murine recombinant | 33 , 34 , 37 | 100 ng/mL | Cloud-Clone Corp. | rpa144muo1 |
| VEGF-A antibody | 32 | 500 ng/mL | R&D. Polyclonal Goat IgG | af493 |
| VEGF-B antibody | 34 , 50 | 500 ng/mL | R&D. Polyclonal Goat IgG | af590 |
| VEGFR1 antibody | 33 , 35 | 10 µg/mL | R&D. Polyclonal Goat IgG | af471 |
| VEGFR2 antibody | 33 , 35 | 10 µg/mL | R&D. Polyclonal Goat IgG | af644 |
| NRP1 antibody | 54 | 30 µg/mL | R&D. Polyclonal Goat IgG | af566 |
| Recombinant mouse IL-1β | 29 , 30 | 20 ng/mL- 24 hours | R&D | 401-ML |
| D-(+)-Glucose/ D-Mannitol | 25 , 26 | 25mM- 72 hours* | Sigma Aldrich | |
| Hypoxia | 27 , 28 | 72 hours | ||
| 4-HNE | 10 µM† or 20 µM‡-24 hours | BioVision | 2083-1 | |
| HC-067047 | 43 , 58 , 60 | 10 µM | Tocris Bioscience | 4100 |
| GSK1016790A | 100 nM | Sigma Aldrich | G0798 |
No changes in cell viability at this length (data not shown).
Sublethal dose of 4-HNE chosen for study the effect of oxidative stress.
Concentration of 4-HNE used in survival studies that induces approximately 15%–25% decrease in cell survival.
Quantitative RT-PCR
Total RNA from murine Müller cells and human MIO-M1 cells was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany) and TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), respectively, according to the manufacturer's instructions. cDNA was synthesized using SuperScript II Reverse Transcriptase Kit (Invitrogen).
Validated TaqMan probes were purchased from Roche (Basel, Switzerland); SYBR Green gene specific primers were designed using NCBI Primer Blast and synthesized by Integrated DNA Technologies (Coralville, IA) (Table 2). Real-time RT-PCR was performed using LightCycler 480 (Roche-Diagnostic) in triplicate. Relative gene expression was calculated using the comparative Ct method (2−ΔΔCT) with data normalized to β-actin or 18s.
Table 2.
Primer Sequences: Genes Whose Expression Was Examined in Müller Cells in Normal or Stress Conditions
| Genes | Forward | Reverse |
|---|---|---|
| Human primers | ||
| hVEGFR-1-s | GGCTGTTTTCTCTCGGATCTC | CATCTCCTCCGAGCCTGAAAG |
| hVEGFR-1-m | TTTGGATGAGCAGTGTGAGC | AGCCCCTCTTCCAAGTGATT |
| hVEGFR-2 | ACCCGAGACATGGAATCACC | AAACTGACTTGGCCTCGGTC |
| hNRP-1 | ACCCAAGTGAAAAATGCGAATG | CCTCCAAATCGAAGTGAGGGTT |
| hVEGF-A | AGGGCAGAATCATCACGAAGT | AGGGTCTCGATTGGATGGCA |
| hVEGF-B | TATATCCCAGTGGGGGAACA | GACAAGGGATGGCAGAAGAG |
| hVEGF-C | GGCTGGCAACATAACAGAGAA | CCCCACATCTATACACACCTCC |
| hVEGF-D | TCCCATCGGTCCACTAGGTTT | AGGGCTGCACTGAGTTCTTTG |
| hPlGF | GGCGATGAGAATCTGCACTGT | CACCTTTCCGGCTTCATCTTC |
| 18s | CTACCACATCCAAGGAAGCA | TTTTTCGTCACTACCTCCCCG |
| Mouse primers | ||
| β-actin | CCTTCCTTCTTGGGTATG | TGTAAAACGCAGCTCAGTAA |
| VEGF-A | CCCACGTCAGAGAGCAAC | TTTCTTGCGCTTTCGTTTTT |
| VEGF-B | GGAGAAGAGTGGAGCACAGG | ATGGCAGCTCTGGGAGATAA |
| VEGF-C | GTGATGTTATAGATGTGGGG | ACGTCTTGCTGAGGTAACCTG |
| VEGF-D | CACTTTTCAGTAGCTGCCTGG | CTTCACTGGTCCATGTTCGC |
| PlGF | TCTGCTGGGAACAACTCAACA | GTGAGACACCTCATCAGGGTAT |
| VEGFR1 | TCACCTGGACTGAGACCAAG | GTACAACACCACGGAGTTGTA |
| VEGFR2 | TGAAAGCCCAGACTGTGTC | AGAAGTCACAGAGGCGGTAT |
| NRP1 | GGACAGAGACTGCAAGTATGA | AGAAAGGGCCCTGAAGACAC |
| Caspase-3 | TGACTGGAAAGCCGAAACTC | AGCCTCCACCGGTATCTTCT |
| GS | TGCCTGCCCAGTGGGAATT | TATTGGAAGGGTTCGTCGCC |
| GFAP | TGCAAGAGACAGAGGAGTGG | GCTCTAGGGACTCGTTCGTG |
| Kir4.1 | TACAGTCAGACGACTCAGACA | GAAGCAGTTTGCCTGTCACCT |
| AQP4 | GAGTCACCACGGTTCATGGA | CGTTTGGAATCACAGCTGGC |
Western Blot
Samples were homogenized in RIPA buffer containing protease inhibitor cocktail. Protein concentration was determined using a Pierce BCA protein assay kit (Thermo Fisher Scientific, Catalog# 23225) or Bradford Assay (Bio-Rad Laboratories, Hercules, CA). The blot was performed using 15 to 20 µg of protein according to previously described methods.23 Primary and secondary antibodies used are detailed in Table 3. Membranes were visualized with enhanced chemiluminescence (Clarity Western ECL Blotting Substrates; Bio-Rad Laboratories) and bands detected using Syngene G-Box imaging system (Syngene, Cambridge, UK).
Table 3.
Antibodies Used for Western Blotting and Immunofluorescence
| Antibody | Company | Catalog Number | Dilution |
|---|---|---|---|
| AQP4 | Santa Cruz | SC-20812 | 1:500 |
| β-actin | Abcam | ab8224 | 1:6000 |
| GFAP | DAKO | z0334 | 1:800 |
| Kir4.1 | Abcam | AB105102 | 1:500 |
| GS | Sigma | G2781 | 1:2500 |
| Vimentin | Biorbyt | orb167196 | 1:1000 |
| TRPV4 | ProSci | 7695 | 1:500(WB)/1:50 (IF) |
| Caspase-3 | ProSci | PSI-3445 | 1:100(IF)/1:30 (IF) |
| AlexaFluor 488 donkey anti-rabbit | Abcam | ab150073 | 1:300 |
| Alexa Fluor 594 Phalloidin | ThermoFisher | A12381 | 1:10,000 |
| Goat Anti-Rabbit IgG H&L (HRP) | Abcam | ab6721 | 1:10,000 |
| Rabbit Anti-Mouse IgG H&L (HRP) | Abcam | ab6728 | 1:10,000 |
| Rabbit Anti-Goat IgG H&L (HRP) | Abcam | ab6741 | 1:7000 |
ELISA
ELISA kits for VEGF-A and VEGFR1 (R&D, DY493 and DY471), NRP1 and VEGF-B (Abcam, Cambridge, UK; ab213880 and ab213897) were used according to the manufacturers’ instructions. VEGFR2 indirect ELISA was performed by adding 20 µg protein or supernatants to the wells and incubated overnight. After several washes and 2 hours of blocking (SuperBlock TBS, Thermo Fisher Scientific), anti-VEGFR2 (1 µg/mL, R&D af644) antibody was added and incubated for 2 hours at room temperature. After thorough washes, wells were incubated with a biotinylated horse anti-goat IgG antibody (1:8,000, Agilent DAKO, Santa Clara, CA) for 2 hours, followed by with HRP-conjudated streptavidin (1:900, DAKO, 20 minutes). The signal was developed by adding TMB (20 minutes, 37ᵒC). Absorbance values were measured at 450 nm/570 nm using a POLARstar OMEGA plate reader (BMG LabTech, Aylesbury, UK). All values were collected from at least four independent samples and each experiment repeated in duplicate.
Cell Viability and Apoptosis
The viability of Müller cells under different conditions was assessed using the AlamarBlue (Thermo Fisher) and YO-PRO uptake assay (YO-PRO-1 Iodide (491/509), Thermo Fisher) according to the manufacturer's instructions. Fluorescence signal was read in a plate reader (POLARstar Omega, BMG Labtech).
Immunofluorescence Staining
Caspase-3/7 expression was measured using the CellEvent caspase-3/7 Green Detection Reagent (Invitrogen) following the manufacturer's instructions. The number of caspase-3/7 positive cells was analyzed using ImageJ.
Immunofluorescence staining of caspase-3 and transient receptor potential vanilloid 4 (TRPV4) in PMCs and QMMuC-1 cells was carried out as previously described23 using antibodies listed in Table 3. Briefly, fixed cells were permeabilized with 0.1% Triton-X-100 and blocked (10% fetal calf serum, 1 hour). Samples were incubated at 4°C overnight with caspase-3 or TRPV4 antibody (ProSci, San Diego, CA). After thorough washes, samples were incubated with Alexa Fluor-conjugated goat anti-rabbit IgG for 1 hour at room temperature. For F-actin staining, Alexa Fluor 594 Phalloidin (Thermo Fisher Scientific) was used. Cells were mounted using Vectashield medium containing DAPI (Vector Laboratories, Burlingame, CA) and imaged using a Nikon C1 Eclipse TE200-U (Nikon, Surrey, UK). Images were processed using ImageJ software.
Intracellular Ca2+ Measurements
Intracellular Ca2+ concentration ([Ca2+]i) was measured in QMMuC-1 mouse Müller cells cultured in black 96-well plates (Corning Inc., Corning, NY, Catalog# 3603) using a Fura-2 QBT Calcium kit (Molecular Devices, San Jose, CA; cat# R8197) and a FlexStation 3 (Molecular Devices) fluorescence microplate reader according to manufacturers’ instructions. Responses to the TRPV4 agonist, GSK1016790A (GSK101, Sigma Aldrich) and/or TRPV4 antagonist, HC067047 (HC06, Tocris Bioscience, Bristol, UK) (Table 1) were recorded. Data were quantified by calculating the baseline to peak fluorescence ratio change (ΔR340/380). Four to eight technical repeats were used in each experiment with two to three biological replicates using different batches of cells.
Statistics
All graphing and statistical analysis was performed using Prism 6 (GraphPad Software, San Diego, CA). An unpaired Student's t test, one-way ANOVA, followed by Dunnett's or Newman-Keuls or two-way ANOVA with Tukey's multiple comparison post-test were utilized to determine the statistical outcome; a P value of less than 0.05 was considered statistically significant.
Results
The Expression Profile of Different VEGF Family Members and Their Receptors in Müller Cells
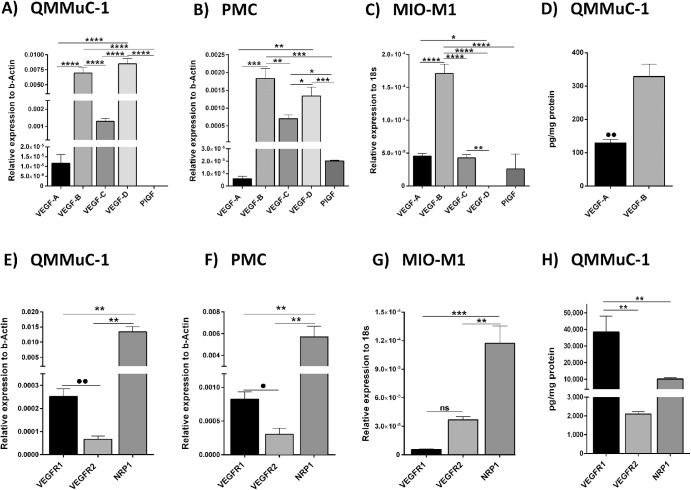
Real-time RT-PCR showed that under normal culture conditions the most abundantly expressed VEGF family members in mouse QMMuC-1 Müller cells (Fig. 1A) and PMCs (Fig. 1B) were VEGF-B and D. VEGF-A was detected at low levels in both QMMuC-1 and PMC. PlGF was absent in QMMuC-1 but expressed at low levels in PMC (Figs. 1A, 1B). In human MIO-M1 Müller cells, VEGF-B expression was also higher than VEGF-A and VEGF-C, VEGF-D and PlGF (Fig. 1C). Using ELISA, we confirmed that a significantly higher level of VEGF-B was detected in QMMuC-1 Müller cell lysates compared with VEGF-A (Fig. 1D) although the levels of VEGF-B and VEGF-A in the supernatants did not differ (Supplementary Fig. S1A) under normal culture conditions.
Figure 1.
The expression of VEGF family members and relevant receptors in Müller cells. The expression of VEGF family member and receptor was assessed at mRNA and protein levels by qPCR and ELISA in murine QMMuC-1 Müller cells, PMCs, and human MIO-M1 cells under normal culture conditions. (A–C), mRNA expression of VEGF-A, -B, -C, -D, and PlGF in QMMuC-1 (A), PMCs (B), and human MIO-M1 (C). (D) Protein quantification by ELISA of VEGF-A and VEGF-B in QMMuC-1 Müller cell lysates. (E–H), VEGF receptor expression in Müller cells. mRNA expression of VEGFR1, VEGFR2, and coreceptor NRP1 in murine Müller cell line QMMuC-1 cells (E), PMCs (F), and in human MIO-M1 Müller cells (G). (H) ELISA measurement of VEGFR1, VEGFR2 and NRP1 in QMMuC-1 cell lysates. n = 3 per group in PCR data and n = 4 in protein. •P < 0.05; ••P < 0.01 Student t test when two groups are compared. ****P < 0.001; ***P < 0.005; **P < 0.01, *P < 0.05. One-way ANOVA followed by Newmann-Keuls post hoc test.
In terms of VEGF receptors, mRNA expression of the coreceptor, NRP1, was markedly higher than VEGFR1 and VEGFR2 in all Müller cell types (Figs. 1E–1G). VEGFR1 mRNA expression was higher than VEGFR2 in murine (Figs. 1E, 1F) but not human (Fig. 1G) Müller cells. However, at the protein level, VEGFR1 (38,368 ± 9552 pg/mg) was significantly higher than the coreceptor NRP1 (10,171 ± 830 pg/mg), and the latter significantly higher than VEGFR2 (2107.0 ± 115.9 pg/mg) in QMMuC-1 Müller cells (Fig. 1H).
Our data suggest that the VEGF-B and its receptors, VEGFR1 and NRP1, are highly expressed at the mRNA and protein levels by Müller cells and hence may play a role in Müller cell physiology.
Expression of VEGF-B and Its Receptors Is Affected by Hypoxia and Oxidative Stress
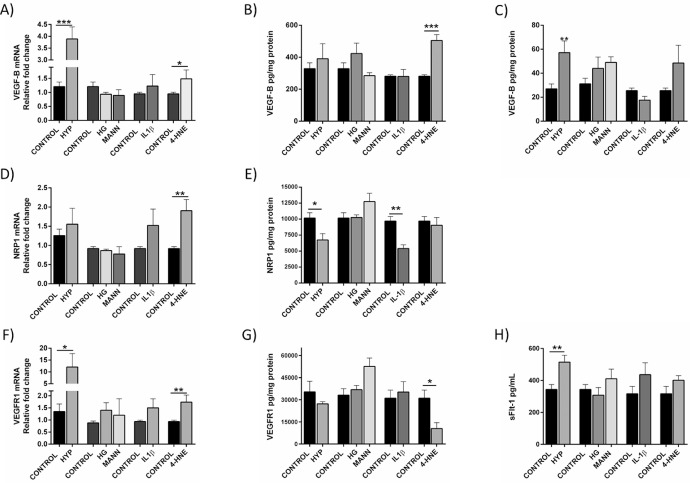
To understand how the expression of VEGF-B and its cognate receptors is altered in Müller cells under conditions resembling retinal disease, QMMuC-1 Müller cells were exposed to hypoxia, hyperglycemia, the inflammatory IL-1β, or oxidative stress. To mimic hyperglycemia that occurs in diabetes, an additional 25 mM of D-glucose was added to the media for 72 hours as previously reported.25,26 This concentration and length did not induce any change in MIO-M1 cell viability (data not shown). A sustained exposure to 1% O2 for 72 hours was used to recapitulate hypoxic conditions.27,28 24 hours treatment with 10 µM 4-hydroxynonenal (4-HNE) was used to induce oxidative stress without reduction in viability (Supplementary Fig. S1B) and 20 ng/mL of IL-1β was used based on previous studies in literature.29,30
Hypoxia significantly upregulated VEGF-B and VEGFR1 mRNA expression (Figs. 2A and 2F) and increased the soluble forms of VEGF-B (Fig. 2C) and VEGFR1 (sFlt-1) (Fig. 2H) proteins. NRP1 expression, however, was decreased under hypoxic conditions (Fig. 2E). These results were also confirmed in MIO-M1 cells (Supplementary Fig. S1F).
Figure 2.
Expression of VEGF-B and its receptors VEGFR1 and NRP1 under different stress conditions. QMMuC-1 cells cultured under hypoxia (HYP, 1% O2), high glucose (HG, 30 mM), or mannitol (MANN), IL-1β (20 ng/mL) and 4-HNE (10 µM) for 72 hours (for HYP and HG/MANN) or 24 hours (for IL-1β and 4-HNE) and the mRNA expression of VEGF-B (A), coreceptor NRP1 (D), and VEGFR1 (F) was determined by qPCR. Protein expression of VEGF-B in QMMuC-1 cell lysates (B) and supernatant (C), NRP1 and VEGFR1 (E and G, respectively) and the soluble receptor sFLT-1 was assessed by ELISA. n = 3–5 per group in PCR, n = 5-8 in ELISA. *P < 0.05; **P < 0.01; ***P < 0.005 Student t test, compared with respective control.
The 4-HNE increased VEGF-B and its receptors NRP1 and VEGFR1 mRNA expression in QMMuC-1 cells (Figs. 2A, 2D, and 2F). However, at the protein level, only VEGF-B (Fig. 2B) was increased by 4-HNE treatment. Regarding other VEGF family members (Supplementary Figs. S1C-D), only VEGF-A mRNA was augmented under hypoxia and after the IL-1β exposure (Supplementary Fig. S1C), as previously reported.31
Blocking VEGF-B Does Not Alter Müller Cell Homeostasis Under Normal Culture Conditions
To ascertain the role of VEGF-B in normal Müller cell function, VEGF-B was neutralized in QMMuC-1 cultures using a neutralizing antibody. The expression of proteins responsible for water and ion homeostasis (inward rectifying K+ channel subtype 4.1 [Kir4.1], aquaporin-4 [AQP4]), glutamate metabolism (glutamine synthetase [GS]) and Müller cell activation (vimentin and glial fibrillary acidic protein [GFAP]) was assessed. Our results showed that blockade of VEGF-B for 24 hours (Supplementary Figs. S2A-D) or 72 hours (Supplementary Figs. S2E–S2H) had no effect on the expression of those proteins.
We also examined QMMuC-1 cell viability after blockade of VEGF-B, NRP1, and VEGFR1, or both VEGFR1 and NRP1 for 72 hours. None of these treatments affected QMMuC-1 cell viability (Supplementary Fig. S2I), indicating that the VEGF-B pathway is not essential for Müller cell survival under normal culture conditions.
VEGF-B Neutralization Compromises Müller Cell Viability and Survival Under Pathologic Conditions
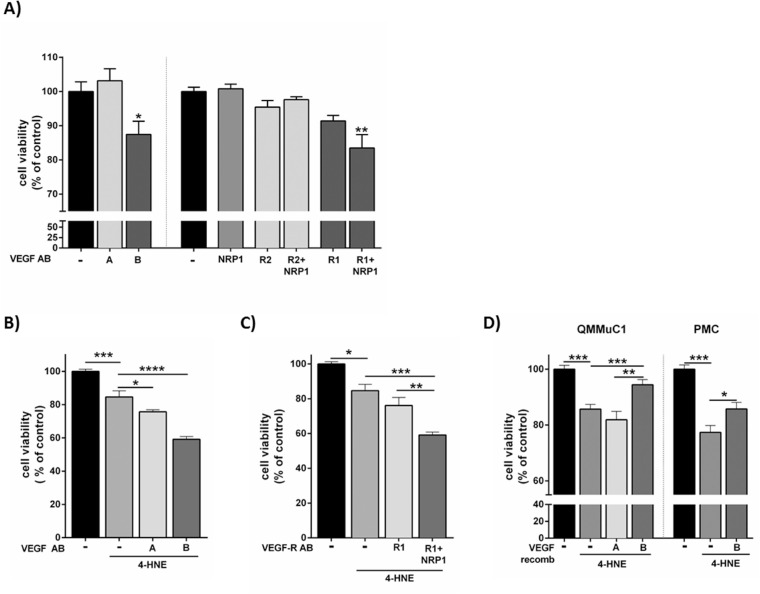
To assess whether VEGF-B plays a role in Müller cell responses to stress, VEGF-B or its receptors were neutralized or inhibited in QMMuC-1 cells cultured in hypoxia or in the presence of 20 µM 4-HNE (dose inducing approximately 15%–25% decrease of viability, Supplementary Fig. S1B) for 72 or 24 hours, respectively, and cell viability measured. Concentration of anti–VEGF-A and -B antibodies, and VEGFR1, VEGFR2, and NRP1 was chosen according to the company's recommendation and in line with others,32–36 in the same way as the selected the doses of recombinant VEGF-A and -B.33,34,37,38
Under hypoxic conditions (Fig. 3A), neutralization of VEGF-B (but not VEGF-A), significantly decreased Müller cell survival. Blocking VEGFR1, VEGFR2, or NRP1 alone or the combination of VEGFR2 and NRP1 did not affect Müller cell survival (Fig. 3A). However, when both VEGFR1 and NRP1 were blocked, cell viability was significantly reduced to a level similar to that observed with VEGF-B neutralization.
Figure 3.
The effect of VEGF-B and its receptors on Müller cell viability under oxidative stress and hypoxic conditions. (A) Effect of the neutralization of VEGF-A and its receptors (VEGFR2 and NRP1) and VEGF-B and its receptors (VEGFR1 and NRP1) on QMMuC-1 Müller cell viability under hypoxic conditions (1% O2 for 72 hours). *P < .05; **P < .01 compared with control (hypoxia only), one-way ANOVA followed by Dunnett's multiple comparison test. (B) Effect of VEGF-A or VEGF-B neutralization on 4-HNE–induced QMMuC-1 Müller cell viability. C, Effect of the neutralization of VEGFR1 and NRP1 on QMMuC-1 and their receptor (VEGFR1 and NRP1) on 4-HNE–induced QMMuC-1 damage. (D) The effect of recombinant VEGF-B and VEGF-A on 4-HNE–induced cell death in QMMuC-1 and PMC Müller cells. The viability of cells was determined by Alamar Blue methods. (A) n = 5–9, (B and C) n = 4–6, (D) n = 5–10 per group. *P < 0.05; **P < 0.01; ***P < 0.005 and ****P < 0.001, one-way ANOVA followed by Newmann-Keuls post hoc test. Antibody, AB. A and B are VEGF-A and VEGF-B respectively. R1 and R2 are VEGFR1 and VEGFR2. Anti-VEGF-A, VEGFR2 500 ng/mL and 10 µg/mL, respectively; NRP1 antibody, 30 µg/mL; VEGF-B and VEGFR1, 500 ng/mL and 10 µg/mL, respectively; recombinant VEGF-A and VEGF-B, 100 ng/mL; 4-HNE 20 µM.
Treatment with 4-HNE decreased QMMuC-1 cell viability by 15% and blocking VEGF-A or VEGF-B further decreased cell viability by 9% and 25%, respectively (Fig. 3B), and similar levels were reached when blocking both VEGFR1 and NRP1 together (Fig. 3C). The results were confirmed in PMCs (Supplementary Fig. S3A and S3B). Yo-Pro uptake assay showed that VEGF-B, but not VEGF-A neutralization, increased 4-HNE–induced apoptosis in QMMuC-1 cells (Supplementary Fig. S3C) via the VEGFR1 and NRP1 pathways (Supplementary Fig. S3D).
Next, the cytoprotective effect of VEGF-B was evaluated (Fig. 3D). Recombinant VEGF-B, but not VEGF-A, significantly increased the viability of Müller cell line QMMuC-1 and PMCs treated with 4-HNE (Fig. 3D).
Our data suggest that the VEGF-B/VEGFR1/NRP1 signaling axis is critically involved in Müller cell survival under hypoxic or oxidative conditions.
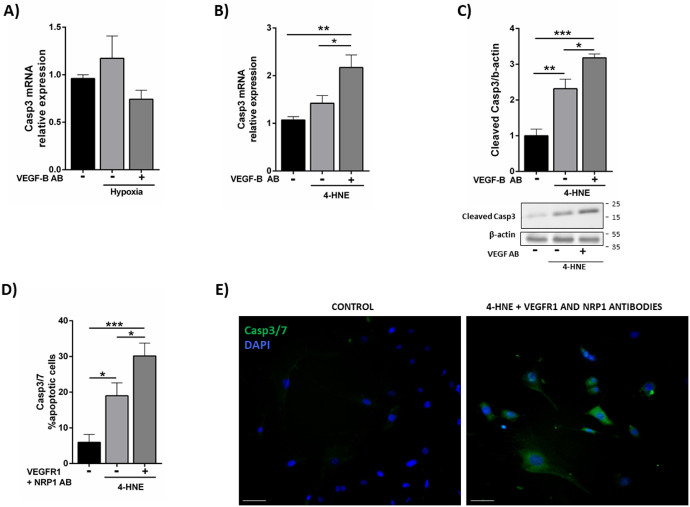
VEGF-B Neutralization Enhances Caspase-3 Expression in Müller Cells Under Oxidative Stress Conditions
To determine the mechanism underlying the decrease in Müller cell viability after VEGF-B pathway blockade, proapoptotic caspase-3 levels were analyzed. As shown in Figure 4A, hypoxia treatment did not affect caspase-3 mRNA expression in QMMuC-1 cells, even after VEGF-B neutralization (Fig. 4A). We found that 4-HNE increased caspase-3 expression and neutralization of VEGF-B further increased caspase-3 mRNA expression and its cleavage (Figs. 4B, 4C). Simultaneous blocking of VEGFR1 and NRP1 also significantly increased caspase 3/7 expression in 4-HNE exposed cells (Figs. 4D and 4E).
Figure 4.
The effect of VEGF-B blockade on caspase-3 expression in Müller cells under hypoxia and oxidative stress conditions. (A) Expression of caspase-3 mRNA by qPCR in QMMuC-1 cells cultured under hypoxic conditions with or without VEGF-B neutralizing antibody (for 72 hours). (B) Caspase-3 mRNA expression in QMMuC-1 Müller cells treated with 4-HNE with or without VEGF-B neutralizing antibody. (C) Western Blot showing cleaved caspase-3 in different groups. (D and E) the effect of VEGF-B receptor blockage (anti-VEGFR1 and anti-NRP1) on the expression of caspase-3/7 in PMCs determined by immunocytochemistry. (E) Representative images showing caspase-3/7 expression in control and 4-HNE + VEGFR blockage group. Anti–VEGF-B and VEGFR1 antibodies, 500 ng/mL and 10 µg/mL, respectively; NRP1 antibody, 30 µg/mL; 4-HNE 10 µM. *P < 0.05; **P < 0.01; ***P < 0.005, one-way ANOVA followed by a Newmann-Keuls post hoc test. Experiments performed at least twice, n = 3–4 per group in qPCR, and n = 4–6 in immunocytochemistry and Western Blot. Scale bar = 50 µM.
Role of VEGF-B in Müller Cell Gliosis and Ion/Water Channel Expression Under Stress Conditions
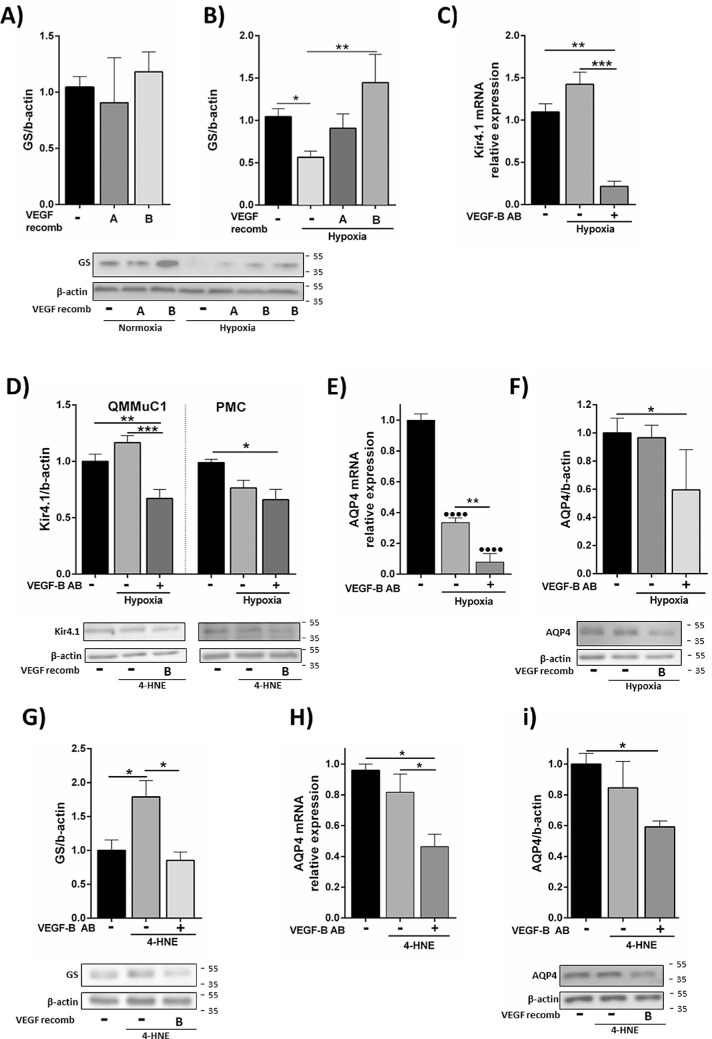
Next, we assessed whether VEGF-B can modulate Müller cell pathologic responses to hypoxia and oxidative stress. Hypoxia did not affect GFAP mRNA and protein expression in Müller cells (Supplementary Figs. S4A and S4B). Decreased GS expression in hypoxia was not affected by VEGF-B neutralization (Supplementary Fig. S4C); however, the addition of recombinant VEGF-B completely prevented hypoxia-induced downregulation of GS (Fig. 5B). This increase in GS with VEGF-B was only observed in hypoxia, because the addition of VEGF-A and VEGF-B recombinant proteins under normal culture conditions did not elicit any changes in GS expression (Fig. 5A). Kir4.1 and AQP4 were not affected by hypoxia treatment but were downregulated after VEGF-B neutralization (Figs. 5C–F). Our results suggest that autocrine VEGF-B is important for Müller cells to maintain the expression of important proteins involved in ion/water homeostasis, whereas exogenous VEGF-B may help to maintain Müller cell glutamate metabolism under hypoxic conditions.
Figure 5.
The effect of VEGF-B on GS, Kir4.1 and AQP4 expression in Müller cells under hypoxia and oxidative stress. (A) QMMuC-1 cells were treated with recombinant VEGF-A or VEGF-B and the expression of GS was measured by Western blotting. (B) Western Blot analysis of GS expression in QMMuC-1 cells cultured in hypoxic conditions for 72 hours with or without recombinant VEGF-A or VEGF-B. (C and D) Kir4.1 mRNA (C) and protein (D) expression in hypoxia (1% O2 for 72 hours) treated QMMuC-1 (C, D, left) and PMCs (D, right) with or without VEGF-B neutralizing antibody. (E and F) AQP4 mRNA (E) and protein (F) expression in QMMuC-1 cells in hypoxia condition with or without VEGF-B neutralization. (G) GS expression in QMMuC-1 cells under oxidative conditions (4-HNE) with/without VEGF-B neutralization. (H and I) AQP4 mRNA (H) and protein (I) expression in 4-HNE treated QMMuC-1 with or without VEGF-B neutralization. VEGF-B antibody, 500 ng/mL. 4-HNE, 10 µM. Data normalized to control values. n = 3–5/group in qPCR; n = 4–10 in Western Blot. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001. One-way ANOVA followed by Newmann-Keuls post hoc test. AB, antibody. Recomb, recombinant.
VEGF-B blockade did not modify the 4-HNE–induced increased mRNA or protein GFAP expression (Supplementary Fig. S4D and S4E). GS was upregulated by 4-HNE and this was abrogated by VEGF-B neutralization (Fig. 5G). Downregulated protein expression of Kir4.1 after 4-HNE was not further modified by VEGF-B neutralization (Supplementary Fig. S4G). AQP4 was not affected by 4-HNE when VEGF-B was present, but was downregulated when VEGF-B was neutralized (Figs. 5H and 5I). Our findings suggest that autocrine VEGF-B is important for Müller cells to maintain water homeostasis and glutamate recycling under oxidative stress conditions.
Role of VEGF-B in Müller Cell TRPV4 Expression and Function Under Pathologic Conditions
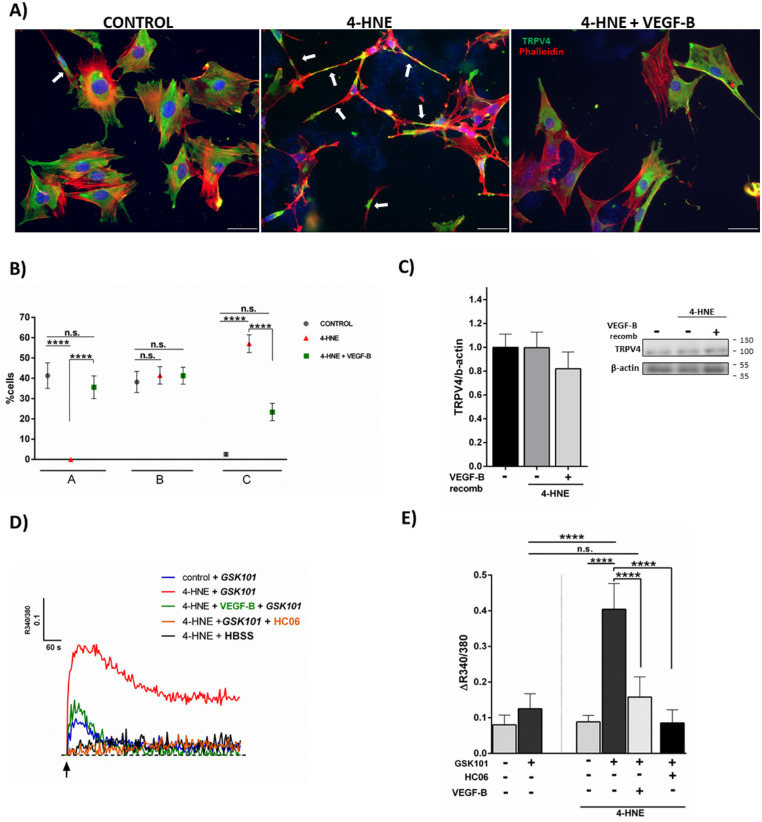
Apart from AQP4 and Kir4.1, the osmosensor, TRPV4, is also critically involved in Müller cell ion/water homeostasis.39–43 Treatment with 4-HNE did not affect the expression level of TRPV4 protein in Müller cells (Fig. 6C). The treatment, however, altered the cellular distribution of TRPV4 in Müller cells (Figs. 6A and 6B). Under normal culture conditions, QMMuC-1 cells spread out on the culture dish and TRPV4 was distributed uniformly across most of the cells (Fig. 6A). Aggregation of TRPV4 proteins was occasionally observed under normal culture conditions (Fig. 6A). After 4-HNE treatment, many QMMuC-1 cells displayed contracted cell bodies with long-banded shaped actin filaments and TRPV4 channel aggregation (arrows in Fig. 6B). VEGF-B protected Müller cells from these changes (Figs. 6A and 6B).
Figure 6.
Effect of VEGF-B on TRPV4 expression and functionality under oxidative stress. (A–C) QMMuC-1 cells were treated with 4-HNE (10 µM) with or without recombinant VEGF-B (100 ng/mL) for 24 hours. The expression of TRPV4 was examined by immunofluorescence staining (A, B) and Western Blot (C). (A) Representative image of TRPV4 (green) and phalloidin (red) in untreated control, 4-HNE treated and 4-HNE + recombinant VEGF-B (100 ng/mL) treated QMMuC-1 cells. Arrow in control indicates cluster aggregation of TRPV4. Arrows in 4-HNE–treated group indicates contracted cell body. (B) Quantitative analysis of different types of TRPV4-expressing QMMuC-1 cells (A, spread cell with evenly distributed TRPV4; B, spread cell with aggregated or uneven TRPV4 expression; C, contracted cell with aggregated TRPV4 expression). ****P < 0.001; n = 4 wells per experiment. Two-way ANOVA followed by Bonferroni post hoc test. (C) Western Blot of TRPV4 expression on 4-HNE–treated QMMuC-1 cells with/without recombinant VEGF-B, n = 6–7. (D and E) Effect of TRPV4 and VEGF-B on intracellular Ca2+ concentration in 4-HNE–treated QMMuC-1 cells and its representative plots (D). Intracellular Ca2+ levels were measured using the FlexStation. The y-axis was the 340 nm/380 nm fluorescence ratio (ΔR340 nm/380 nm). (D and E) TRPV4 agonist GSK101 (100 nM) alone did not elicit a measurable calcium response in QMMuC-1 cells. A 24 hours 4-HNE (10 µM) treatment significantly increased the GSK101-evoked response, which was fully blocked by the TRPV4 antagonist HC06 (10 µM) and by recombinant VEGF-B (100 ng/mL). Mean ± SD, n = 4–8 ****P < 0.001; one-way ANOVA followed by Newmann-Keuls post hoc test. All experiments were performed at least twice.
To further explore the role of TRPV4 in 4-HNE–induced Müller cell damage, intracellular Ca2+ recording was conducted. Under normal culture conditions, the TRPV4 agonist GSK101 did not affect [Ca2+]i in QMMuC-1 cells (Figs. 6D and 6E). Treatment with 4-HNE alone had no effect on [Ca2+]i. However, preincubation of the cells with this aldehyde facilitated [Ca2+]i response to GSK101 (Fig. 6D), which was fully blocked by the TRPV4 inhibitor, HC06, confirming the involvement of these channels (Figs. 6D and 6E). VEGF-B completely abrogated TRPV4-dependent [Ca2+]i signals in 4-HNE treated Müller cells (Figs. 6D and 6E). Our results suggest that 4-HNE can facilitate TRPV4 channel activity in Müller cells and that this effect can be prevented by VEGF-B.
Discussion
In this study, we demonstrate for the first time that Müller cells produce more VEGF-B than VEGF-A, -C, -D, or PlGF. Blocking VEGF-B did not alter Müller cell viability or expression of key functional proteins, indicating that VEGF-B might not be required for normal Müller cell function. These findings concur with a previous report that was unable to identify any obvious function for VEGF-B under physiologic conditions.13 However, it is also possible that VEGF-B may support Müller cells through an autocrine pathway in normal physiologic conditions as the levels of VEGF-B in Müller cell lysates were significantly higher than those in the supernatants. The autocrine support of VEGF-B to Müller cells may be supplemented significantly by the paracrine pathway in stress conditions, evidenced by the protective effects of recombinant VEGF-B and detrimental effects of blocking antibodies. Nevertheless, our results suggest that VEGF-B plays an important protective role in Müller cells under hypoxic or oxidative conditions.
Oxidative stress and hypoxia are known to be involved in several retinal diseases such as glaucoma, AMD, diabetic retinopathy, and retinal detachment to name but a few.1,44–46 Müller cells are one of the key responding cells of the retina to oxidative stress and hypoxia.1–3,46 Under hypoxic conditions, such as retinal detachment, the expression of GS was decreased and the TRPV4 activity was increased.41 Similarly, oxidative stress and hypoxia are implicated in the development of diabetic retinopathy.47,48 In this condition, an impaired Müller cell GS and dysregulation K+ and water transportation have been reported; ultimately, this process may lead to glutamate excitotoxicity, Müller cell swelling, and degeneration of the inner retina.2,47,48 Diabetic retinopathy also increases the production of neurotrophic factors such as VEGF-A, and was found to be one of the few pathologies that affects Müller cell viability.1,2
In this study, VEGF-B production in Müller cells was increased under both oxidative stress and hypoxic conditions. Blockade of VEGF-B or its cognate receptors, VEGFR1 and NRP1, compromised Müller cell viability in hypoxic and oxidative conditions most likely through induction of caspase-3 activation. Furthermore, treatment with recombinant VEGF-B attenuated 4-HNE–induced Müller cell death. This finding is in line with previous observations that showed that the survival of motor neurons,49 peripheral neurons,33 vascular cells,34 and retinal ganglion cells50 was promoted by VEGF-B.
Müller cells respond to retinal stress by initiating reactive gliosis, whereby they express high levels of gliosis markers such as GFAP and vimentin.46,48 Acute and/or sustained gliosis hinders retinal function via inflammatory cytokine induction, which accelerates disease progression.46,51,52 Interestingly, VEGF-B did not affect Müller cell gliosis under hypoxic or oxidative stress conditions. It did, however, regulate other Müller cell responses under pathologic conditions.
One vital function of Müller cells is glutamate transportation and recycling in the retina. GS regulates extracellular glutamate levels by converting internalized glutamate molecules into nontoxic glutamine. Expression of GS is decreased in retinal diseases, leading to glutamate accumulation and toxicity.2,48,53 We found that hypoxia decreased GS expression in Müller cells and that this effect was completely prevented by recombinant VEGF-B, but not VEGF-A.
Müller cells also maintain K+ and water homeostasis, via inward rectifying potassium channels Kir4.1 and the water channel AQP4.1,54 Dysregulation in K+ and water homeostasis has been linked with a myriad of retinal diseases, such as diabetic retinopathy, retinal detachment, and diabetic macular edema.46,48,54 Kir4.1 and/or AQP4 dysfunction can result in K+ dysregulation, impaired synaptic activity, Müller cell osmotic swelling, and retinal excitotoxicity and neurodegeneration.1,55,56 We found that VEGF-B blockade downregulated the expression of Kir4.1 and AQP4 when Müller cells were subjected to hypoxia and oxidative stress. Therefore, therapies like aflibercept, which targets different VEGF family members in the retina, could be problematic as they may exacerbate ongoing retinal disease via inhibition of the essential potassium and water homeostasis mechanisms.
TRPV4, member of the TRP channels superfamily, can function as an osmosensor and/or mechanosensor.39–42,57,58 TRPV4 colocalizes with AQP4 in the end feet of Müller glial cells.39 Activation of TRPV4 in swollen Müller cells has been reported to contribute significantly to the development of different retinal diseases, such as retinal detachment,41 glaucomatous retinopathy,40,57 and photoreceptor death.41 In this study, although 4-HNE treatment did not alter TRPV4 expression in Müller cells, it significantly disrupted the distribution of actin filaments and altered TRPV4 localization patterns. This finding is in line with a previous report on the interaction between TRPV4 and the microtubule cytoskeleton.59 In this study, 4-HNE facilitated TRPV4-dependant Ca2+ influx in Müller cells and this effect was blocked by VEGF-B. The restorative effect of VEGF-B on TRPV4 responses may occur via normalization of the cytoskeleton.
Owing to their prominent role in retinal homeostasis, Müller cells are an important therapeutic target in pathologies.3 We have demonstrated that VEGF-B is a potent gliotrophic factor for Müller cells under pathologic conditions, and that the neutralization of this factor may exacerbate diseases processes. Because VEGF-B does not have a significant angiogenic effect, and its overexpression does not elicit any adverse effects,9 VEGF-B is, therefore, a promising protective molecule and may be a valuable therapy for oxidative stress- and hypoxia-related retinal diseases.
Supplementary Material
Acknowledgments
Supported by Diabetes UK (13/0004729) and Fight for Sight (1574/1575, 5057/5058).
Disclosure: M. Llorián-Salvador, None; P. Barabas, None; E.M. Byrne, None; J. Lechner, None; J. Augustine, None; T.M. Curtis, None; M. Chen, None; H. Xu, None
References
- 1. Bringmann A, Pannicke T, Grosche J, et al.. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006; 25: 397–424, doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 2. Coughlin BA, Feenstra DJ, Mohr S. Müller cells and diabetic retinopathy. Vision Res. 2017; 139: 93–100, doi: 10.1016/j.visres.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devoldere J, Peynshaert K, De Smedt SC, Remaut K. Müller cells as a target for retinal therapy. Drug Discov Today 2019; 24: 1483–1498, Published online 2019, doi: 10.1016/j.drudis.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 4. Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab. 2013; 33: 1685–1695, doi: 10.1038/jcbfm.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010; 59: 2297–2305, doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hombrebueno JR, Ali IH, Xu H, Chen M. Sustained intraocular VEGF neutralization results in retinal neurodegeneration in the Ins2(Akita) diabetic mouse. Sci Rep. 2015; 5: 18316, doi: 10.1038/srep18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olofsson B, Pajusola K, Kaipainen A, et al.. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA. 1996; 93: 2576–2581, doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nilsson M, Heymach J V. Vascular endothelial growth factor (VEGF) pathway. J Thorac Oncol. 2006; 1: 768–770. [PubMed] [Google Scholar]
- 9. Bry M, Kivelä R, Leppänen V-M, Alitalo K. Vascular endothelial growth factor-B in physiology and disease. Physiol Rev. 2014; 94: 779–794, doi: 10.1152/physrev.00028.2013. [DOI] [PubMed] [Google Scholar]
- 10. McColl BK, Baldwin ME, Roufail S, et al.. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J Exp Med. 2003; 198: 863–868, doi: 10.1084/jem.20030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haiko P, Makinen T, Keskitalo S, et al.. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol. 2008; 28: 4843–4850, doi: 10.1128/MCB.02214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baldwin ME, Catimel B, Nice EC, et al.. The specificity of receptor binding by vascular endothelial growth factor-d is different in mouse and man. J Biol Chem. 2001; 276: 19166–19171, doi: 10.1074/jbc.M100097200. [DOI] [PubMed] [Google Scholar]
- 13. Li X, Kumar A, Zhang F, Lee C, Tang Z. Complicated life, complicated VEGF-B. Trends Mol Med. 2012; 18: 119–127, doi: 10.1016/j.molmed.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 14. Jensen LD, Nakamura M, Bräutigam L, et al.. VEGF-B-Neuropilin-1 signaling is spatiotemporally indispensable for vascular and neuronal development in zebrafish. Proc Natl Acad Sci. 2015; 112: E5944–E5953, doi: 10.1073/pnas.1510245112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arjunan P, Lin X, Tang Z, et al.. VEGF-B is a potent antioxidant. Proc Natl Acad Sci USA. 2018; 115: 10351–10356, doi: 10.1073/pnas.1801379115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le YZ. VEGF production and signaling in Müller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vis Res. 2017; 139: 108–114, doi: 10.1016/j.visres.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010; 6: 107–114, doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caballero B, Sherman SJ, Falk T. Insights into the mechanisms involved in protective effects of VEGF-B in dopaminergic neurons. Parkinsons Dis. 2017; 2017: 4263795, doi: 10.1155/2017/4263795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Llorián-Salvador M, González-Rodríguez S.. Painful understanding of VEGF. Front Pharmacol. 2018; 9: 1267, doi: 10.3389/fphar.2018.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schoch HJ, Fischer S, Marti HH. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. 2002; 125: 2549–2557, doi: 10.1093/brain/awf257. [DOI] [PubMed] [Google Scholar]
- 21. Chen R, Lee C, Lin X, Zhao C, Li X. Novel function of VEGF-B as an antioxidant and therapeutic implications. Pharmacol Res 2019; 143: 33–39, Published online 2019, doi: 10.1016/j.phrs.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 22. Mesquita J, Castro de Sousa JP, Vaz-Pereira S, et al.. VEGF-B Levels in the Vitreous of Diabetic and Non-Diabetic Patients with Ocular Diseases and Its Correlation with Structural Parameters. Med Sci (Basel). 2017; 5: 17, doi: 10.3390/medsci5030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Augustine J, Pavlou S, O'Hare M, et al.. Characterization of a spontaneously immortalized murine Müller glial cell line QMMuC-1. Invest Ophthalmol Vis Sci. 2018; 59: 1666–1674, doi: 10.1167/iovs.17-23293. [DOI] [PubMed] [Google Scholar]
- 24. Limb GA, Salt TE, Munro PM, Moss SE, Khaw PT. In vitro characterization of a spontaneously immortalized human Müller cell line (MIO-M1). Invest Ophthalmol Vis Sci. 2002; 43: 864–869, https://www.ncbi.nlm.nih.gov/pubmed/11867609. [PubMed] [Google Scholar]
- 25. Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 3784–3791, doi: 10.1167/iovs.10-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madonna R, Giovannelli G, Confalone P, Renna F V, Geng YJ, De Caterina R. High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: implications for diabetic retinopathy. Cardiovasc Diabetol. 2016; 15: 18, doi: 10.1186/s12933-016-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maneu V, Gerona G, Fernández L, Cuenca N, Lax P. Evidence of alpha 7 nicotinic acetylcholine receptor expression in retinal pigment epithelial cells. Vis Neurosci. 2010; 27: 139–147, doi: 10.1017/S0952523810000246. [DOI] [PubMed] [Google Scholar]
- 28. Kurihara T, Westenskow PD, Gantner ML, et al.. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife. 2016; 5: e14319, doi: 10.7554/eLife.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arroba AI, Valverde ÁM. Inhibition of protein tyrosine phosphatase 1B improves IGF-I receptor signaling and protects against inflammation-induced gliosis in the retina. Invest Ophthalmol Vis Sci. 2015; 56: 8031–8044, doi: 10.1167/iovs.15-17234. [DOI] [PubMed] [Google Scholar]
- 30. Yoshida S, Sotozono C, Ikeda T, Kinoshita S. Interleukin-6 (IL-6) production by cytokine-stimulated human Müller cells. Curr Eye Res. 2001; 22: 341–347, doi: 10.1076/ceyr.22.5.341.5498. [DOI] [PubMed] [Google Scholar]
- 31. Tanaka T, Kanai H, Sekiguchi K, et al.. Induction of VEGF gene transcription by IL-1β is mediated through stress-activated MAP kinases and Sp1 sites in cardiac myocytes. J Mol Cell Cardiol. 2000; 32: 1955–1967, doi: 10.1006/jmcc.2000.1228. [DOI] [PubMed] [Google Scholar]
- 32. Di Bernardini E, Campagnolo P, Margariti A, et al.. Endothelial lineage differentiation from induced pluripotent stem cells is regulated by microRNA-21 and transforming growth Factor β2 (TGF-β2) Pathways. J Biol Chem. 2014; 289: 3383–3393, doi: 10.1074/jbc.M113.495531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guaiquil VH, Pan Z, Karagianni N, Fukuoka S, Alegre G, Rosenblatt MI. VEGF-B selectively regenerates injured peripheral neurons and restores sensory and trophic functions. Proc Natl Acad Sci USA. 2014; 111: 17272–17277, doi: 10.1073/pnas.1407227111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Zhang F, Nagai N, et al.. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008; 118: 913–923, doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohamedali KA, Poblenz AT, Sikes CR, et al.. Inhibition of prostate tumor growth and bone remodeling by the vascular targeting agent VEGF121/rGel. Cancer Res. 2006; 66: 10919–10928, doi: 10.1158/0008-5472.CAN-06-0459. [DOI] [PubMed] [Google Scholar]
- 36. Esquibies AE, Karihaloo A, Quaggin SE, Bazzy-Asaad A, Cantley LG. Heparin binding VEGF isoforms attenuate hyperoxic embryonic lung growth retardation via a FLK1-neuropilin-1-PKC dependent pathway. Respir Res. 2014; 15: 1–15, doi: 10.1186/1465-9921-15-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang F, Tang Z, Hou X, et al.. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009; 106: 6152–6157, doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oosthuyse B. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001; 28: 131–138, 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 39. Jo AO, Ryskamp DA, Phuong TT, et al.. TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal Müller glia. J Neurosci. 2015; 35: 13525–13537, doi: 10.1523/jneurosci.1987-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ryskamp DA, Frye AM, Phuong TT, et al.. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci Rep. 2016; 6: 30583, doi: 10.1038/srep30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsumoto H, Sugio S, Seghers F, et al.. Retinal detachment-induced Müller glial cell swelling activates TRPV4 ion channels and triggers photoreceptor death at body temperature. J Neurosci. 2018; 38: 8745–8758, doi: 10.1523/JNEUROSCI.0897-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Netti V, Fernández J, Kalstein M, et al.. TRPV4 contributes to resting membrane potential in retinal Müller cells: implications in cell volume regulation. J Cell Biochem. 2017; 118: 2302–2313, doi: 10.1002/jcb.25884. [DOI] [PubMed] [Google Scholar]
- 43. Ryskamp DA, Jo AO, Frye AM, et al.. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci. 2014; 34: 15689–15700, doi: 10.1523/JNEUROSCI.2540-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen R, Lee C, Lin X, Zhao C, Li X. Novel function of VEGF-B as an antioxidant and therapeutic implications. Pharmacol Res. 2019; 143: 33–39, doi: 10.1016/j.phrs.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 45. Cheng L, Yu H, Yan N, Lai K, Xiang M. Hypoxia-inducible factor-1α target genes contribute to retinal neuroprotection. Front Cell Neurosci. 2017; 11: 20, doi: 10.3389/fncel.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bringmann A, Wiedemann P.. Müller glial cells in retinal disease. Ophthalmologica. 2012; 227: 1–19, doi: 10.1159/000328979. [DOI] [PubMed] [Google Scholar]
- 47. Pannicke T, Iandiev I, Wurm A, et al.. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006; 55: 633–639, doi: 10.2337/diabetes.55.03.06.db05-1349. [DOI] [PubMed] [Google Scholar]
- 48. Gu L, Xu H, Zhang C, Yang Q, Zhang L, Zhang J. Time-dependent changes in hypoxia- and gliosis-related factors in experimental diabetic retinopathy. Eye. 2019; 33: 600–609, doi: 10.1038/s41433-018-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caballero B, Sherman SJ, Falk T. Insights into the mechanisms involved in protective effects of VEGF-B in dopaminergic neurons. Park Dis. 2017; 2017: 4263795, doi: 10.1155/2017/4263795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang D, Zhao C, Ju R, et al.. VEGF-B inhibits hyperglycemia- and Macugen-induced retinal apoptosis. Sci Rep. 2016; 6: 26059, doi: 10.1038/srep26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu KH, Madigan MC, Billson FA, Penfold PL. Differential expression of GFAP in early v late AMD: a quantitative analysis. Br J Ophthalmol. 2003; 87: 1159–1166, doi: 10.1136/bjo.87.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hippert C, Graca AB, Barber AC, et al.. Müller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS One. 2015; 10: e0120415, doi: 10.1371/journal.pone.0120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vohra R, Kolko M.. Neuroprotection of the inner retina: Müller cells and lactate. Neural Regen Res. 2018; 13: 1741–1742, doi: 10.4103/1673-5374.238612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Noël G, Belda M, Guadagno E, Micoud J, Klöcker N, Moukhles H. Dystroglycan and Kir4.1 coclustering in retinal Müller glia is regulated by laminin-1 and requires the PDZ-ligand domain of Kir4.1. J Neurochem. 2005; 94: 691–702, doi: 10.1111/j.1471-4159.2005.03191.x. [DOI] [PubMed] [Google Scholar]
- 55. Zhao M, Bousquet E, Valamanesh F, et al.. Differential regulations of AQP4 and Kir4.1 by triamcinolone acetonide and dexamethasone in the healthy and inflamed retina. Invest Ophthalmol Vis Sci. 2011; 52: 6340–6347, doi: 10.1167/iovs.11-7675. [DOI] [PubMed] [Google Scholar]
- 56. Liu XQ, Kobayashi H, Jin ZB, Wada A, Nao-I N. Differential expression of Kir4.1 and aquaporin 4 in the retina from endotoxin-induced uveitis rat. Mol Vis. 2007; 13: 309–317, https://www.ncbi.nlm.nih.gov/pubmed/17356517. [PMC free article] [PubMed] [Google Scholar]
- 57. Gao F, Yang Z, Jacoby RA, Wu SM, Pang JJ. The expression and function of TRPV4 channels in primate retinal ganglion cells and bipolar cells. Cell Death Dis. 2019; 10: 364, doi: 10.1038/s41419-019-1576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci USA. 2013; 110: 6157–6162, doi: 10.1073/pnas.1216514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goswami C, Kuhn J, Heppenstall PA, Hucho T. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS One. 2010; 5: e11654, doi: 10.1371/journal.pone.0011654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jo AO, Lakk M, Frye AM, et al.. Differential volume regulation and calcium signaling in two ciliary body cell types is subserved by TRPV4 channels. Proc Natl Acad Sci USA. 2016; 113: 3885–3890, doi: 10.1073/pnas.1515895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.