Key Points
COVID-19 convalescent plasma (CP) may be a safe and effective treatment option in SARS-CoV-2 infection refractory to remdesivir.
Infants may benefit from CP despite developing immune systems and donor variability emphasizes the need for characterization prior to use.
Introduction
Although <2% of coronavirus disease 2019 (COVID-19) infections are reported in the pediatric population, children with comorbidities such as congenital heart disease and those at a younger age are more likely to become critically ill.1-3 Remdesivir has been reported to be efficacious in adults with COVID-194; however, there are no studies in children. Convalescent plasma (CP) can contain neutralizing antibodies to viruses,5 and has been used during previous viral epidemics with clinical improvement.6-11 COVID-19 CP (C19-CP) may be useful in critically ill adults, resulting in improvement in inflammatory markers, pulmonary lesions, and mortality.12 However, the impact of C19-CP in pediatric patients, particularly infants with developing immune systems and significant comorbidities, is completely unknown.
We present an infant with cardiopulmonary failure secondary to unrepaired congenital heart disease exacerbated by COVID-19. Given postsurgical complications of children with viral respiratory infection,13-17 the patient required clearance of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for surgical candidacy. We hypothesized that C19-CP administration may clear SARS-CoV-2 following failure of remdesivir.
Case description
A 3.1-kg term 9-week-old female with trisomy 21 and unrepaired balanced complete atrioventricular canal defect with baseline mild to moderate common atrioventricular valve regurgitation (AVVR) presented in acute hypoxemic respiratory failure secondary to progressive decompensated heart failure. Notable findings included lymphopenia, β-natriuretic peptide of 530 pg/mL (reference range <100 pg/mL), worsened AVVR (moderate to severe), and cardiomegaly and pulmonary edema on chest X-ray. Initial treatment, including bilevel noninvasive positive pressure ventilation, milrinone infusion, and IV diuretics, resulted in restoration of organ function. By hospital day 8, her common AVVR returned to baseline; she transitioned from milrinone to captopril, carvedilol, and IV diuretics and had weaned to high-flow nasal cannula; and she was transferred to the general cardiology floor.
On hospital day 12, she developed acute hypoxemic respiratory failure and decompensated heart failure, with atelectasis on chest X-ray and new rhinorrhea requiring bilevel noninvasive positive pressure ventilation. The result of SARS-CoV-2 nucleic acid testing (NAT) (DiaSorin Molecular Simplexa direct real-time PCR of viral RNA, Cypress, CA) on a nasopharyngeal (NP) swab was positive; a residual sample from a respiratory viral panel performed on hospital day 5 for intermittent desaturations was retested for SARS-CoV-2 and likewise had a positive result (Figure 1A). The source of the infection remains unclear. While the mother and siblings were asymptomatic, they were quarantined for 14 days after infection was identified in the patient but never tested. Nosocomial infection is certainly possible but less likely, as the hospital had very few infected patients, none of those infected were in the cardiac intensive care unit, and there was no shortage of personal protective gear at the hospital prior or following the patient’s infection. Additional investigation did not reveal macrophage activation syndrome or new bacterial or viral infection. As her prolonged QTc prohibited use of hydroxychloroquine, remdesivir (5 mg/kg per day on hospital day 15 and 2.5 mg/kg per day on hospital days 16-25) was given. Her hospital course was complicated by staphylococcal scalded skin syndrome, treated with nafcillin, and stage II necrotizing enterocolitis (Bell criteria), treated with piperacillin-tazobactam (Figure 1A). Throughout this time, she continued to have persistent lymphopenia, a C-reactive protein (CRP) level of 5 mg/dL (reference range, <1.0 mg/dL) with normal fibrinogen, and a common AVVR that remained unchanged with preserved biventricular function managed with IV diuretics.
Figure 1.
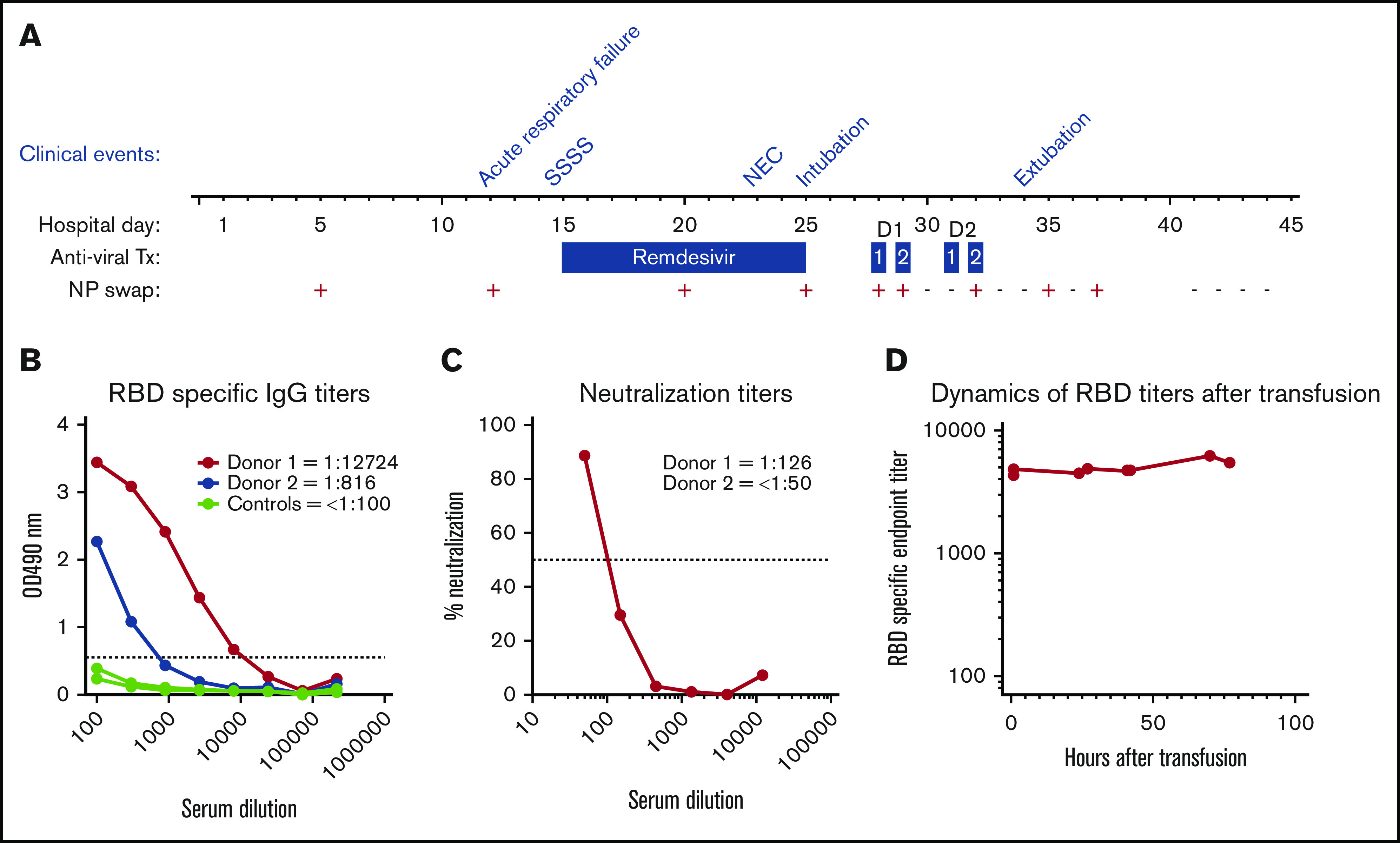
Hospital course summary and donor and patient plasma characterization. (A) Hospital course by clinical event, antiviral therapy (Tx), and C19-CP transfusion in relation to NP swab SARS-CoV-2 RNA results. Clinical events are as follows: acute respiratory failure on hospital day 12, staphylococcal scalded skin syndrome (SSSS) on hospital day 14, necrotizing enterococcus (NEC) on hospital day 23, intubation on hospital day 25, and extubation on hospital day 34. Dosages of antiviral therapy are as follows: remdesivir: 5 mg/kg per day on hospital day 15 and 2.5 mg/kg/day on hospital days 16 to 25; C19-CP donor 1: 10 mL/kg per day over 4 hours ∼20 hours apart on hospital days 28 and 29; and C19-CP donor 2: 10 mL/kg per day over 4 hours ∼20 hours apart on hospital days 31 and 32. (B) Enzyme-linked immunosorbent assays showing titration of samples from the 2 C19-CP donors and 3 negative controls collected before 2019, against SARS-CoV-2 spike RBD. End point titers were calculated using a cutoff of the average of the control samples, plus 3 times standard deviation value, marked with a dotted line. End points were calculated as the intersection of samples with this cutoff. Titers are shown numerically in the legend. (C) Neutralization activity of C19-CP donor samples against SARS-CoV-2. The focus reduction neutralization test (FRNT)50 titers of the C19-CP donor samples were determined by an FRNT assay using an immunostain to detect infected foci. The dotted line represents the maximum concentrations of the serum tested (1/50). (D) Dynamics of RBD-specific end-point titers in the recipient after transfusion.
Despite completion of remdesivir, on hospital day 25, she required endotracheal intubation for worsening respiratory failure. Repeat investigation was notable for persistent lymphopenia, persistent SARS-CoV-2, CRP 7.7 mg/dL, but no evidence of new secondary infection or macrophage activation syndrome was found. Milrinone was reinitiated in addition to IV diuretics. Given her lack of response to remdesivir and deteriorating clinical status, 2 aliquots of CP (10 mL/kg per aliquot) from 2 COVID-19 recovered donors (C19-CP) were transfused in accordance with the World Health Organization guidelines for Ebola CP treatment in children9 (Figure 1A). She received increased IV diuretics, daily monitoring of serum chemistries, and intermittent monitoring of coagulopathy. Daily surveillance of SARS-CoV-2 NAT by NP swab was performed, and the results were negative following the second aliquot from donor 1 (Figure 1A) with concurrent reduced ventilator support. She was extubated on hospital day 34. The infant tolerated all transfusions without anaphylaxis, transfusion-related circulatory overload, transfusion-related acute lung injury, or hypercoagulability. The results of daily NP swabs were intermittently positive before becoming persistently negative (Figure 1A). Other notable findings post–C19-CP transfusion include CRP of 1.3 mg/dL and intermittent normalization of absolute lymphocyte count. The patient had an uneventful complete repair 47 days (hospital day 91) after SARS-CoV-2 infection resolution and is doing well.
Methods
The patient is blood group O RhD negative. Donor 1 is a 63-year-old male who donated 35 days following COVID-19 symptom resolution and is blood type A RhD positive. Donor 2 is a 56-year-old male who donated 55 days following COVID-19 symptom resolution and is blood type O RhD negative. Both had mild COVID-19 infection, and neither was hospitalized. The plasma donor units were transfused without prior characterization per emergency use. To understand the impact of C19-CP treatment, both donor units were tested for titers of IgG against the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein (Emory University and Children’s Healthcare of Atlanta, Atlanta, GA), which has been shown to correlate with neutralizing titers,18 and subsequent microneutralization assays were performed in a biosafety level 3 laboratory.19 Donor 1 had an immunoglobulin G (IgG) titer of 1:12 724 and neutralizing titer of 1:126. Donor 2 had an IgG titer of 1:816 and a neutralizing titer of <1:50 (Figure 1B-C, respectively). Following the first aliquot from donor 1, the recipient showed robust IgG anti-SARS-CoV-2 antibody levels of ∼1:5000, which was sustained after all subsequent transfusions (Figure 1D).
Results and discussion
Young age, unrepaired congenital heart disease, trisomy 21, persistent lymphopenia, and naive immune system likely increased this patient’s SARS-CoV-2 vulnerability. While this represents only 1 case, her improved respiratory status, increase in lymphocytes, and decrease in CRP after C19-CP transfusion suggest a positive clinical correlation, especially in the setting of subsequent SARS-CoV-2 clearance.
Logistical constraints prevented anti-SARS-CoV-2 antibody characterization of C19-CP prior to transfusion, which likely reflects common current approaches to emergent C19-CP administration. C19-CP from donor 1 likely provided the most significant impact on SARS-CoV-2 eradication given the higher neutralizing titer (Figure 1C), which is comparable to US Food and Drug Administration recommendations20 and may be sufficient when treating COVID-19 in adults.10-21 This case suggests that such titers may likewise be sufficient in infants. The delay in viral clearance may reflect the time required for C19-CP therapy to effectively eliminate the virus and is consistent with reports in adults with persistent SARS-CoV-2 NAT by NP samples beyond symptom resolution.22,23
Factors influencing high anti-SARS-CoV-2 titers with effective characteristics remain incompletely understood. Severe illness would be presumed to correlate with higher anti-SARS-CoV-2 antibodies. However, even though neither C19-CP donor was hospitalized, donor 1 possessed higher titers than donor 2, whose titers were below the limit of detection. This variability illustrates the challenge of current C19-CP transfusion practice, making it difficult to determine the potential benefit without adequate testing prior to C19-CP transfusion in place.
While neutralizing antibodies are critical for antibody-mediated protection against viral infection, the impact of CP on resolution of active infection remains incompletely understood. However, beyond direct neutralization of the virus, other mechanisms, such as antibody-dependent cellular cytotoxicity, may also play a role in the ability of C19-CP to aid in the clearance of an active infection. Such considerations may be especially important in infants with developing or suppressed immune systems that may attenuate the ability of other antibody effector systems to effectively clear COVID-19 following C19-CP therapy. While we were unfortunately unable to acquire neutralizing titer data from the patient prior to C19-CP administration to determine potential changes in antibody levels prior to and following administration of the first dose of C19-CP, data obtained following serial SARS-CoV-2 NAT evaluation provides uncommon insight into the temporal relationship between C19-CP and its potential impact on SARS-CoV-2 infection. However, this is a single case, and understandable uncertainties remain. Future studies will certainly be needed to fully define the efficacy of C19-CP in this vulnerable patient population.
Acknowledgments
The authors thank their colleagues at LifeSouth for collection of donor plasma. Parental consent was obtained for treatment with C19-CP. Review of medical records, publication of results, and research use of biospecimens per the Declaration of Helsinki under the protocols were approved by the Institutional Review Board of Emory University School of Medicine and the Department of Health and Human Services.
Footnotes
Requests for data sharing should be e-mailed to the corresponding author at rodriguezz@kidsheart.com.
Authorship
Contribution: Z.R., S.R.S., and C.D.J. conceived of and wrote the manuscript; S.R.S., C.D.J., Z.R., M.S., and J.W. prepared the figures; A.L.S., H.V., C.L., M.G.Z., M.S., J.W., H.M., M.W., S.C., J.D.R., C.M.A., S.R.S., and C.D.J. critically reviewed, analyzed, and interpreted the data; M.G.Z., J.W., M.S., H.M., C.M.A., and S.R.S. performed the immunologic assays; and C.L. collected the donor units and information on blood donors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zahidee Rodriguez, Emory University, Children’s Healthcare of Atlanta–Egleston, 1405 Clifton Rd, 2nd Floor, Tower 1, Atlanta, GA 30322; e-mail: rodriguezz@kidsheart.com.
References
- 1.Dong Y, Mo X, Hu Y, et al. . Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 2.CDC COVID-19 Response Team Coronavirus disease 2019 in children: United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBiasi RL, Song X, Delaney M, et al. . Severe COVID-19 in children and young adults in the Washington, DC metropolitan region. J Pediatr. 2020;223:199-203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledford H. Hopes rise for coronavirus drug remdesivir [published online ahead of print 29 April 2020]. Nature. 10.1038/d41586-020-01295-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JS, Chen JT, Liu YX, et al. . A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol. 2005;77(2):147-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599-609. [DOI] [PubMed] [Google Scholar]
- 7.Hung IF, To KK, Lee CK, et al. . Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung IFN, To KKW, Lee CK, et al. . Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144(2):464-473. [DOI] [PubMed] [Google Scholar]
- 9.van Griensven J, Edwards T, de Lamballerie X, et al. ; Ebola-Tx Consortium . Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Wong R, Soo YO, et al. . Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh KM, Chiueh TS, Siu LK, et al. . Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen C, Wang Z, Zhao F, et al. . Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malviya S, Voepel-Lewis T, Siewert M, Pandit UA, Riegger LQ, Tait AR. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98(3):628-632. [DOI] [PubMed] [Google Scholar]
- 14.Tait AR, Malviya S, Voepel-Lewis T, Munro HM, Seiwert M, Pandit UA. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95(2):299-306. [DOI] [PubMed] [Google Scholar]
- 15.Mallory MD, Travers C, McCracken CE, Hertzog J, Cravero JP. Upper respiratory infections and airway adverse events in pediatric procedural sedation. Pediatrics. 2017;140(1):e20170009. [DOI] [PubMed] [Google Scholar]
- 16.Parnis SJ, Barker DS, Van Der Walt JH. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11(1):29-40. [DOI] [PubMed] [Google Scholar]
- 17.Tanne JH. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368:m1256. [DOI] [PubMed] [Google Scholar]
- 18.To KK, Tsang OT, Leung WS, et al. . Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suthar MS, Zimmerman M, Kauffman R, et al. . Rapid generation of neutralizing antibody responses in COVID-19 patients. medRxiv. 2020;1(3):100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FDA. Recommendations for investigational COVID-19 convalescent plasma. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma. Accessed 1 May 2020.
- 21.Bloch EM, Shoham S, Casadevall A, et al. . Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249. [DOI] [PubMed] [Google Scholar]
- 23.Wajnberg A, Mansour M, Leven E, et al. . Humoral immune response and prolonged PCR positivity in a cohort of 1343 SARS-CoV 2 patients in the New York City region [published online ahead of print 5 May 2020]. medRxiv. 10.1101/2020.04.30.20085613. [DOI] [Google Scholar]


