Key Points
In this cohort of patients with suspected HIT, plasma sGPVI levels were significantly higher in patients with major bleeding than those without.
sGPVI may be marker of elevated bleeding risk in patients with suspected HIT.
Abstract
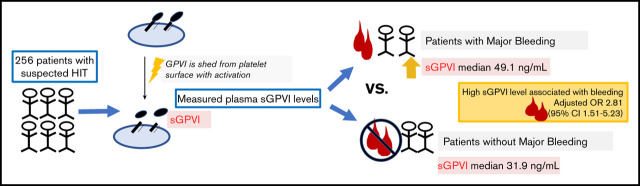
We have shown that patients with suspected heparin-induced thrombocytopenia (HIT) have a high incidence of major bleeding. Recent studies have implicated elevated soluble glycoprotein VI (sGPVI) levels as a potential risk factor for bleeding. We sought to determine if elevated sGPVI plasma levels are associated with major bleeding events in patients with suspected HIT. We used a cohort of 310 hospitalized adult patients with suspected HIT who had a blood sample collected at the time HIT was suspected. Plasma sGPVI levels were measured by using enzyme-linked immunosorbent assay. Patients were excluded who had received a platelet transfusion within 1 day of sample collection because of the high levels of sGPVI in platelet concentrates. We assessed the association of sGPVI (high vs low) with International Society on Thrombosis and Haemostasis major bleeding events by multivariable logistic regression, adjusting for other known risk factors for bleeding. Fifty-four patients were excluded due to recent platelet transfusion, leaving 256 patients for analysis. Eighty-nine (34.8%) patients had a major bleeding event. Median sGPVI levels were significantly elevated in patients with major bleeding events compared with those without major bleeding events (49.09 vs 31.93 ng/mL; P < .001). An sGPVI level >43 ng/mL was independently associated with major bleeding after adjustment for critical illness, sepsis, cardiopulmonary bypass surgery, and degree of thrombocytopenia (adjusted odds ratio, 2.81; 95% confidence interval, 1.51-5.23). Our findings suggest that sGPVI is associated with major bleeding in hospitalized patients with suspected HIT. sGPVI may be a novel biomarker to predict bleeding risk in patients with suspected HIT.
Visual Abstract
Introduction
Clinical practice guidelines suggest that patients with suspected heparin-induced thrombocytopenia (HIT) and an intermediate to high probability 4Ts score be treated with a nonheparin anticoagulant while awaiting HIT confirmatory laboratory test results.1 Although HIT is a prothrombotic condition, patients with suspected HIT have a high incidence of major bleeding.2,3 Thus, the decision to initiate empiric alternative anticoagulation (often at therapeutic-intensity) in patients with suspected HIT is a challenging one. Clinicians must weigh the risk of bleeding vs the risk of thrombosis for the individual patient. Bleeding risk assessment is particularly difficult in the absence of well-validated bleeding prediction models for hospitalized patients. Studies have identified some risk factors based on routine clinical laboratory findings, but bleeding risk scores for inpatients have not been widely adopted in clinical practice nor have they been applied to patients with suspected HIT.4 Identifying additional biomarkers to predict bleeding risk is important to help guide management and may also shed light on mechanisms of bleeding.
Glycoprotein VI (GPVI) is a platelet-specific surface marker shed from the platelet surface during platelet activation or other causes.5,6 Soluble GPVI (sGPVI) can be measured in the plasma by using an enzyme-linked immunosorbent assay and typically exhibits little variation among healthy subjects, ranging from 11 to 24 ng/mL.6 Increased sGPVI levels are linked to adverse outcomes in sepsis, thermal injury patients, and trauma.7 Elevated sGPVI levels before implantation of a left ventricular assist device have been found to be associated with bleeding events.8,9
The goal of the present study was to determine if sGPVI levels were associated with bleeding outcomes in a cohort of patients with suspected HIT. We hypothesized that elevated levels would be predictive of bleeding.
Patients and methods
Patients
This study used a previously described cohort of 310 hospitalized adult patients with suspected HIT recruited from 2 hospitals, the Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center.10 The ultimate determination of cases as “HIT positive” or “HIT negative” was made by the consensus of an expert panel based on clinical information and HIT laboratory testing as described in Pishko et al.10 The original study was approved by the University of Pennsylvania institutional review board. All patients provided written informed consent for the original study.
sGPVI measurement
All patients had a citrated plasma sample collected at the time HIT was suspected. Plasma sGPVI levels were measured by enzyme-linked immunosorbent assay of platelet-depleted plasma in duplicate, and the average result was used for analysis.11 Patients’ transfused platelets within 1 day of sample collection were excluded because of the potential presence of high levels of sGPVI in stored platelet concentrates.12
Clinical and demographic variables
Clinical and demographic variables were extracted retrospectively from the electronic health record, including platelet count on day of sGPVI specimen collection, age, race, hospitalization in a critical care unit, primary team caring for the patient (medical or surgical), and surgery before specimen collection. We used International Classification of Diseases, Ninth Revision, codes during the patient’s admission to identify the presence or absence of sepsis using the codes 038 (septicemia), 995.91 (sepsis), 995.92 (severe sepsis), or 785.52 (septic shock).13
Outcomes
The study’s primary outcome was major bleeding according to International Society of Thrombosis and Haemostasis (ISTH) criteria, which includes fatal bleeding, hemorrhage occurring at a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a drop in hemoglobin (Hb) of ≥2 g/dL, or leading to transfusion of 2 or more units of whole blood or packed red blood cells (pRBCs).14 This definition was modified to limit the 2 g/dL drop in Hb or 2-unit pRBC transfusion to have occurred within a 24-hour period so as to avoid the gradual reductions in Hb that can occur in hospitalized patients. We assessed for the primary outcome from time of sGPVI sample collection to hospital discharge or death. Patients could only have 1 bleeding event, and their bleeding event was classified according to the highest severity of ISTH criteria fulfilled. For example, if a patient had a critical organ and fatal bleed, the patient was categorized as having a fatal bleed only. Secondary outcomes included the development of arterial/venous thrombosis (confirmed by imaging) following sGPVI sample collection until hospital discharge or death and mortality at 30 days.
Statistical analysis
In patients with and without bleeding events, categorical variables and continuous variables were compared between patients with and without bleeding events by using a Wilcoxon rank sum test (continuous variables) and a χ2 test (categorical variables). Among all patients in the cohort, we assessed for associations with sGPVI levels as a continuous variable and binary demographic/clinical variables (age [≥65 or <65 years], sex, race [white vs non-white], HIT positivity, and sepsis) using the Wilcoxon rank sum test. Missing values were not imputed. We then performed receiver-operating curve (ROC) analysis for sGPVI levels as a predictor of major bleeding events and selected a dichotomous cut-point for sGPVI that maximized the associated Youden’s index.15 Univariate logistic regression was used to assess the association of sGPVI (high vs low) and other clinical and demographic variables with the binary outcome of bleeding event (yes/no). Variables from the univariate analysis with P < .2 and interactions between these variables were included in the full multivariable model of the association of sGPVI and bleeding outcomes. We also built univariate logistic regression models to assess the association of sGPVI levels (high vs low) with the secondary outcomes of thrombosis and 30-day mortality.
All results are presented with 95% confidence intervals (CIs), and P < .05 was considered statistically significant. Statistical analyses were performed by using Stata version 14.0 (Stata Corp, College Station, TX) and GraphPad Prism version 8.4 (GraphPad Software, San Diego, CA).
Results
Cohort description
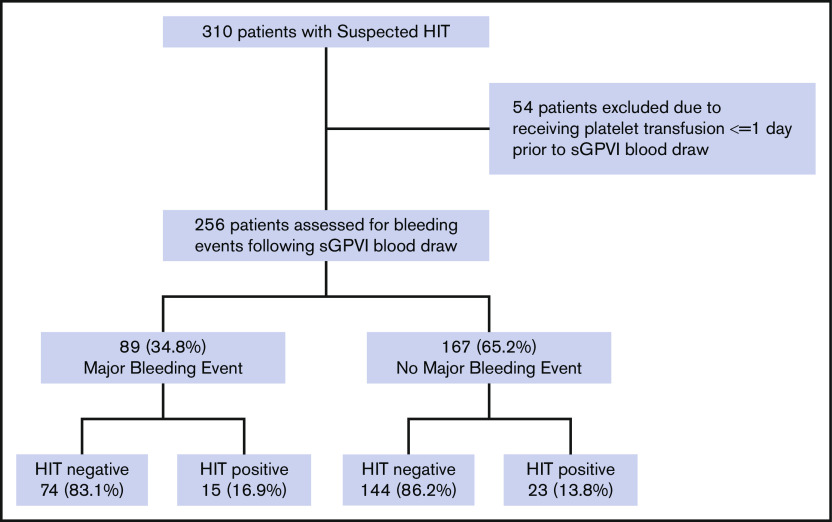
Of the 310 patients in the original cohort, 54 (17.4%) were excluded because they received a platelet transfusion within 24 hours of sample collection (Figure 1). Among the remaining 256 patients, 89 (34.8%) had an ISTH major bleeding event during the time from sample collection to hospital discharge or death. The majority (76.4% [68 of 89]) fulfilled criteria for major bleeding due to need for 2 units of pRBCs or a 2 g/dL decrease in Hb within a 24-hour period; 18.0% (16 of 89) of patients had a critical organ bleed, and 5.6% (5 of 89) had a fatal bleeding event. The time from sGPVI sample collection to first major bleeding event ranged from 0 to 28 days (median, 3 days).
Figure 1.
Study cohort selection flowchart. Study design and cohort allocation are shown.
Demographic and clinical characteristics of patients with and without major bleeding are listed in Table 1. There was no significant difference in the percentage of HIT-positive patients among those with and without major bleeding (16.9% vs 13.8%; P = .51). The majority of patients who had a major bleeding event (74.2%) were hospitalized in the intensive care unit (ICU). Patients with a major bleeding event were more likely to be on a surgical service than those without a major bleeding event (62.9% vs 47.9%; P = .02) and more likely to have sepsis during their hospital stay (41.6% vs 19.8%; P < .001). Patients with major bleeding had a lower median platelet count on the day of sGPVI blood draw than patients without major bleeding events (54.5 × 109/L vs 79 × 109/L; P = .004).
Table 1.
Demographic and clinical characteristics of patients with and without major bleeding events
| Characteristic | Bleeding event (n = 89) | No bleeding event (n = 167) | P |
|---|---|---|---|
| Age, median (range), y | 65 (19-86) | 67 (24-92) | .373 |
| Race, n (%) | .140 | ||
| White | 72 (80.9) | 113 (67.8) | |
| Hispanic | 1 (1.1) | 2 (1.2) | |
| African American | 14 (15.7) | 48 (28.7) | |
| Asian | 2 (2.3) | 4 (2.4) | |
| ICU, n (%) | 66 (74.2) | 68 (40.7) | <.001 |
| Primary service, n (%) | .022 | ||
| Surgical | 56 (62.9) | 80 (47.9) | |
| Medical | 33 (37.1) | 87 (52.1) | |
| Sepsis, n (%)* | 37 (41.6) | 33 (19.8) | <.001 |
| Platelet count on day of blood sample collection, median (range), ×109/L† | 54.5 (8-200) | 79 (13-422) | .0004 |
| Serotonin release assay, n (%) | .863 | ||
| Positive | 10 (11.2) | 15 (8.9) | |
| Indeterminate | 1 (1.1) | 1 (0.6) | |
| Negative | 77 (86.5) | 150 (89.8) | |
| Missing | 1 (1.1) | 1 (0.6) | |
| PF4/H ELISA OD, n (%) | .749 | ||
| <0.4 | 63 (70.8) | 117 (70.1) | |
| 0.4-1.0 | 7 (7.9) | 16 (9.6) | |
| 1.0-2.0 | 7 (7.9) | 17 (10.2) | |
| >2.0 | 12 (13.5) | 16 (9.6) | |
| Missing | 0 (0) | 2 (1.2) | |
| HIT status, n (%) | .509 | ||
| Positive | 15 (16.9) | 23 (13.8) | |
| Negative | 74 (83.5) | 144 (86.2) |
ELISA, enzyme-linked immunosorbent assay; OP, optical density; PF4/H, platelet factor 4/heparin.
International Classification of Diseases, Ninth Revision, code for sepsis during admission that sGPVI was drawn.
Platelet count on day of blood sample collection (first count of day). Nine patients were missing a platelet count on day of blood sample collection.
sGPVI levels in the study cohort
In the cohort, sGPVI levels ranged from 7.0 to 97.7 ng/mL, with a median of 35.8 ng/mL. sGPVI levels did not vary significantly by gender (P = .81), age (≥65 or <65 years) (P = .91), or race (white vs non-white) (P = .47). Median sGPVI levels were higher in patients with sepsis than those without sepsis (46.5 [interquartile range (IQR), 15.5-86.3] ng/mL vs 32.1 [IQR, 13.3-70.9] ng/mL; P = .001). Median sGPVI levels were also significantly higher in patients admitted to the ICU vs the wards (41.7 [IQR, 25.9-56.4] ng/mL vs 31.9 [IQR, 20.6-43.1] ng/mL; P = .0002). There was no significant difference in sGPVI levels in HIT-positive vs HIT-negative patients (P = .10).
Association of sGPVI levels and bleeding outcomes
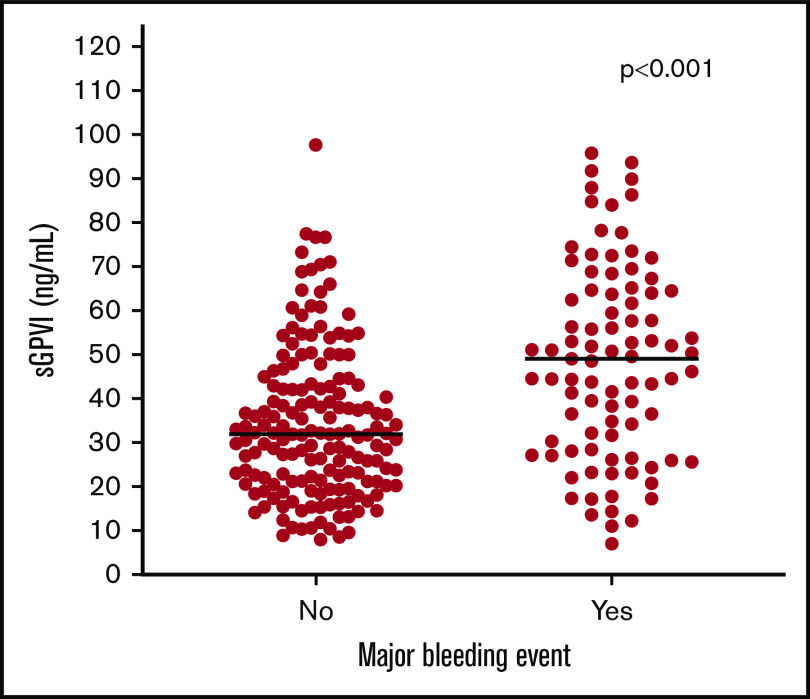
Median sGPVI levels were significantly elevated in patients with major bleeding events compared with those without (49.1 [IQR, 28.5-64.5] ng/mL vs 31.9 [IQR, 21.2-43.1] ng/mL; P < .001) (Figure 2). ROC analysis of sGPVI levels for predicting bleeding events revealed an area under the curve of 0.69 (95% CI, 0.62-0.76) (supplemental Figure 1). The cut-point that maximized Youden’s index was 43.4 ng/mL. We thus defined sGPVIlow as <43 ng/mL and sGPVIhigh as ≥43 ng/mL. Other clinical and demographic variables associated with a major bleeding event were admission to the ICU, cardiopulmonary bypass surgery, sepsis, and platelet count (supplemental Table 1). In the final multivariable model, after adjustment for ICU status, sepsis, cardiopulmonary bypass, and platelet count, sGPVIhigh remained independently associated with major bleeding with an odds ratio (OR) of 2.81 (95% CI, 1.51-5.23) (Table 2).
Figure 2.
Distribution of sGPVI levels in patients with and without bleeding events. Distribution of sGPVI levels in patients with suspected HIT who did and did not have an ISTH major bleeding event. Blue line indicates median plasma sGPVI level for each group.
Table 2.
Univariate and multivariable logistic regression model for predictors of bleeding
| Variable | Univariable logistic regression | Multivariable logistic regression | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| sGPVI | ||||
| <43 ng/mL | 1 (Ref) | — | 1 (Ref) | — |
| ≥43 ng/mL | 4.59 (2.65-7.96) | <.001 | 2.81 (1.51-5.23) | .001 |
| ICU status* | ||||
| No | 1(Ref) | — | 1 (Ref) | — |
| Yes | 4.18 (2.37-7.36) | <.001 | 2.76 (1.46-5.21) | .002 |
| Sepsis† | ||||
| No | 1 (Ref) | — | 1 (Ref) | — |
| Yes | 2.89 (1.64-5.10) | <.001 | 2.27 (1.13-4.56) | .021 |
| Cardiopulmonary bypass surgery | ||||
| No | 1 (ref) | — | 1 (Ref) | — |
| Yes | 1.79 (1.04- 3.07) | .036 | 1.50 (0.76-2.93) | .238 |
| Platelet count, ×109/L‡ | ||||
| ≥151 | 1 (Ref) | — | 1 (Ref) | — |
| 150-100 | 1.44 (0.44-4.67) | .542 | 0.89 (0.25- 3.22) | .858 |
| 50-100 | 1.26 (0.42-3.74) | .680 | 0.86 (0.26- 2.81) | .801 |
| ≤49 | 3.58 (1.20-10.66) | .222 | 1.72 (0.52- 5.66) | .376 |
Ref, reference.
Hospitalized in a medical or surgical critical care unit at time of enrollment in study.
International Classification of Diseases, Ninth Revision, code for sepsis during hospitalization sGPVI level as drawn.
Platelet count on day of sGPVI blood draw.
Association of sGPVI with thrombosis and mortality
Thirty-seven (14.45%) of 256 patients had a venous and/or arterial thrombosis after sGPVI blood collection. sGPVIhigh was not significantly associated with the development of venous and/or arterial thrombosis after sGPVI sample collection (OR, 1.71; 95% CI, 0.85-3.45; P = .13). Sixteen (6.25%) patients were lost to follow-up at 30 days. Among the remaining 240 patients, 25% were deceased. sGPVIhigh was associated with increased 30-day mortality (OR, 2.14; 95% CI, 1.18-3.87).
Discussion
We found that plasma sGPVI levels were significantly higher in patients with suspected HIT who had a major bleeding event compared with patients with suspected HIT who did not have a bleeding event. sGPVIhigh remained a significant predictor of bleeding events when adjusted for the presence of sepsis, hospitalization in the ICU, cardiopulmonary bypass, and degree of thrombocytopenia. sGPVIhigh was also found to be significantly associated with 30-day mortality.
Our results are consistent with the findings of Muthiah et al,8 who reported that mean sGPVI levels before left ventricular assist device implantation were higher in patients with bleeding events vs those without bleeding events (86 ± 52 vs 52 ± 19 ng/mL; P = .008). A proposed mechanism in patients with cardiac devices is that shear forces lead to excessive shedding of the receptor and platelet dysfunction.8 In patients without such devices, other disease processes such as sepsis may lead to pathologic platelet activation and fibrin deposition, which in turn causes inappropriate shedding of sGPVI and platelet dysfunction.5 The current study found significantly higher sGPVI levels in patients with sepsis vs those without sepsis. This aligns with previous research by Montague et al,7 which likewise reported the association of higher sGPVI levels in patients who experienced a thermal injury and developed sepsis.
In our analysis of secondary outcomes, we found a significant association between sGPVIhigh levels and 30-day mortality. Montague et al7 also observed an association between elevated sGPVI and 28-day mortality in ICU patients and patients with thermal injury. Notably, in our study, we used a higher sGPVI cutoff (≥43 ng/mL) than Montague et al (>22.3 ng/mL), who based their cutoff on levels in healthy control subjects. We selected our sGPVI cutoff based on ROC analysis for prediction of bleeding. sGPVI as a predictor of mortality requires further study in a large cohort of patients to determine the ideal cutoff for prognostication of this outcome.
A limitation of our study includes the retrospective nature of the data collection. Although we used a standardized definition of major bleeding, other causes of a 2 g/dL Hb drop or the need for a 2-unit pRBC transfusion (eg, hemolysis/dilution) can be difficult to ascertain from chart review. Furthermore, we chose the time of sGPVI blood draw as the starting point for our observation period. We did not record whether patients had active bleeding before the sGPVI blood draw; thus, it is possible that some patients may have experienced major bleeding before this time point. In addition, the time period required after platelet transfusion for sGPVI levels to return to baseline is not known. We excluded patients receiving platelet transfusions within 24 hours, but it is not known if transfusions at 48 or 72 hours could also affect results. Further prospective analyses with serial measurements of sGPVI in individual patients are required to establish sGPVI as a predictor of major bleeding rather than a marker of ongoing or recent bleeding.
Despite these limitations, the current study supports previous literature that elevated sGPVI levels may predict bleeding risk.8 A biomarker for bleeding risk in patients with suspected HIT could prove useful for clinicians in weighing the risks and benefits of empiric anticoagulation. For example, among patients with suspected HIT and an intermediate-probability 4Ts score, the American Society of Hematology clinical practice guidelines on HIT suggests treatment with a nonheparin anticoagulant at prophylactic-intensity if the patient is judged to be at high bleeding risk and at therapeutic-intensity if the patient is deemed not to be at elevated risk of bleeding.1 This recommendation is challenging to implement in practice owing to a lack of validated predictors of bleeding in this population. If sGPVI is ultimately validated as a predictor of bleeding in patients with suspected HIT, it could be used to inform estimation of bleeding risk and decisions about intensity of anticoagulation.
In summary, we identified sGPVI levels as an independent marker of bleeding risk among patients with suspected HIT. sGPVI levels could prove useful in clinical practice as a means of estimating bleeding risk and guiding empiric treatment in this population, although prospective validation is needed.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgment
This work was supported by a HTRS Mentored Research Award (A.M.P.) from the Hemostasis and Thrombosis Research Society supported by an educational grant from Sanofi Genzyme.
Footnotes
Requests for original de-identified data should be submitted to the corresponding author (Allyson M. Pishko; e-mail: allyson.pishko@pennmedicine.upenn.edu).
Authorship
Contribution: A.M.P. and A.C. collected and analyzed data and drafted the manuscript; D.S.L. collected data; E.E.G. and R.K.A. performed the laboratory assays; all authors contributed and critically reviewed the manuscript; and A.M.P. and A.C. had full access to the data and take responsibility for the integrity of the study.
Conflict-of-interest disclosure: A.M.P. received an educational grant from Sanofi Genzyme. A.C. has served as a consultant for Synergy CRO; and his institution has received research support on his behalf from Alexion, Bayer, Novo Nordisk, Pfizer, Sanofi, Spark, and Takeda. The remaining authors declare no competing financial interests
Correspondence: Allyson M. Pishko, University of Pennsylvania, 3400 Spruce St, 3rd Floor, Dulles Building, Philadelphia, PA 19104; e-mail: allyson.pishko@pennmedicine.upenn.edu.
References
- 1.Cuker A, Arepally GM, Chong BH, et al. . American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pishko AM, Lefler DS, Gimotty P, et al. . The risk of major bleeding in patients with suspected heparin-induced thrombocytopenia. J Thromb Haemost. 2019;17(11):1956-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuter DJ, Konkle BA, Hamza TH, et al. . Clinical outcomes in a cohort of patients with heparin-induced thrombocytopenia. Am J Hematol. 2017;92(8):730-738. [DOI] [PubMed] [Google Scholar]
- 4.Decousus H, Tapson VF, Bergmann JF, et al. ; IMPROVE Investigators . Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. [DOI] [PubMed] [Google Scholar]
- 5.Montague SJ, Hicks SM, Lee CS, et al. . Fibrin exposure triggers αIIbβ3-independent platelet aggregate formation, ADAM10 activity and glycoprotein VI shedding in a charge-dependent manner. J Thromb Haemost. 2020;18(6):1447-1458. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner EE, Andrews RK. Plasma sGPVI: changing levels in human disease. Thromb Res. 2014;133(3):306-307. [DOI] [PubMed] [Google Scholar]
- 7.Montague SJ, Delierneux C, Lecut C, et al. . Soluble GPVI is elevated in injured patients: shedding is mediated by fibrin activation of GPVI. Blood Adv. 2018;2(3):240-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthiah K, Connor D, Ly K, et al. . Longitudinal changes in hemostatic parameters and reduced pulsatility contribute to non-surgical bleeding in patients with centrifugal continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2016;35(6):743-751. [DOI] [PubMed] [Google Scholar]
- 9.Lukito P, Wong A, Jing J, et al. . Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J Thromb Haemost. 2016;14(11):2253-2260. [DOI] [PubMed] [Google Scholar]
- 10.Pishko AM, Fardin S, Lefler DS, et al. . Prospective comparison of the HEP score and 4Ts score for the diagnosis of heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Tamimi M, Mu FT, Moroi M, Gardiner EE, Berndt MC, Andrews RK. Measuring soluble platelet glycoprotein VI in human plasma by ELISA. Platelets. 2009;20(3):143-149. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini E, Ghasemzadeh M, Nassaji F, Jamaat ZP. GPVI modulation during platelet activation and storage: its expression levels and ectodomain shedding compared to markers of platelet storage lesion. Platelets. 2017;28(5):498-508. [DOI] [PubMed] [Google Scholar]
- 13.Liu V, Escobar GJ, Greene JD, et al. . Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90-92. [DOI] [PubMed] [Google Scholar]
- 14.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. [DOI] [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32-35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.