Key Points
Cumulative fracture incidence after R-CHOP in older patients (>70 years) is high (11.4% at 18 months).
Bone involvement, prephase steroids, or either reduced bone mineral density, RhA, or prior fracture are independent risk factors.
Abstract
Diffuse large B-cell lymphoma (DLBCL) and osteoporotic fracture are both more common in older patients. Exposure to R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) is likely to increase the risk of fracture, but evidence is lacking to define fracture incidence in this group. Data on consecutive patients with DLBCL aged ≥70 years treated with 1 to 8 cycles of full or attenuated R-CHOP were retrospectively collected across 10 UK centers (2009-2019). Patients were followed up from starting R-CHOP for a minimum of 6 months and censored at 18 months; at last follow-up if <18 months; or at progression or death. Of 877 patients identified, 148 were excluded: 121 had progression or died before 6 months; 23 had follow-up <6 months. Across 729 remaining patients, the median age was 77 years, and 68% had an Eastern Cooperative Oncology Group performance status of 0 to 1. Eighty-one fractures occurred within 18 months of follow-up; 42 were symptomatic, including 30 requiring hospital attendance or admission. The cumulative fracture incidence was 6.2% (95% confidence interval [CI], 4.7-8.2) at 6 months; 9.7% (95% CI, 7.8-12.1) at 12 months; and 11.4% (95% CI, 9.3-14.0) at 18 months. Multivariate analysis identified a predisposing history (osteoporosis, osteopenia, prior fracture, and rheumatoid arthritis [RhA]), DLBCL bone involvement at baseline, and receipt of prephase steroids as independent risk factors for fracture. There is a clinically relevant fracture risk and significant associated morbidity in older patients receiving R-CHOP. Careful attention to bone health is warranted in older patients receiving R-CHOP. Randomized studies are required to better define the most effective strategies to reduce fracture risk.
Visual Abstract
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoid malignancy, with the highest incidence in older patients.1 Full-dose or attenuated R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) produces durable remissions in 60% to 70% of older patients.2-4 Long-term treatment complications therefore remain important. Historical epidemiologic studies have identified an association between the treatment of non-Hodgkin lymphoma (NHL) with steroid-containing chemotherapy and increased fracture rates during the following year.5 Follow-up of 111 adult patients treated with R-CHOP, the most commonly used front-line regimen for DLBCL, showed a reduction in bone mineral density (BMD) at 1 year with incomplete recovery at 2 years and a rate of vertebral fracture of 14% on routine surveillance computed tomography (CT) scans; additional fracture sites and symptomatic fracture events were not assessed, however.6
Recent Danish population-based data have been reported in 2589 patients receiving high-dose steroids for DLBCL or follicular lymphoma diagnosed between 2000 and 2012; a composite end point of “osteoporotic events” (osteoporosis treatment or low-impact fracture) was found to be increased compared with an age and sex-matched population (n = 12 945) (hazard ratio [HR], 1.61; 95% confidence interval [CI], 1.40-1.84).7 The 5- and 10-year cumulative risks of osteoporotic events for patients with lymphoma were 10.0% and 16.3%, compared with matched population rates of 6.8% and 13.5%, respectively. Patients without an osteoporotic event within the first 2 years’ posttreatment were not at higher risk of osteoporotic events in subsequent years.
The use of corticosteroids in the treatment of DLBCL likely contributes to an increased fracture incidence given the well-described association between high-dose or prolonged courses of glucocorticoids and increased fracture risk,8 reflected in the validated Fracture Risk Assessment Tool (FRAX) recommended by many national guidelines.9-11 R-CHOP includes 5 days of prednisolone 40 mg/m2 to 100 mg/d in each 21-day cycle and is frequently given with a prephase steroid of similar dose.2,12 A total cumulative dose of 2400 to 3000 mg across 18 weeks would therefore be anticipated in six R-CHOP cycles.
Historical evidence from randomized clinical trials has shown the benefits to bone health in terms of improvement in BMD and fracture risk for bisphosphonate therapy in patients receiving long-term glucocorticoids.13-15 Current national guidelines10,11,16 relating to prevention and management of glucocorticoid-induced osteoporosis (GIO) are based on evidence from patients receiving long-term lower dose steroids and recommend a risk-stratified approach incorporating measurement of BMD, a technique not routinely included before lymphoma treatment. Simpler interventions addressing calcium and vitamin status and lifestyle modifications are recommended for most patients, with oral bisphosphonates or more complex interventions reserved for higher risk patients.16
Osteoporotic fracture is associated with significant morbidity and mortality arising from pain, hospitalization, and loss of mobility and independence17; in addition, accumulated vertebral fractures have been shown to progressively worsen health-related quality of life.18 These issues are particularly pertinent in elderly patients.
In light of the data outlined and the relative lack of published data that specifically define the risk and characteristics of fracture after R-CHOP in elderly patients with DLBCL, this large observational, retrospective study was conducted to describe the incidence and characteristics of fractures occurring after R-CHOP in an older consecutively treated cohort and to analyze associations with potential risk factors for fracture.
Methods
Data on consecutive DLBCL patients aged ≥70 years treated with R-CHOP were retrospectively collected across 10 large UK centers. Patients were included from 2009 onward, and follow-up was censored in October 2019. All patients had untreated, de novo DLBCL or untreated transformed (to DLBCL) indolent B-cell NHL. Patients with posttransplant lymphoproliferative disorder, HIV, or pretreated NHL were excluded. We were specifically interested in the patient fracture risk for those who had survived without progression beyond 6 months; therefore, any patient who had progressive disease (PD) or died within 6 months of the start of cycle 1 R-CHOP (R-CHOP1) were excluded. A detailed, anonymized database included Eastern Cooperative Oncology Group (ECOG) performance status; body mass index (BMI); history of osteoporosis, osteopenia, type 2 diabetes, or rheumatoid arthritis (RA); documented prephase steroid; vitamin D, calcium, and/or bisphosphonate supplementation; sites of bone DLBCL involvement; and calcium and alkaline phosphatase levels categorized as below, within, or above the local reference range.
Local institutional review board approval was given at each participating site: Oxford University Hospitals NHS Foundation Trust, The Christie Hospital NHS Foundation Trust, Guy’s and St. Thomas’ NHS Foundation Trust, Norfolk and Norwich NHS Foundation Trust, Southampton University Hospital NHS Foundation Trust, Royal Berkshire Hospital NHS Foundation Trust, Great Western Hospital NHS Foundation Trust, Frimley Health NHS Foundation Trust, Milton Keynes University Hospital NHS Foundation Trust, and Buckinghamshire Healthcare NHS Trust.
All patients received full or attenuated R-CHOP given with curative intent at a minimum interval of 21 days. Standard dosing consisted of 375 mg/m2 rituximab, 50 mg/m2 doxorubicin, 750 mg/m2 cyclophosphamide, and 1.4 mg/m2 vincristine (capped at 2 mg) on day 1 of each cycle; and 40 mg/m2 or 100 mg oral prednisolone on days 1 to 5 according to local practice. At the treating clinicians’ discretion, attenuated doses of 25 mg/m2 doxorubicin, 400 mg/m2 cyclophosphamide, and/or 1 mg vincristine were given. To be eligible for analysis, patients must have completed their treatment course and remain alive, progression free, and in follow-up at 6 months. Twenty-six percent (186 of 712) of patients received prephase steroids according to local practice (typically oral prednisolone 50-100 mg daily for 7-14 days) before commencing R-CHOP.
Data on fractures at DLBCL diagnosis (classified as DLBCL related or unrelated) and fractures preceding the DLBCL diagnosis were collected. Fractures (including number, anatomical bone site, and clinical consequences) occurring during 18 months’ post–R-CHOP1 were identified from detailed examination of radiology records. Patients were censored at 18 months from R-CHOP1. Patients were intentionally not followed up beyond 18 months because the mechanistic relationship to R-CHOP from theoretical increased risk of fragility-related fracture associated with glucocorticoids, immunochemotherapy, and the catabolic nature of an aggressive lymphoma is less clear with increasing time following R-CHOP1.11,19 The finding that risk of osteoporotic events from 2 years after glucocorticoid-containing immunochemotherapy was not increased compared with the baseline population supports this assumption.7
Statistical analysis
Baseline patient characteristics were summarized descriptively. Survival analyses were performed by using Kaplan-Meier methods20 and Cox regression with comparisons between categories using the log-rank test. The primary endpoint was the 18-month cumulative fracture incidence. Time to fracture was measured from R-CHOP1 until fracture event. Deaths and progressions occurring between 6 and 18 months were treated as competing risks, and patients without an event were censored at 18 months or the last date seen if before this point. Patients without a fracture event and with follow-up of <6 months were excluded. Univariable analysis of potential influencing factors relevant to fracture included: sex, BMI, prephase steroid, calcium and alkaline phosphatase levels, history of osteopenia, osteoporosis, and other medical comorbidities; DLBCL bone involvement at diagnosis; and DLBCL-related fracture at diagnosis. Multivariate analysis was performed by using Cox regression (stepwise backward selection; P = .05 cut off for inclusion). All analyses were performed by using Stata 15.1 (StataCorp, College Station, TX), and 95% CIs are presented for all estimates with P < .05 taken as significant.
Results
A total of 877 consecutive patients were identified; 148 were excluded, including 121 patients who had PD or died before 6 months, and 23 patients with follow-up <6 months. Four other patients were excluded (Burkitt lymphoma [n = 1], not receiving R-CHOP [n = 2], and unknown [n = 1]) (supplemental Figure 1). Baseline characteristics of the remaining 729 patients are displayed in Table 1. Within the first 18 months of follow-up, 87 patients progressed or died, and 86 patients were censored without an event. Supplemental Table 1 provides details of R-CHOP and prephase steroid treatment according to patient group (fracture vs no fracture). Supplemental Table 2 provides details of recent bone-protective medication (vitamin D, calcium, and bisphosphonates) divided by patients with or without a subsequent fracture.
Table 1.
Baseline characteristics
| Characteristic | All patients | Fracture | No fracture | |
|---|---|---|---|---|
| (N = 729)* | (n = 81)* | (n = 648)* | ||
| Age, median (range), y | 76.8 (69.5-96.4) | 76.7 (69.5-96.4) | 77.2 (9.5-90.5) | |
| Sex, n (%) | ||||
| Male | 353 (48.4) | 29 (35.8) | 324 (50.0) | |
| Female | 376 (51.6) | 52 (64.2) | 324 (50.0) | |
| ECOG performance score, n (%) | ||||
| 0-1 | 491 (68.0) | 56 (71.8) | 435 (67.5) | |
| 2-4 | 231 (32.0) | 22 (28.2) | 209 (32.5) | |
| IPI score grouped, n (%) | ||||
| IPI 1-2 | 328 (46.4) | 34 (43.0) | 294 (46.8) | |
| IPI 3-5 | 379 (53.6) | 45 (57.0) | 334 (53.2) | |
| BMI group, n (%) | ||||
| Underweight | 32 (4.4) | 5 (6.2) | 27 (4.2) | |
| Normal | 317 (43.8) | 32 (39.5) | 285 (44.4) | |
| Overweight | 237 (32.8) | 25 (30.9) | 212 (33.0) | |
| Obese | 137 (18.9) | 19 (23.5) | 118 (18.4) | |
| Relevant comorbidities and predisposing history, n (%) | ||||
| Current smoker | ||||
| No | 617 (95.1) | 72 (94.7) | 545 (95.1) | |
| Yes | 32 (4.9) | 4 (5.3) | 28 (4.9) | |
| Known osteoporosis | ||||
| No | 680 (94.3) | 71 (87.7) | 609 (95.2) | |
| Yes | 41 (5.7) | 10 (12.3) | 31 (4.8) | |
| Known osteopenia | ||||
| No | 689 (96.1) | 74 (91.4) | 615 (96.7) | |
| Yes | 28 (3.9) | 7 (8.6) | 21 (3.3) | |
| Family history of osteoporosis | ||||
| No | 329 (99.1) | 29 (93.5) | 300 (99.7) | |
| Yes | 3 (0.9) | 2 (6.5) | 1 (0.3) | |
| RA | ||||
| No | 690 (95.8) | 74 (91.4) | 616 (96.4) | |
| Yes | 30 (4.2) | 7 (8.6) | 23 (3.6) | |
| Type 2 diabetes | ||||
| No | 635 (88.3) | 70 (86.4) | 565 (88.6) | |
| Yes | 84 (11.7) | 11 (13.6) | 73 (11.4) | |
| Inflammatory bowel disease | ||||
| No | 707 (98.6) | 81 (100.0) | 626 (98.4) | |
| Yes | 10 (1.4) | 0 (0.0) | 10 (1.6) | |
| History of excess alcohol use | ||||
| No | 614 (95.5) | 76 (98.7) | 538 (95.1) | |
| Yes | 29 (4.5) | 1 (1.3) | 28 (4.9) | |
| Prior steroids in last 12 mo | ||||
| No | 614 (95.5) | 76 (98.7) | 538 (95.1) | |
| Yes | 29 (4.5) | 1 (1.3) | 28 (4.9) | |
| Bone-protective medication (any)† | ||||
| No medication, no predisposing history‡ | 534 (75.9) | 48 (62.3) | 486 (77.5) | |
| No medication, predisposing history | 69 (9.8) | 12 (15.6) | 57 (9.1) | |
| Medication, no predisposing history | 48 (6.8) | 4 (5.2) | 44 (7.0) | |
| Medication and predisposing history | 53 (7.5) | 13 (16.9) | 40 (6.4) | |
| Fracture history and bone involvement, n (%) | ||||
| Prior fracture | ||||
| No | 617 (85.3) | 62 (76.5) | 555 (86.4) | |
| Yes | 44 (6.1) | 4 (4.9) | 40 (6.2) | |
| Fracture at baseline | 62 (8.6) | 15 (18.5) | 47 (7.3) | |
| Non-lymphoma–related fracture at diagnosis | ||||
| No | 705 (97.2) | 77 (95.1) | 628 (97.5) | |
| Yes | 20 (2.8) | 4 (4.9) | 16 (2.5) | |
| Baseline fracture due to DLBCL | ||||
| No | 678 (93.5) | 68 (85.0) | 610 (94.6) | |
| Yes | 47 (6.5) | 12 (15.0) | 35 (5.4) | |
| Bone involvement | ||||
| None | 559 (76.7) | 46 (56.8) | 513 (79.2) | |
| Involvement, no fracture | 123 (16.9) | 23 (28.4) | 100 (15.4) | |
| Involvement, fracture | 47 (6.4) | 12 (14.8) | 35 (5.4) | |
| Biochemical parameters, n (%) | ||||
| ALP level, high | ||||
| No | 593 (82.8) | 65 (81.3) | 528 (83.0) | |
| Yes | 123 (17.2) | 15 (18.8) | 108 (17.0) | |
| Calcium level | ||||
| Low | 9 (1.3) | 1 (1.3) | 8 (1.3) | |
| Normal | 600 (87.1) | 69 (88.5) | 531 (86.9) | |
| High | 80 (11.6) | 8 (10.3) | 72 (11.8) | |
ALP, alkaline phosphatase; ECOG, Eastern Cooperative Oncology Group; IPI, international prognostic index.
*Missing data were excluded from denominators.
Bone-protective medication includes vitamin D, calcium, and/or bisphosphonates.
Predisposing history defined by known osteoporosis, osteopenia, RA, or prior fracture.
The median age across all included patients was 77 years (range, 70-96 years) and the median follow-up was 18 months. Sex balance was equal (male 48%). Sixty-eight percent had an Eastern Cooperative Oncology Group performance status of 0 to 1. The median number of R-CHOP cycles was 6 (range, 1-8). Twenty-six percent (186 of 712) of patients received prephase steroids. Prednisolone doses ranged from 40 mg/m2 (7 centers, 469 patients) to 100 mg fixed dose (3 centers, 260 patients). The median BMI was 25.3 kg/m2 (range, 13.6-50.7 kg/m2). Sixty-four patients (8.9%) had a historic fracture before DLBCL diagnosis, and 9.8% had a history of osteopenia or osteoporosis, 4.9% were current smokers, 4.2% had a history of RA, 11.7% had type 2 diabetes, and 4.5% had a documented history of excess alcohol use. At baseline, 23.3% had positron emission tomography (PET) or CT imaging–assessed cortical DLBCL bone involvement, including 47 (6.5%) resulting in a pathologic fracture.
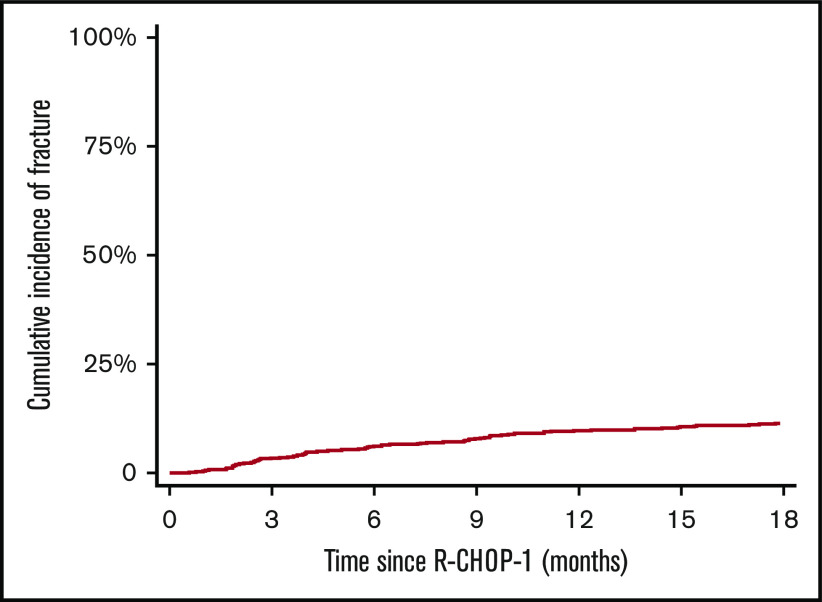
Overall, there were 81 fractures documented within 18 months of follow-up; 44 events before 6 months and 37 between 6 and 18 months’ follow-up. The cumulative fracture incidence was 6.2% (95% CI, 4.7-8.2) at 6 months, 9.7% (95% CI, 7.8-12.1) at 12 months, and 11.4% (95% CI, 9.3-14.0) at 18 months, respectively (Figure 1).
Figure 1.
Cumulative incidence of fracture up to 18 months from R-CHOP1 for all patients.
Fracture events
Fracture sites and clinical data regarding the 81 patients with fracture events are shown in Table 2. Sixty-two (78.5%) patients had a single fracture site, and 14 (17.3%) patients had 2 sites. Thoracic vertebrae and lumbar vertebrae were the dominant fracture sites occurring in 40.7% and 32.1% of patients, respectively. Fifty-three (65.4%) patients had a vertebral fracture of at least 1 anatomic level. Less common sites included femur (12.3%), rib/clavicle (8.6%), forearm/wrist (6.2%), and humerus (4.9%). Baseline DLBCL bone involvement was present in 35 (43.2%) of 81 patients with fracture; in 14 of these, fracture occurred at a site of initial DLBCL bone involvement. Just over one-half (51.9%) of fractures were identified by investigation of local symptoms, and 48.1% were found incidentally on follow-up imaging. Twenty-nine (54.7%) of the 53 vertebral fractures were found incidentally. Low-impact trauma (a fall from standing height or less) preceded the fracture in 28.2% (22 of 78). Patients sought emergency hospital care in 37% (30 of 81), with 17.3% of patients requiring emergency hospitalization. Emergency surgery was required in 11.1% of cases, and opiate analgesia was required in 43.5% (27 of 62) of cases in which prescribing practice was known.
Table 2.
Details of fracture events during follow-up
| Variable | Patients with a fracture (N = 81) |
|---|---|
| Was the site of fracture involved at baseline?, n (%) | |
| Initial DLBCL bone involvement but not at fracture site | 21 (25.9) |
| Initial DLBCL bone involvement at subsequent fracture site | 14 (17.3) |
| No initial DLBCL bone involvement | 46 (56.8) |
| Multiple sites, n (%) | |
| 1 | 63 (77.8) |
| 2 | 14 (17.3) |
| 3 | 3 (3.7) |
| 4 | 1 (1.2) |
| Thoracic vertebrae, n (%) | |
| No | 48 (59.3) |
| Yes | 33 (40.7) |
| Lumbar vertebrae, n (%) | |
| No | 55 (67.9) |
| Yes | 26 (32.1) |
| Any vertebrae, n (%) | |
| No | 28 (34.6) |
| Yes | 53 (65.4) |
| Femur/acetabulum, n (%) | |
| No | 71 (87.7) |
| Yes | 10 (12.3) |
| Rib/clavicle, n (%) | |
| No | 74 (91.4) |
| Yes | 7 (8.6) |
| Forearm/wrist, n (%) | |
| No | 76 (93.8) |
| Yes | 5 (6.2) |
| Humerus, n (%) | |
| No | 77 (95.1) |
| Yes | 4 (4.9) |
| Shoulder/scapula, n (%) | |
| No | 78 (96.3) |
| Yes | 3 (3.7) |
| Sacrum/pubic rami, n (%) | |
| No | 78 (96.3) |
| Yes | 3 (3.7) |
| Ankle, n (%) | |
| No | 78 (96.3) |
| Yes | 3 (3.7) |
| What was the method of diagnosis?, n (%) | |
| Symptomatic | 42 (51.9) |
| Incidental | 39 (48.1) |
| Was A&E attendance or admission required due to fracture?, n (%) | |
| No | 51 (63.0) |
| Yes, A&E or other unplanned attendance | 16 (19.8) |
| Yes, admission | 14 (17.3) |
| Was there a history or trauma associated with the fracture?, n (%) | |
| No | 56 (71.8) |
| Yes, fall from standing or sitting | 22 (28.2) |
| Missing | 3 |
| Was surgery needed?, n (%) | |
| No | 72 (88.9) |
| Yes | 9 (11.1) |
| Were bisphosphonates used?, n (%) | |
| No | 51 (75.0) |
| Yes | 17 (25.0) |
| Unclear | 13 |
| Was opiate analgesia used?, n (%) | |
| No | 35 (56.5) |
| Yes | 27 (43.5) |
| Unclear | 19 |
A&E, accident and emergency department.
Univariable analysis
Univariable risk factors for fracture are displayed in supplemental Table 3 and are divided into 2 groups: all patients (N = 729) and cases with no missing observations (n = 554). Univariable risk factors include female sex (HR, 1.71; 95% CI, 1.09-2.70; P = .019), known osteopenia or osteoporosis (HR, 2.74; 95% CI, 1.58-4.73; P < .001), and history of RA (HR, 2.24; 95% CI, 1.03-4.87; P = .036). Patients with DLBCL bone involvement at baseline had an increased risk of fracture compared with those without involvement (HR, 2.41; 95% CI, 1.46-3.98), with a greater risk still in those with a DLBCL-related fracture at diagnosis and bone involvement (HR, 3.51; 95% CI, 1.86-6.63; P < .001). Patients with a fracture of any cause at baseline had an increased risk of a further fracture event compared vs those with no fracture (HR, 2.68; 95% CI, 1.52-4.71; P = .0014).
To dissect the relative influence of bone-protective medication (vitamin D, and/or calcium, and/or bisphosphonates) and the fracture risk of those with a predisposing medical history defined by osteoporosis, osteopenia, prior fragility fracture, or RA, patients were grouped into 4 categories: (1) patients with no predisposing history or medication (n = 534, used as reference group; HR, 1.00); (2) patients with a predisposing history not taking medication (n = 69); (3) patients taking medication with no predisposing history (n = 48); and (4) patients with a predisposing history on medication (n = 53). Patients with a predisposing history had an increased risk of fracture whether they did take supplementation (HR, 2.02; 95% CI, 1.07-3.81) or did not take supplementation (HR, 2.86; 95% CI, 1.55-5.28; P = .0015). Patients receiving prephase steroids (n = 128) exhibited a trend to increased risk of fracture (HR, 1.54; 95% CI, 0.97-2.43; P = .064) across all patients. Prephase steroids did meet significance as a fracture risk factor within the population with no missing data (P = .03) (ie, the cohort included in the multivariable analysis).
Multivariable analysis and fracture risk score
Multivariable analysis showed that 3 key independent variables were associated with increased fracture risk (Table 3). Patients with a predisposing history whether taking bone-protective medication (HR, 2.84; 95% CI, 1.49-5.43) or not (HR, 2.12; 95% CI, 1.08-4.16; P = .0042) were at higher risk. PET or CT imaging–assessed DLBCL bone involvement at baseline, both in association with a baseline pre–R-CHOP1 fracture (HR, 2.76; 95% CI, 1.37-5.58) and without a baseline fracture (HR, 2.28; 95% CI, 1.29-4.03), was an independent risk factor. Finally, use of prephase steroid was an independent risk factor (HR, 1.84; 95% CI, 1.11-3.05). An additional analysis was conducted with supplements split further into bisphosphates or calcium/vitamin D; univariable and multivariable analysis results remained the same (data not shown).
Table 3.
Multivariable analysis of risk factors for fracture
| Risk factor | Events/N | Univariable (complete cases) | Multivariable* | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Bone-protective medication† | ||||||
| No predisposing history‡ | 60/603 | 1.00 | .0022 | 1.00 | .0042 | |
| Predisposing history, no medication | 11/56 | 2.11 (1.08-4.12) | 2.12 (1.08-4.16) | |||
| Predisposing history and medication | 12/43 | 3.02 (1.58-5.76) | 2.84 (1.49-5.43) | |||
| DLBCL bone involvement | ||||||
| None | 46/559 | 1.00 | .001 | 1.00 | .0026 | |
| Involvement, no fracture | 18/94 | 2.23 (1.27-3.90) | 2.28 (1.29-4.03) | |||
| Involvement, fracture | 10/37 | 3.31 (1.65-6.63) | 2.76 (1.37-5.58) | |||
| Prephase steroid | ||||||
| No | 43/415 | 1.00 | .0320 | 1.00 | .021 | |
| Yes | 24/139 | 1.73 (1.05-2.84) | 1.84 (1.11-3.05) | |||
Variables included: age, sex, Eastern Cooperative Oncology Group performance status (0, 1, 2, and 3-4), BMI group, smoking status, type 2 diabetes, alcohol use history, previous steroids within 12 mo, alkaline phosphatase, calcium (low, normal, or high), bone-protective medication (4 groups including comorbidities as in supplemental Table 3), bone involvement (3 groups as per univariable analysis table), prephase steroid, and non-lymphoma fracture at baseline. Stepwise backward selection with a cutoff of 0.05 was used.
Bone-protective medication includes vitamin D, calcium, and/or bisphosphonates.
Predisposing history: known osteoporosis, osteopenia, RA, or prior fracture.
Multivariable using complete cases from the backward selection model.
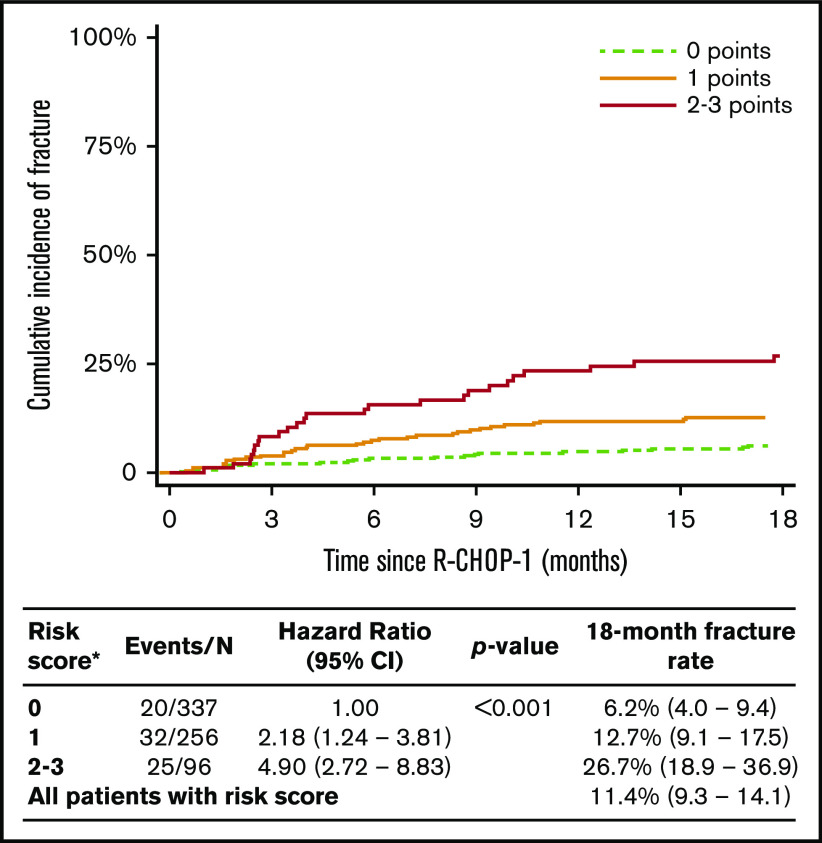
Given the broadly similar HR weighting to each independent risk factor, a simple scoring system (score, 0-3) was devised to further define 18-month fracture risk. A single point score was given to: (1) those with a predisposing history (eg, osteoporosis, osteopenia, prior fracture, RA); (2) DLBCL bone involvement at baseline; and (3) patients receiving prephase steroids. A risk score was subsequently calculated in 662 patients with complete data for these variables, of which there were 77 fracture events. Eighteen-month fracture cumulative incidence for these patients was 11.4% (95% CI, 9.3-14.1), suggesting this patient sample has a risk similar to that of the whole cohort. Patients with a score of 0 (n = 337), 1 (n = 256), and 2 or 3 (n = 96/n = 6) had an 18-month cumulative incidence of 6.2% (95% CI, 4.0-9.4), 12.7% (95% CI, 9.1-17.5), and 26.7% (95% CI, 18.9-36.9), respectively (Figure 2).
Figure 2.
Cumulative incidence of fracture up to 18 months from R-CHOP1 according to risk group in the model derived from multivariate analysis of fracture risk factors. *Creating a simple score based on one point for each risk factor (score range, 0-3). One point for history of predisposing medical condition (known osteoporosis, osteopenia, RA, or prior fracture) regardless of use of bone-protective medication; one point for DLBCL bone involvement at baseline, regardless of whether associated with DLBCL-related fracture at baseline; and one point for use of prephase steroids.
Discussion
To our knowledge, the current analysis is the largest published series reporting the incidence, identifiable risk factors, and characteristics of fractures occurring after R-CHOP in this specific age cohort of patients with DLBCL. We provide a detailed analysis of fracture events and comprehensive number of potential associated risk factors in consecutive patients included across a geographically wide range of district and tertiary referral centers over a 10-year period. We report a significant risk of fracture in early follow-up of 11.4%, highlighting this clinically relevant complication. A previous UK-based population analysis of fracture risk across 11.3 million people exhibited an increased risk in older individuals and female subjects.21 Male subjects between age 70 and 90 years had an incidence of 50 to 200 fractures per 10 000 patient-years. Female subjects of similar age had an incidence of 150 to 350 fractures per 10 000 patient-years. Broadly, across this whole population aged 70 to 90 years and assuming an equivalent sex balance, this equates to an annual risk of ∼2%. Although our data set has no specific control cohort, these data suggest that the risk following R-CHOP is considerably higher.
Many patients experienced substantial morbidity following these fracture events, with a significant number requiring opioid analgesia, surgery, or hospitalization. A previous smaller study reported a rate of 14% for vertebral fracture, as assessed by retrospective review of routine CT imaging during the first year after R-CHOP.6 In our data, thoracic and lumbar vertebral fracture accounted for only 61% of fractures, and incidental fractures on routine imaging accounted for only 48.1% of fractures, with 51.9% being symptomatic. The most clinically significant fractures as assessed by requirement for admission, surgery, or opioid analgesia were fractures of the hip or femoral shaft or humerus, which together accounted for 17.2% of events.
Current guidelines for GIO focus on long-term steroid use, owing to a paucity of data to define risk with pulsed high-dose steroid regimens such as R-CHOP. The 2017 American College of Rheumatology GIO guidance recommends any patient receiving prednisolone >2.5 mg/d for ≥3 months to be advised regarding lifestyle modifications, undergo BMD testing, vitamin D testing and replacement where indicated, and to check adequate calcium intake.11 Oral bisphosphonates are reserved for patients aged >40 years with a FRAX-defined 10-year risk of hip fracture >1% or risk of major osteoporotic fracture >10%.22 The National Comprehensive Cancer Network B-cell NHL guidelines sets a threshold of 20% 10-year risk of major osteoporotic fracture for bisphosphonate therapy,16 as do the UK Osteoporosis Guidelines, although in these, bisphosphonates are recommended without the need for BMD in those with a risk >25%.10 The effect of glucocorticoids on BMD and fracture risk is dose dependent, and therefore FRAX in this setting may be an underestimate in those on high doses of glucocorticoids, although risk adjustments for this population have been described.22 Our data show that risk of fracture in older patients receiving R-CHOP is sufficiently high to warrant routine assessment of risk factors, vitamin D levels, and calcium intake and consideration of BMD testing or intervention with bisphosphonates in higher risk patients.
We show that DLBCL bone baseline involvement, predisposing medical history, and prephase steroids are independent risk factors for subsequent fracture. Although female subjects are at higher risk, they are significantly more likely to have a history of osteoporosis or osteopenia and are also more likely to have a baseline fracture, and therefore sex did not remain as an independent risk factor. Although prospective and independent validation is required, we show for the first time that these risk factors can be used to provide a simple and usable predictive clinical scoring system. Patients with a score of 2 to 3 (14% of all patients) have a substantial fracture risk (18-month cumulative incidence 26.7% and approximately one-third of all events). If this score were validated, prospective identification of a high-risk population would allow targeting of oral bisphosphonates or more complex prophylatic interventions.
Evidence regarding the use of bisphosphonates in lymphoma is currently limited. Two previous small randomized studies have examined intravenous bisphosphonates. One study included 74 patients of all ages with newly diagnosed lymphoma of variable histologic subtypes and a baseline BMD ≥ −2.0 to receive oral calcium and vitamin D with or without zoledronate at enrollment and 6 months later. Although 21 patients were nonevaluable due to lymphoma progression, death, or other reasons, patients receiving zoledronate stabilized BMD compared with a BMD decrease in the control subjects (P < .05 at lumbar spine and bilateral femoral neck).23 The other study randomized 50 patients with advanced-stage lymphomas treated with chemotherapy to receive pamidronate or placebo; they reported statistically significant reductions in loss of lumbar spine BMD and vertebral fractures. This study also included a variety of histologies, and the majority of patients were treated with the more intensive ProMACE-CytaBOM regimen (prednisolone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, and methotrexate).24 These are relatively small studies in a broader clinical setting but provide some evidence of the efficacy of pamidronate in patients undergoing chemotherapy for lymphoma. A large randomized controlled trial would be required to fully answer the question of the value and safety of prophylactic interventions such as bisphosphonates, which is not yet defined in this group.25 A small randomized study comparing alendronate vs placebo in patients across all ages receiving glucocorticoid-containing immunochemotherapy at initial diagnosis or relapse is ongoing (EudraCT #2015-005688-18).
Data specific to R-CHOP–treated patients are valuable because current cancer-specific guidance is derived from data published in patients treated with long-term lower steroid doses.16 Whether these data can be extrapolated to R-CHOP use in other therapeutic settings, different histologies, and across different age categories has not been specifically examined here and remains an open question.
It is unclear from our data which components of the R-CHOP or attenuated R-CHOP regimen contribute most to fracture risk; a comparison across regimens would be necessary to resolve this question. Whether this risk can be extrapolated to R-CHOP use in other therapeutic settings, different histologies, and across age categories also remains unresolved. Mechanistically, however, steroids are likely to play a predominant role, and it is notable that prephase steroids contribute significantly in our series. We did not examine the role of relative dose intensity of chemotherapy agents within R-CHOP, and in particular we were not able to collect detailed information on cumulative steroid dose, including prephase steroids within our series, which may be of relevance and should be considered in future studies in this area.
Our data suggest that prephase steroids are an independent risk factor for fracture events. This novel finding is important in developing our understanding of fracture risk. Although the multivariable analysis accounts for many of the typical reasons why prephase steroids might be used, potential unmeasured confounding factors such as tumor proliferation, organ compromise, or frailty as assessed by more detailed measures may be relevant. Similarly, we did not examine lactate dehydrogenase independently from the international prognostic index or serum albumin, which were reportedly associated with increased BMD loss at the lumbar spine and total hip, respectively, in a cohort of 41 patients with lymphoma undergoing chemotherapy; only 24 of these patients, however, had high-grade B-cell lymphoma.26 We did not collect specific details on prephase indications which vary among clinicians.
The beneficial impact of prephase steroids in addition to vincristine has been shown in two LYSA attenuated CHOP plus anti-CD20 monoclonal antibody phase 2 trials in patients aged >80 years.4,27 The introduction of prephase therapy contributed to a reduction of treatment-related mortality from 12% to 0% in patients with otherwise comparable baseline characteristics. Prephase steroids has rightly become standard practice for selected patients (in our series, 26%), and we believe this approach continues to play a key role in the early management of DLBCL. We do not advocate excluding patients from prephase therapy but rather that consideration be given to their fracture risk when prephase therapy is used.
A further relevant consideration is the role of factors other than bone health in determining fracture risk after R-CHOP in elderly patients. In 28% of fracture events reported in our data, fractures were associated with falls, and unmeasured potential risk factors such as unresolved peripheral neuropathy, deconditioning, or reduced performance status after chemotherapy toxicity may be of significance. The relationship between these factors and fracture risk is, however, less clearly defined than for the included risk factors, but these are nevertheless pertinent issues to consider in patient care.
Our data have other limitations inherent to a retrospective study, including missing data, the potential for unknown confounders, and the possibility of medical record and radiologic misinterpretation. It is possible that some asymptomatic vertebral fractures were not included in follow-up imaging reports. Bone involvement was assessed only by local radiology reports without histologic confirmation of concordant high-grade lymphoma. PET-CT imaging was not used in all patient cases, and radiology was not centrally reviewed. Our analysis was also limited by a lack of routine measurement of vitamin D levels, precluding examination of vitamin D deficiency as a risk factor, and it was not possible to assess compliance with bone-protective medication. BMD was also not routinely measured, and there are likely to have been cases in which undiagnosed osteoporosis was a factor contributing to fracture risk. It was also not possible to retrospectively determine all the factors required to accurately determine FRAX score before R-CHOP. A future prospective study would be required to address many of these issues. Finally, in our study, 14 of 81 fracture events occurred at a site of DLBCL bone involvement at baseline, in which the mechanism of increased fracture risk may be different from that in patients without DLBCL bone involvement.
Despite these limitations, our data clearly show a high risk of fracture early in follow-up for older patients treated with R-CHOP or attenuated R-CHOP associated with significant morbidity. A particularly high-risk group can be defined by routinely available clinical data. We conclude that careful attention to bone health is warranted in older patients undergoing R-CHOP or similar immunochemotherapy, and we welcome further studies to better define the most effective strategies to reduce the risk of fracture.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
T.A.E. acknowledges funding from the Julian Starmer‐Smith Lymphoma Fund. G.P.C. and T.A.E. acknowledge support by the National Institute for Health Research Oxford Biomedical Research Centre Programme. G.P.C. and S.B. acknowledge support from the Oxford CRUK Experimental Cancer Medicine Centre.
The views expressed are those of the authors and not necessarily those of the funding bodies.
Footnotes
Presented in abstract form at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2019.
All requests for original data should be submitted to the corresponding author (Toby A. Eyre; e-mail: toby.eyre@ouh.nhs.uk).
Authorship
Contribution: T.A.E. designed and coordinated the study; S.B., T.A.E., H.P., J.M.O., U.K., B.T., J.B., L.C., J. Willan, J. Wolf, A.G., T.E., D.G.-W., and C.H. collected the majority of the data; T.A.E., P.F., R.L., N.S., K.M.L., G.P.C., and J.K. managed patients on the study and oversaw data collection; A.A.K. performed the statistical analysis; and T.A.E. and S.B. wrote the manuscript, which all authors critically reviewed.
Conflict-of-interest disclosure: G.P.C. declares honoraria for speaker/consultancy work for Celleron, Roche, Takeda, Gilead, BMS, MSD, and Pfizer; and received research funding from Celgene, BMS, MSD, Celleron, Pfizer, and Amgen. T.A.E. received honoraria from Roche, Gilead, AbbVie, and Janssen; holds an advisory board role for Gilead, AbbVie, and AstraZeneca; has received research funding from Gilead; and traveled to conferences for Takeda, AbbVie, and Janssen. N.S. declares advisory/consulting roles for AbbVie, Janssen, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Toby A. Eyre, Department of Haematology, Oxford University Hospitals NHS Trust, Cancer and Haematology Centre, Churchill Hospital, Oxford OX3 7LE, United Kingdom; e-mail: toby.eyre@ouh.nhs.uk.
References
- 1.Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK’s Haematological Malignancy Research Network. Br J Cancer. 2015;112(9):1575-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Schubert J, Ziepert M, et al. ; German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) . Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105-116. [DOI] [PubMed] [Google Scholar]
- 3.Eyre TA, Martinez-Calle N, Hildyard C, et al. Impact of intended and relative dose intensity of R-CHOP in a large, consecutive cohort of elderly diffuse large B-cell lymphoma patients treated with curative intent: no difference in cumulative incidence of relapse comparing patients by age. J Intern Med. 2019;285(6):681-692. [DOI] [PubMed] [Google Scholar]
- 4.Peyrade F, Jardin F, Thieblemont C, et al. ; Groupe d’Etude des Lymphomes de l’Adulte (GELA) investigators . Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12(5):460-468. [DOI] [PubMed] [Google Scholar]
- 5.Cabanillas ME, Lu H, Fang S, Du XL. Elderly patients with non-Hodgkin lymphoma who receive chemotherapy are at higher risk for osteoporosis and fractures. Leuk Lymphoma. 2007;48(8):1514-1521. [DOI] [PubMed] [Google Scholar]
- 6.Svendsen P, Shekhrajka N, Nielsen KL, et al. R-CHOP(-like) treatment of diffuse large B-cell lymphoma significantly reduces CT-assessed vertebral bone density: a single center study of 111 patients. Leuk Lymphoma. 2017;58(5):1105-1113. [DOI] [PubMed] [Google Scholar]
- 7.Baech J, Hansen SM, Jakobsen LH, et al. Increased risk of osteoporosis following commonly used first-line treatments for lymphoma: a Danish Nationwide Cohort Study. Leuk Lymphoma. 2020;61(6):1345-1354. [DOI] [PubMed] [Google Scholar]
- 8.Majumdar SR, Morin SN, Lix LM, Leslie WD. Influence of recency and duration of glucocorticoid use on bone mineral density and risk of fractures: population-based cohort study. Osteoporos Int. 2013;24(9):2493-2498. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A; National Osteoporosis Guideline Group . Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19(10):1395-1408. [DOI] [PubMed] [Google Scholar]
- 10.Compston J, Cooper A, Cooper C, et al. ; National Osteoporosis Guideline Group (NOGG) . UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69(8):1095-1110. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817-1826. [DOI] [PubMed] [Google Scholar]
- 13.Saag KG, Emkey R, Schnitzer TJ, et al. ; Glucocorticoid-Induced Osteoporosis Intervention Study Group . Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339(5):292-299. [DOI] [PubMed] [Google Scholar]
- 14.Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44(1):202-211. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res. 1998;13(9):1431-1438. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network, Inc. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas, version 1.2020. Plymouth Meeting, PA: National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 26 August 2020.
- 17.Fox C, Edwards MH, Dennison EM, Cooper C. Personal and societal burden of osteoporotic fractures. Clin Rev Bone Miner Metab. 2015;13(2):53-60. [Google Scholar]
- 18.Oleksik A, Lips P, Dawson A, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000;15(7):1384-1392. [DOI] [PubMed] [Google Scholar]
- 19.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. June, 2000. J Bone Miner Res. 2005;20(8):1487-1494. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 21.Curtis EM, van der Velde R, Moon RJ, et al. Epidemiology of fractures in the United Kingdom 1988-2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanis JA, Johansson H, Oden A, McCloskey EV. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int. 2011;22(3):809-816. [DOI] [PubMed] [Google Scholar]
- 23.Westin JR, Thompson MA, Cataldo VD, et al. Zoledronic acid for prevention of bone loss in patients receiving primary therapy for lymphomas: a prospective, randomized controlled phase III trial. Clin Lymphoma Myeloma Leuk. 2013;13(2):99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SH, Lim SK, Hahn JS. Effect of pamidronate on new vertebral fractures and bone mineral density in patients with malignant lymphoma receiving chemotherapy. Am J Med. 2004;116(8):524-528. [DOI] [PubMed] [Google Scholar]
- 25.Suresh E, Pazianas M, Abrahamsen B. Safety issues with bisphosphonate therapy for osteoporosis. Rheumatology (Oxford). 2014;53(1):19-31. [DOI] [PubMed] [Google Scholar]
- 26.Paccou J, Merlusca L, Henry-Desailly I, et al. Alterations in bone mineral density and bone turnover markers in newly diagnosed adults with lymphoma receiving chemotherapy: a 1-year prospective pilot study. Ann Oncol. 2014;25(2):481-486. [DOI] [PubMed] [Google Scholar]
- 27.Peyrade F, Bologna S, Delwail V, et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: an open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematol. 2017;4(1):e46-e55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.