Abstract
Inflammatory myofibroblastic tumor (IMT) is an uncommon, usually benign, mesenchymal tumor. IMT affects people of all ages, but it more commonly occurs in children and adolescents. Also, it has the potential to arise in any part of the body, though, it frequently develops in the lungs and mesentery. In this report, we discuss a rare clinical manifestation of mesenteric IMT presented as intussusception of the small intestine in a 7-year-old child.
INTRODUCTION
Inflammatory myofibroblastic tumor (IMT) is a rare borderline mesenchymal tumor, histologically made up of proliferated fibroblasts and myofibroblasts admixed with various types of inflammatory cells. IMT was previously known as inflammatory pseudotumor, plasma cell granuloma, omental mesenteric myxoid hamartoma and inflammatory fibrosarcoma, which reflects a heterogeneity in the clinicopathological findings of these tumors [1]. In the last three decades, a subset of these lesions was proved to be neoplastic, and a clonal chromosomal abnormality, especially of the anaplastic lymphoma kinase (ALK) gene on chromosome 2p23, was documented. ALK gene rearrangement is more common in children and young adults than in old patients and ranges from 33 to 67% [2–4]. IMTs have a local recurrence rate of ~10–25% and a low risk of metastasis, ~5% [2, 5]. People of any age can be affected by IMT, but it tends to occur in children and young adults [2]. Its etiology remains unknown, though it is indicated to occur secondary to viral infection, trauma or surgery [6]. The tumor was first reported in the lungs and was later described in other organs [1, 2]. Clinical manifestations of IMTs vary depending on the size and location of the tumor, but generally, there are no specific symptoms and the definitive diagnosis of IMT is established on pathological examination.
CASE REPORT
A 7-year-old child was hospitalized due to an inability to pass feces and gas. The patient's mother mentioned that he has suffered from diffuse abdominal pain, fever (unmeasured) and vomiting for 3 days.
On physical examination, the patient’s general condition was stable; he was pale and was suffering from abdominal pain, yet he had no fever or chills. Abdominal examination revealed localized tenderness in the right iliac fossa. He had no other complaints, and his medical record was clear except for an orchiopexy 2 years ago.
Plain abdominal radiography revealed signs that suggested a small intestinal intussusception, and the ultrasound examination revealed further signs of intussusception (target sign), as well as signs of appendicitis. Laboratory studies showed elevated white blood cells (WBC) count and c- reactive protein (CRP) level (WBC = 15.7 × 109/L, Neutrophils = 84.9%, CRP = 16 mg/L).
Laparotomy was performed, and the patient’s abdomen was explored, revealing an intussusception ~70 cm from the ileocecal valve. During the correction of the intussusception, the surgeon noticed a segmental enlargement of the ileum with the mesentery being focally depressed and palpated there an intraluminal mass (Fig. 1). The enlarged intestinal segment and the appendix were resected.
Figure 1.
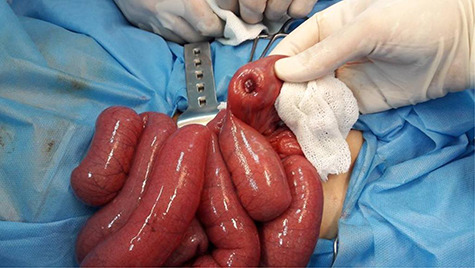
Exploration of the ileum revealed a focally depressed region where a small intraluminal mass was palpated.
The resected segment measuring 3 × 1.5 × 2 cm was opened, revealing a firm, sessile polypoid mass measuring 2.5 × 2 × 1.5 cm. The cut-section of the tumor was whitish-grey with a whorled appearance (Fig. 2). Microscopic examination of the H&E-stained sections revealed the proliferation of interlacing bundles of spindle cells containing large elongated and vacuolated nuclei with prominent nucleoli. Sparse mitotic figures were observed. The vascularized stroma contained a mix of inflammatory cells (lymphocytes, plasmocytes, neutrophils and eosinophils) (Fig. 3).
Figure 2.
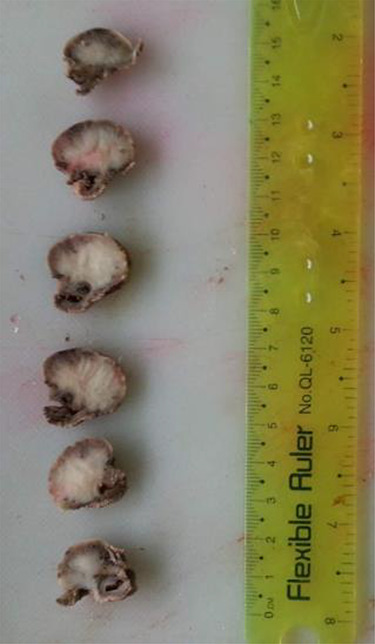
Gross section of the tumor reveals whitish-grey surface with whorled appearance.
Figure 3.
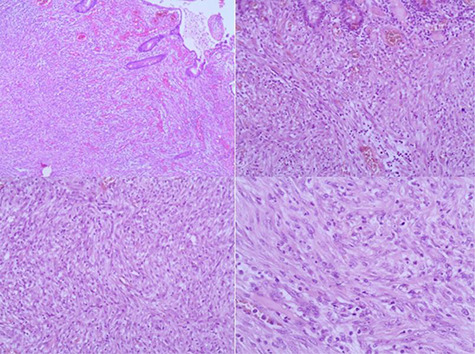
(A) Microscopic findings of IMT. (B, C and D) Fibroblasts and myofibroblasts intermixed with different types of inflammatory cells on a background of abundant blood vessels. The tumor cells containing large elongated and vacuolated nuclei with prominent nucleoli. (Hematoxylineosin, original magnification ×40 [A] ×100 [B, C] ×200 [D]).
The spindle cell proliferation extended through all layers of the intestinal wall and projected as a polypoid mass into the lumen of the ileum and was covered by an ulcerated mucosa.
Immunohistochemically, the spindle cells were positive for ALK1 and vimentin, with weak positivity for smooth muscle actin (SMA). C-kit, CD34, Desmin, epithelial membrane antigen (EMA) and Cytokeratin (CK) were all negative in the tumor cells (Fig. 4). Finally, IMT was diagnosed based on the immunohistochemical results and routine microscopic findings. The stained section of the appendix vermiformis showed mild acute inflammation with intraluminal Enterobius vermicularis.
Figure 4.
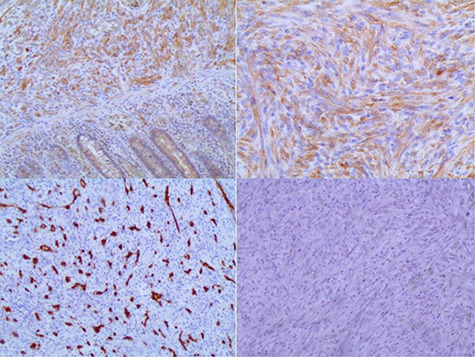
(A) Positivity of ALK1 in the tumor cells. (B) Vimentin is diffusely positive in the tumor cells. (C) Negativity of CD34 in the tumor cells. (D) Tumor cells are negative for CD117 (Immunohistochemistry, original magnification ×100 [A, C, D] ×200 [B]).
DISCUSSION
IMT is a rare mesenchymal tumor that usually presents as firm, well-circumscribed and multi-nodular masses ranging in size from 0.4 to 36 cm. The tumor could be polypoid, sessile or pedunculated, and is covered with either eroded or ulcerated mucosa [2]. The tumor in our 7-year-old patient had presented as an intraluminal polypoid mass.
Intestinal IMTs lack specific clinical manifestations, and they are usually accompanied by weight loss, malaise and a variety of laboratory abnormalities [7]. Several other atypical presentations have been reported in the literature such as portal venous thrombosis, intussusceptions and anemia [2]. The intussusception of the small intestine was the primary presentation in our patient. Microscopically, the main proliferating cells in IMTs are myofibroblasts and fibroblasts, and immunohistochemically, they show variable positivity for SMA, and vimentin with negativity for CD34, Desmin, S100 and CD117 [8]. The abnormalities of the ALK gene lead to the expression of ALK1 and p80 in the spindle cell components, which can aid in diagnosing these tumors and differentiating them from other tumorous or tumor-like lesions [2, 5]. In our case, the tumor cells were positive for ALK1 and vimentin with weak positivity for SMA. CD117, CD34, Desmin, EMA and CK were negative in the tumor cells.
In addition to other soft tissue tumors that can arise in the mesentery and intestinal wall, the differential diagnoses include other lesions that present as intestinal polyps. All epithelial tumors and hamartomatous lesions are easily excluded as they arise from mucosal membranes, unlike IMTs. A constellation of clinical and microscopic findings in addition to the results of immunohistochemical stains aid in ruling out the other mesenchymal tumors and tumor-like lesions, such as gastrointestinal stromal tumors, mesenteric fibromatosis and sclerosing mesenteritis [2].
In most cases, complete surgical resection is adequate; however, in cases where the tumor has locally recurred or metastasized, other treatment modalities might be used such as chemotherapy, radiation treatment, nonsteroidal anti-inflammatory drugs, steroid and cyclosporin-A [2, 5]. After 2 years of the surgery, our patient is in well condition without any complaints.
CONCLUSION
Diagnosing mesenteric IMT as a cause of intestinal intussusceptions can be very challenging due to the rarity of the tumor and the unusual presentation. The pathological examination remains the essential tool for accurate diagnosis of IMT in all cases.
Contributor Information
Zeina Alabbas, Department of Pathology, Tishreen University, Lattakia, Syria.
Mohsen Issa, Al Andalus Private University, Tartus, Syria.
Ammar Omran, Department of Pediatric Surgery, Tishreen University, Lattakia, Syria.
Rana Issa, Department of Pathology, Tishreen University, Lattakia, Syria.
REFERENCES
- 1. Yi E, Aubry M-C. Pulmonary pseudoneoplasms. Arch Pathol Lab Med 2010;134:417–26. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhary P. Mesenteric inflammatory myofibroblastic tumors. Ann Gastroenterol 2015;28:49–54. [PMC free article] [PubMed] [Google Scholar]
- 3. Cessna MH, Zhou H, Sanger WG, Perkins SL, Tripp S, Pickering D, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol 2002;15:931–8. [DOI] [PubMed] [Google Scholar]
- 4. Snyder CS, Dell 'Aquila M, Haghighi P, Baergen RN, Suh YK, Yi ES. Clonal changes in inflammatory pseudotumor of the lung: a case report. Cancer 1995;76:1545–9. [DOI] [PubMed] [Google Scholar]
- 5. Karnak I, Senocak ME, Ciftci AO, Cağlar M, Bingöl-Koloğlu M, Tanyel FC, et al. Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg 2001;36:908–12. [DOI] [PubMed] [Google Scholar]
- 6. Groenveld RL, Raber MH, Oosterhof-Berktas R, Eijken E, Klaase JM. Abdominal inflammatory myofibroblastic tumor. Case Rep Gastroenterol 2014;8:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Appak YÇ, Sahin GE, Ayhan S, Taneli C, Kasırga E. Inflammatory myofibroblastic tumor of the colon with an unusual presentation of intestinal intussusception. European J Pediatr Surg Rep 2014;2:54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical Spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol 2000;13:1134–42. [DOI] [PubMed] [Google Scholar]


