Abstract
Objective
To compare survival, readmissions, and end-of-life care after palliative procedures vs. medical management for malignancy-associated bowel obstruction (MBO).
Background
MBO is a late complication of intra-abdominal malignancy for which surgeons are frequently consulted. Decisions about palliative treatments, which include medical management, surgery, or venting gastrostomy tube (VGT), are hampered by the paucity of outcomes data relevant to patients approaching the end of life.
Methods
Retrospective study using 2001-2012 SEER-Medicare data of patients ≥65 years of age with stage IV ovarian or pancreatic cancer who were hospitalized for MBO. Multivariate competing-risks regression models were used to compare the following outcomes: survival, readmission for MBO, hospice enrollment, ICU care in the last days of life, and location of death in an acute care hospital.
Results
Median survival after MBO admission was 76 days (IQR 26-319 days). Survival was shorter after VGT (38 days [IQR 23-69]) than medical management (72 days [23-312]) or surgery (128 days [42-483]). As compared to medical management, patients treated with VGT had fewer readmissions (subdistribution hazard ratio 0.41[0.29-0.58]), increased hospice enrollment (1.65[1.42-1.91]), and less ICU care (0.69[0.52-0.93]) and in-hospital death (0.47[0.36-0.63]). Surgery was associated with fewer readmissions (0.69[0.59-0.80]), decreased hospice enrollment (0.84[0.76-0.92]), and higher likelihood of ICU care (1.38[1.17-1.64]).
Conclusions
VGT is associated with fewer readmissions and lower intensity healthcare utilization at the end of life than medical management or surgery. Given the limited survival, regardless of management, hospitalization with MBO carries prognostic significance and presents a critical opportunity to identify patients’ priorities for end-of-life care.
Keywords: End-of-life care, palliative surgery, geriatric patients, cancer, malignant bowel obstruction, palliative care
MINI ABSTRACT
This is a retrospective population-based comparison of survival, readmissions, and end-of-life care after palliative treatment for malignancy-associated bowel obstruction with medical management, surgery, or venting gastrostomy. Venting gastrostomy was associated with fewer readmissions and lower intensity health care utilization at the end of life than medical or surgical management.
BACKGROUND
Among patients with cancer, the estimated prevalence of malignancy-associated bowel obstruction (MBO) is 3-15%,1 including up to 51% in ovarian cancer and up to 28% in cancers of the gastrointestinal tract.1–4 MBO is among the most common palliative indications for surgical consultation5 and typically signifies a poor prognosis, with mean survival of 3-8 months in surgical cases and 4-5 weeks in those with inoperable MBO.1, 4, 6–8 Managing the considerable symptom burden associated with MBO frequently requires hospitalization and contributes to high-intensity healthcare utilization.1, 6–10 In light of national efforts to address the poor quality and high cost of care near the end of life,11 it is critical to understand the relationship between treatment for complications of terminal cancer, such as MBO, and end-of-life (EOL) care.
The primary objectives of palliative treatment for MBO are to relieve suffering and to support quality of life.12 While medical management is the mainstay of treatment, prior studies have reported beneficial outcomes after treatment for MBO with surgery or venting gastrostomy tube (VGT), including relief of obstructive symptoms, nasogastric tube removal, ability to tolerate oral diet, and discharge to home.1, 3, 4, 12–17 However, the current evidence is predominantly derived from single-institution experiences, and other relevant outcomes related to high-quality end-of-life care, such as hospice enrollment, avoidance of ICU care in the last days of life, and death outside an acute care hospital, have not been studied in a national population.4, 17 Despite the prevalence of MBO and its importance as a clinical marker for prognosis that can be measured in months, the association between treatment for MBO and subsequent survival, healthcare utilization, and type of EOL care patients receive remains poorly understood. As a consequence, clinical decisions are hampered by the paucity of data from a national population about outcomes relevant to patients approaching the end of life.4, 12, 18, 19
To address these knowledge gaps, the present study sought to use data from a large national population linked to Medicare Claims to compare the following outcomes after treatment for MBO among patients with stage IV ovarian or pancreatic cancer: 1) survival; 2) readmission for MBO; 3) EOL care outcomes, including hospice enrollment, ICU care in the last days of life, and location of death in an acute care hospital.
METHODS
This study was approved by the Partners Human Research Committee.
Study design and data source
This retrospective study used data from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registry linked with Medicare claims data. The SEER registry is a large clinical database, which includes approximately 26% of the U.S. population.20 Linkage of the SEER registry with Medicare claims permits longitudinal analysis of health services utilization. As such, the SEER-Medicare linked dataset is uniquely suited to examine longitudinal outcomes of medical care in patients with cancer. Among patients reported by SEER registries as being diagnosed with cancer at age 65 years or older, 94% were matched with Medicare enrollment records.21 The Patient Entitlement and Diagnosis Summary File (PEDSF) was used to identify primary cancer site, month and year of diagnosis, race, sex, Medicare enrollment, and date of death. Medicare claims from the Medicare Provider and Review (MedPAR), Outpatient, and Hospice files were linked to SEER to extract diagnosis and treatment data based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (eTable 1). Hospital characteristics were obtained from the SEER-Medicare Hospital file.
Study cohort
There were three separate analytic cohorts used for this study. All cohorts included Medicare beneficiaries ≥ 65 years who: 1) had ovarian or pancreatic cancer diagnosed at stage IV in 2001-2011, 2) were continuously enrolled in Medicare Parts A and B without HMO coverage, and 3) were hospitalized for a bowel obstruction subsequent to cancer diagnosis. Medicare claims do not specify the cancer stage at the time of hospital admission, and patient prognosis could impact choice of treatment for MBO as well as care at the end of life. Therefore, the cohort was restricted to patients who were diagnosed at stage IV in order to analyze the associations between treatment and outcomes among those with a more predictable disease trajectory. The most common malignancies associated with MBO are colon (25-40%), ovarian (16-29%) gastric (6-19%) and pancreatic cancers (6-13%);1, 3, 15 however, patients with obstructing colorectal primary lesions may also be candidates for curative resection, and prior therapy or anatomic location of gastric malignancies may preclude treatment with VGT. Therefore, this study focused on patients with ovarian and pancreatic cancer diagnoses. Hospital admissions for MBO were identified based on prior epidemiological studies of patients with MBO using SEER-Medicare data.7, 8, 22 All ICD-9-CM codes used for cohort selection and variable definitions appear in eTable 1.
Patients were excluded from all analytic cohorts if they were missing either the month or year of cancer diagnosis, were diagnosed at autopsy, underwent both surgery and VGT during the same hospital stay, underwent gastrostomy placement as an outpatient procedure, or underwent gastrostomy placement as an inpatient procedure during a hospital admission in which bowel obstruction was not listed as a diagnosis.
For the first study aim, survival after the first MBO admission after cancer diagnosis was analyzed among all patients in the cohort. For the second study aim—which was to compare readmissions for MBO based on treatment during the first MBO admission—a second analytic cohort was created from the survival cohort, excluding patients who died in-hospital during their first MBO admission.
To compare EOL care after treatment for MBO, a third analytic cohort was created from the survival cohort, including only patients who died on or before December 31, 2012. Patients were categorized based on treatment with surgery or VGT at any MBO admission (including the first MBO admission or readmissions for MBO). If patients had more than one procedure for MBO, the last procedure prior to death was presumed to have the most proximate impact on EOL care and was used and the MBO admission in which the procedure occurred served as the index MBO admission for analyses of EOL care outcomes. The comparison group consisted of patients who did not undergo either procedure, with their first MBO admission serving as the index MBO admission for analysis of EOL care outcomes.
Variables
Patient characteristics
Demographic characteristics included age (stratified into three groups as 65-74 years, 75-84 years, or ≥ 85 years), sex, and race (categorized as white, black, or other/unknown). A modified Charlson Comorbidity Index (CCI) was calculated based on diagnosis codes for 15 disease conditions from the Deyo adaptation for administrative data, omitting weighted scores for cancer and metastatic cancer diagnoses.23 The weighted summary score was then used to compute CCI scaled from 0-2, with 2 representing greater comorbidity. A single primary malignancy and its stage at diagnosis was identified for each patient. For three patients who had both qualifying malignancies, the later stage diagnosis served as the primary malignancy in analyses. In addition, palliative care consultation during the hospital admission was identified using claims codes for encounter for palliative care (ICD-9-CM V66.7).
Hospital Characteristics
Prior studies have identified regional and hospital-related characteristics which contribute to variation in intensity and surgical care in the year before death.24 To account for these factors, the following hospital characteristics were identified: U.S. census region (Northeast, Midwest, South, or West), hospital size (< 400 beds or ≥ 400 beds), and teaching status (teaching or non-teaching).
Treatment for MBO
The primary independent variable was treatment for MBO with medical management, surgery, or VGT. Based on prior studies, bowel obstruction surgery was identified using procedure claims for gastro-enterostomy, entero-enterostomy, bowel resection, enterostomy, or lysis of peritoneal adhesions.7, 8 Absent a secondary claim, laparoscopy or laparotomy as a standalone procedure did not qualify as surgery for MBO.7 VGT was identified based on procedure claims for percutaneous or open surgical gastrostomy during a MBO admission. Claim codes do not provide the indication for gastrostomy placement (e.g. feeding vs. venting). However, based on prior work demonstrating that 95% of gastrostomy procedures for patients with cancer during hospitalization for bowel obstruction are indicated for venting (Lilley EJ, Columbus AB, Cooper Z. Inferring palliative intent from administrative data: Validation of a claims-based case definition for venting gastrostomy tube. Under review at Journal of Pain and Symptom Management), gastrostomy placement during a MBO admission was presumed to represent VGT.
Outcomes
Mortality rate in-hospital and at 30, 90, and 180-days were determined after the first MBO admission. Survival duration was the number of days after the first MBO admission until the date of death or December 31, 2012. Among patients discharged alive from the first MBO admission, readmission for MBO was a binary outcome whereby any subsequent MBO admission was counted as a readmission. Time, in days, from the date of discharge from the first MBO admission until readmission for MBO, death, or December 31, 2012 was determined for each patient.
EOL care outcomes included: 1) enrollment in hospice, 2) ICU care in the last days of life, and 3) location of death in an acute care hospital. ICU care in the last days of life was defined as ICU stay during the 30 days prior to death or during the period from admission to death for those who survived less than 30 days after admission to the index MBO admission. The lack of validated quality metrics for outcomes after palliative surgery remains an important measurement gap. We chose these outcomes based on other studies in cancer patients which found that earlier hospice enrollment, avoidance of ICU care at the end of life, and death outside of an acute care hospital were associated with favorable perceptions of EOL care following the death of a family member with advanced cancer, 25 and studies in the general population showing that increasing intensity of EOL care is discordant with most Americans preferences for death and dying.11, 26 In addition, Earle and colleagues27 proposed claims-based measures of EOL care intensity as quality indicators for patients which cancer, including the proportion of patients with cancer who are not enrolled in hospice prior to death and the proportion admitted to an ICU in the last 30 days of life. These have been endorsed by the National Quality Forum28 and are high priority measures in the Centers for Medicare and Medicaid (CMS) Merit-based Incentive Payment System (MIPS).29 Location of death in a hospital was chosen as a third EOL care outcome because most Americans state a preference to die at home.27, 30
Analyses
Patient and hospital characteristics were summarized and compared based on treatment at the first MBO admission using Chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables. Given the small number of patients who received palliative care consultation, this variable was omitted from regression models. Survival after the first MBO admission was analyzed using multivariable Cox proportional hazards regression and adjusting for patient covariates, hospital covariates, and time from cancer diagnosis until the first MBO admission. Following Fine and Gray’s proportional subdistribution hazards model,31 subdistribution hazards analysis was performed using multivariable competing risks regression models and adjusting for patient and hospital covariates to examine the association between treatment and readmission for MBO and EOL care outcomes, accounting for the competing risk of death.31–33 Plots of cumulative incidence estimates were used to illustrate these associations.
Based on the finding that much of the morbidity occurred during the MBO admission in which treatment with surgery or VGT occurred, a sensitivity analysis was performed, excluding patients who died in-hospital during their index MBO admission.
Analyses were performed using SAS/STAT, version 9.3 (SAS Institute Inc., Cary, NC) and Stata, version 14.0 (StataCorp, College Station, TX) where α = 0.05.
RESULTS
Survival after first MBO admission
From 2001-2012, 35,606 Medicare beneficiaries were diagnosed with pancreatic or ovarian cancers at stage IV, of whom 21% of patients with ovarian cancer and 5% of patients with pancreatic cancer were hospitalized with bowel obstruction after cancer diagnosis: 118 patients were excluded based on an ineligible gastrostomy procedure, 118 patients were excluded because they underwent surgery and VGT during a single hospital admission for MBO, and 3,583 were included in the cohort (Figure 1). The median (IQR) age was 75 years (71-81 years), 87% were female, and 89% were white. Ovarian cancer was the primary malignancy for 73% and 27% had pancreatic cancer. At their first MBO admission, 5% of patients had palliative care consultations, 69% were treated with medical management, 24% underwent surgery, and 7% underwent VGT. Hospital U.S. Census region was West for 43%, Northeast for 23%, South for 23%, and Midwest for 12%. The majority were teaching hospitals (65%) and 45% had ≥ 400 beds. Patient and hospital characteristics are summarized by treatment for MBO in Table 1.
Figure 1.
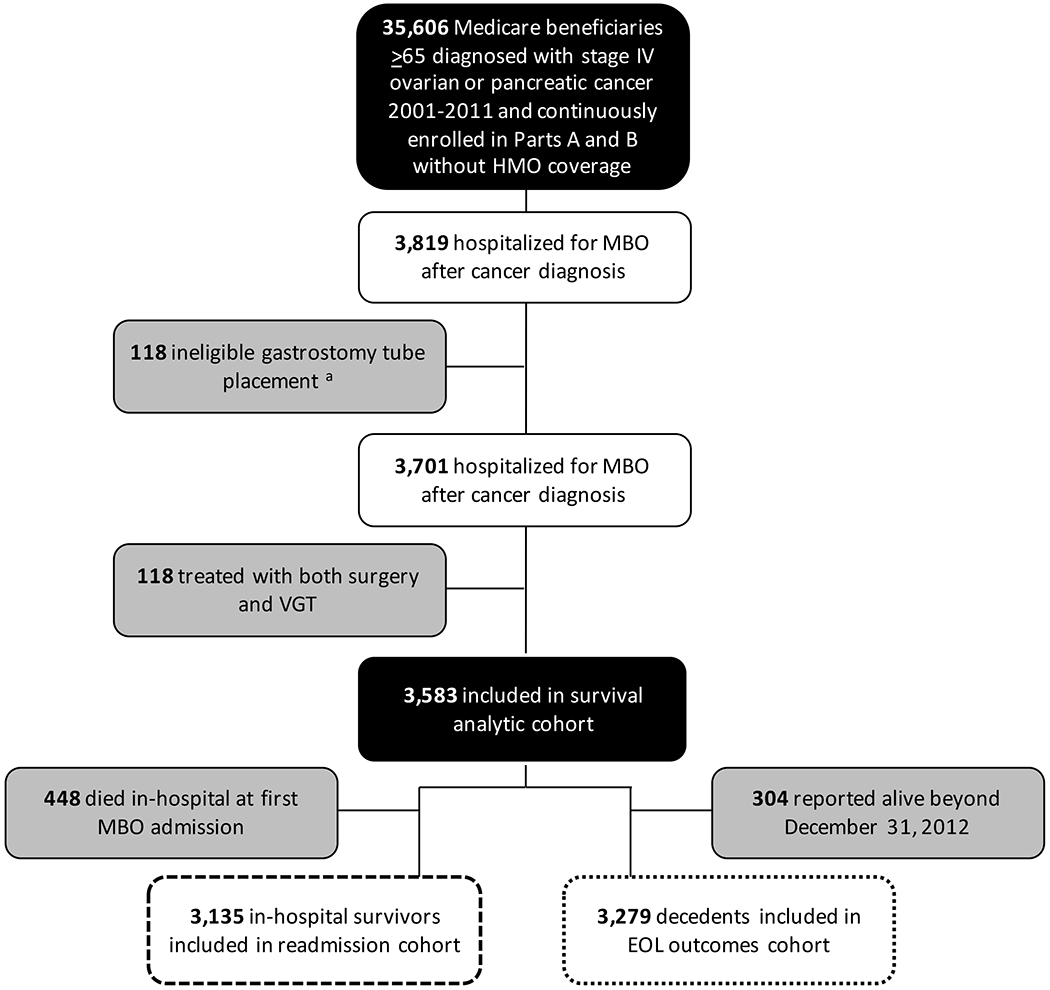
Flowchart for creation of the analytic cohorts from the Surveillance, Epidemiology, and End Results (SEER) Medicare-linked dataset. a Ineligible VGT refers to gastrostomy placement as an outpatient procedure or during a admission in which bowel obstruction was not a diagnosis code; b Patients in top quartile of survival duration after first MBO admission were excluded from analysis of EOL care outcomes; Abbreviations: EOL, end-of-life; HMO, health maintenance organization; MBO, malignancy-associated bowel obstruction; VGT, venting gastrostomy tube
Table 1.
Patient and hospital characteristics based upon treatment at the first bowel obstruction admission following diagnosis of stage IV ovarian or pancreatic cancer
|
Treatment at the first MBO admission |
||||
|---|---|---|---|---|
| Medical Management (n = 2,463) % | Surgery (n = 871) % | VGT (n = 249) % | ||
| Patient characteristics | ||||
| Age group | 65-74 y | 45.4 | 41.5 | 47.8 |
| 75-84 y | 42.8 | 45.1 | 43.4 | |
| ≥ 85 y | 11.8 | 13.4 | 8.8 | |
| Sex* | Female | 86.4 | 89.7 | 85.1 |
| Race*, a | White | 88.2 | 91.2 | 88.8 |
| Black | 7.5 | 5.7 | 9.6 | |
| Other / Unknown | 4.3 | 3.1 | - | |
| Charlson comorbidity index | 0 | 67.1 | 71.0 | 70.1 |
| 1 | 24.2 | 21.8 | 20.9 | |
| 2 | 8.7 | 7.2 | 8.4 | |
| Primary malignancy | Ovarian | 71.6 | 75.7 | 72.7 |
| Pancreas | 28.4 | 24.3 | 27.3 | |
| Palliative care consultation | 5.4 | 2.2 | 8.4 | |
| Hospital characteristics | ||||
| Region* | Northeast | 22.4 | 22.5 | 25.3 |
| Midwest | 12.0 | 12.3 | 13.3 | |
| South | 21.8 | 22.4 | 32.5 | |
| West | 43.8 | 42.8 | 28.9 | |
| Hospital size* | ≥ 400 beds | 44.0 | 44.2 | 62.7 |
| Teaching status | Teaching | 64.4 | 65.9 | 68.3 |
p < 0.05
Cell values representing < 11 patients were suppressed in accordance with SEER-Medicare data use agreement.
Abbreviations: MBO, malignancy-associated bowel obstruction; VGT, venting gastrostomy tube
After hospital admission for the first MBO admission, mortality rates in-hospital and at 30, 90, and 180-days were 13%, 29%, 53%, and 65%, respectively. The overall median (IQR) survival was 76 days (26-319 days). Mortality rates and survival duration after the first MBO admission are summarized by treatment in Table 2. Surgery was associated with reduced hazard of death after the first MBO admission (adjusted hazard ratio (HR) 0.84 [0.77-0.91], p < 0.001) and VGT was associated with increased hazard of death (adjusted HR 1.86 [1.62-2.12], p < 0.001) compared with medical management (Figure 2).
Table 2.
Survival and readmission after the first bowel obstruction admission following diagnosis of stage IV ovarian or pancreatic cancer
| Treatment at the first MBO admission |
||||
|---|---|---|---|---|
| Medical Management % | Surgery % | VGT % | ||
| Survival outcomes | n = 2,463 | n = 871 | n = 249 | |
| In-hospital mortality* | 12.9 | 12.4 | 9.2 | |
| 30-day mortality* | 31.4 | 18.1 | 41.9 | |
| 90-day mortality* | 54.3 | 41.8 | 81.4 | |
| 180-day mortality* | 65.9 | 57.2 | 89.9 | |
| Survival (days)*, a | 72 (23-312) | 128 (42-483) | 38 (23-69) | |
| Readmission outcomesb | n = 2,146 | n = 763 | n = 226 | |
| Readmission for MBO* | None | 67.4 | 75.0 | 85.0 |
| One | 20.6 | 18.1 | 10.6 | |
| Two or more | 11.9 | 7.0 | 4.4 | |
p < 0.05
Time from hospital admission date until date of death, reported as median (interquartile range)
Readmission for MBO is measured among patients who were discharged alive from their first MBO admission (N = 3,135)
Abbreviations: MBO, malignancy-associated bowel obstruction; VGT, venting gastrostomy tube
Figure 2.
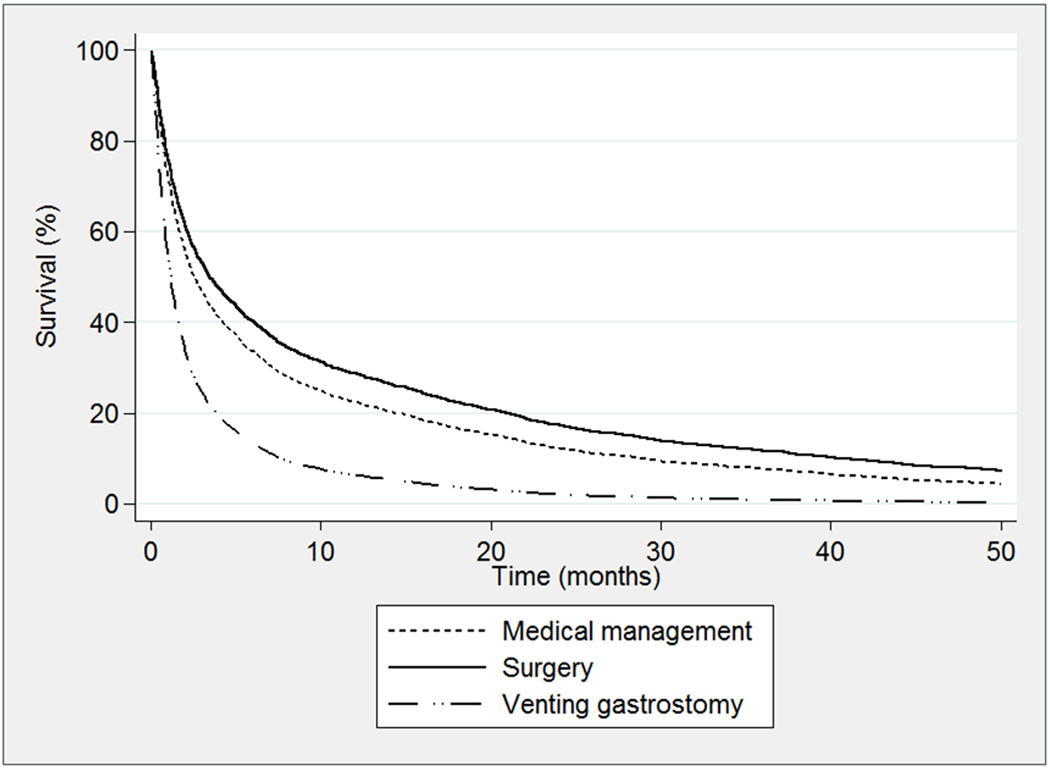
Survival after date of admission for the first malignancy-associated bowel obstruction admission occurring after cancer diagnosis
Readmission for MBO
There were 3,135 patients (87%) who were discharged alive from the first MBO admission and were included in the analytic cohort for readmission for MBO. After discharge from the first MBO admission, 29% of patients had one or more readmission for MBO (Table 2). Compared with medical management, the relative risk of readmission for MBO was lower after treatment with either surgery (adjusted subdistribution hazard ratio (SDHR) 0.69 [0.59-0.80], p < 0.001) or VGT (adjusted SDHR 0.41 [0.23-0.58], p < 0.001) (Figure 3) during the first MBO admission.
Figure 3.
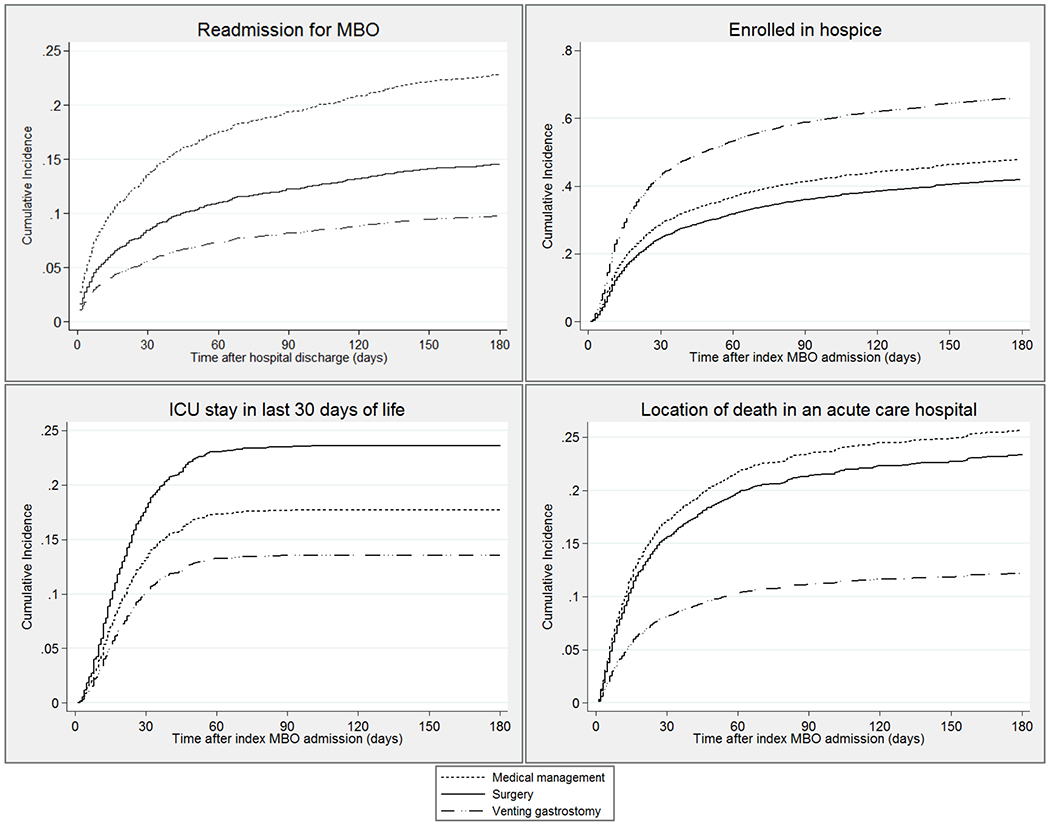
Cumulative incidence estimates of malignancy-associated bowel obstruction readmission and end-of-life outcomes based upon treatment for malignancy-associated bowel obstruction
End-of-life care outcomes
EOL care outcomes were studied among 3,279 patients (92%) who died on or before December 31, 2012. Prior to death, 845 patients (30%) had multiple MBO admissions, of whom 18% underwent surgery and 20% underwent VGT during readmissions for MBO. The last procedure prior to death was surgery for 27% and VGT for 12%. The remaining patients were treated with medical management during all MBO admissions. Overall, 65% enrolled in hospice, 19% had an ICU stay in their last 30 days of life, and 25% died in an acute care hospital.
EOL care outcomes of patients, grouped by treatment, are compared in Table 3. Compared with medical management, VGT was associated with increased relative risk of enrolling in hospice prior to death and reduced relative risk of having an ICU stay in the last days of life or dying in an acute care hospital. Surgery was associated with reduced relative risk of enrolling in hospice and increased relative risk of having an ICU stay in the last days of life. Cumulative incidence competing-risks estimates for each EOL care outcome are depicted in Figure 4.
Table 3.
A comparison of end-of-life outcomes based upon treatment for malignancy-associated bowel obstruction among decedents with stage IV ovarian or pancreatic cancer using multivariate competing risks regressiona (N = 3,279)
| Treatment for MBO |
|||||||
|---|---|---|---|---|---|---|---|
| Medical Management % | % | Surgery SDHRb | 95% CI | % | VGT SDHRb | 95% CI | |
| Enrolled in hospice | 64.3 | 63.0 | 0.84* | 0.76-0.92 | 76.4 | 1.65* | 1.42-1.91 |
| ICU care in last days of life | 18.2 | 24.4 | 1.38* | 1.17-1.64 | 12.8 | 0.69* | 0.52-0.93 |
| Death in an acute care hospital | 27.8 | 25.3 | 0.91 | 0.78-1.06 | 13.8 | 0.47* | 0.36-0.63 |
p < 0.05
Regression models are adjusted for age group, sex, race, Charlson Comorbidity Index, primary cancer site, hospital size, teaching status, and region
Reference group included patients treated with medical management during all MBO admissions
Abbreviations: CI, confidence interval; ICU, intensive care unit; MBO, malignancy-associated bowel obstruction; SDHR, subdistribution hazards ratio; VGT, venting gastrostomy tube
In a sensitivity analysis excluding patients who died during their index MBO admission, there was no significant difference between medical management and surgery in terms of the relative risk of having an ICU stay in the last days of life. Analyses of all other associations produced qualitatively similar results (eTable 2).
DISCUSSION
The results of this study corroborate previous work demonstrating that MBO typically occurs in the last months of life, with 65% of patients dying within 180 days of their first MBO admission. In this population-based, retrospective cohort study of older Medicare patients with MBO in the setting of ovarian or pancreatic cancer, the overall median survival after the first MBO admission was less than 3 months, underscoring the relevance of EOL care outcomes in delivering patient-centered care for these patients. Nonetheless, fewer than 5% had palliative care consultation. Patients treated with surgery at their first MBO admission had the longest survival. Those treated with medical management had the highest rate of readmission for MBO. Treatment with VGT was associated with lower intensity healthcare utilization at the end of life than medical management or surgery.
This study expands the existing literature by comparing outcomes of palliative treatment modalities for MBO in a large, population-based cohort. We found large differences in observed survival after treatment at the first MBO admission: As in prior studies, survival was longest after surgical treatment for MBO, which likely reflects selection of patients for surgery who were felt to have a better prognosis.1, 7, 8 Similarly, the short survival duration after VGT placement may indicate its use in patients who were expected to die sooner. Unfortunately, prognostic estimates from the clinicians’ perspective, which could explain these differences are unavailable in secondary data.
Current treatment algorithms for MBO recommend using VGT as a last resort when medical management fails to control symptoms.17 However, the results of this study indicated that VGT may be preferable to medical management for avoidance of hospital readmissions. The rates of readmission for MBO in this study after VGT are comparable to what has been previously reported in single-institution case series and cohort studies,9, 13, 14, 34–41 and were significantly lower than among those treated with medical management. Our analytic approach accounted for the competing risk of death; therefore, this finding is not explained by the shorter survival duration after VGT. Nonetheless, only 20% of patients with multiple MBO admissions underwent VGT, which substantiates prior assertions that VGT is an underutilized treatment modality for MBO.17 Prospective primary data is needed to delineate optimal timing of VGT and identify which patients are most likely to receive benefit from this procedure. Surgery was also associated with fewer readmissions for MBO; however, the increased risk of receiving ICU care in the last days of life underscores the potential tradeoffs of surgery and need for careful patient selection.
Treatment of MBO with VGT was associated with more hospice, less ICU care, and fewer deaths in acute care hospitals. These outcomes are associated with more favorable perceptions of death and dying among family members25 and are quality metrics for EOL care of cancer patients.28, 29 In contrast with surgical intervention, VGT does not alleviate the obstruction and is unlikely to permit adequate nutrition. Prior studies have shown that patients with cancer who have an accurate understanding of terminal prognosis often choose comfort-directed care over life-prolongation.42–45 The decision for VGT may reflect improved prognostic awareness on the part of patients and their clinicians, which in-turn prompted communication about goals of care. Our findings argue that because patients can be expected to die in weeks to months after a diagnosis of MBO regardless of management, conversations about priorities for EOL care and discussions about treatment options in the context of these priorities are appropriate for all patients. Introducing conversations about EOL care priorities into surgical decision making can improve patient selection and outcomes after palliative treatment and align EOL care with patients’ preferences.
Limitations
This study had several important limitations. This study was retrospective and is limited by the unavailability of information regarding clinical decisions (i.e. technical challenges, patient preferences, patent physical status, and prognostic estimates), which can influence treatment selection. In addition, these data do not describe other elements of the patient experience that are important patient-centered outcomes after palliative procedures. Large, prospective studies are needed to characterize other favorable outcomes, including quality of life, resolution of symptoms, functional status, and time with loved ones. The methods in this study were modeled after prior research on malignant bowel obstruction;7, 8, 22 however, claims-based definitions do not distinguish the cause of obstruction; therefore, we are not able to determine whether patients had a malignant bowel obstruction, as defined by the International Conference on Malignant Bowel Obstruction.46 Nonetheless, this uncertainty mirrors clinical practice, where the cause of obstruction is not always evident based on clinical history and imaging studies.1 Finally, the study population was limited to older patients with pancreatic and ovarian cancer and may therefore not be generalizable to younger patients and those with other primary malignancies.
Conclusion
This study found that VGT placement was associated with fewer readmissions than medical management and lower intensity EOL care than either medical or surgical management. Given the limited survival, hospitalization with MBO is a prognostically significant event and decisions regarding management should be informed by patients’ priorities for outcomes and preferences for care at the end of life. Implementation of these findings in clinical practice may lead to improved communication about options for managing MBO and better align management decisions with patients’ treatment goals.
Supplementary Material
ACKNOWLEDGEMENT
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement # U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding: This work was supported, in part, by funding from the Brigham Research Institute
Declaration of Interests
This work was supported, in part, by funding from the Brigham Research Institute (Lilley). Unrelated to this work, Dr. Cauley received grant support from the National Cancer Institute at the NIH (R25CA092203) during the conduct of the study. Dr. Cooper is supported by the Cambia Foundation. Dr. Haider is a co-founder and equity holder of a Johns Hopkins University supported start up known as Patient Doctor Technologies which owns and operates the website www.doctella.com. The study design and analytic protocol were developed at the Massachusetts General Hospital Supportive Oncology Workshop, which is sponsored by a National Cancer Institute R25 grant. Drs. Scott, Goldberg, Temel, Epstein, Lipsitz, Smalls, Bader, and Weissman report no conflicts of interest.
REFERENCES
- 1.Tuca A, Guell E, Martinez-Losada E, et al. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res 2012; 4:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakraborty A, Selby D, Gardiner K, et al. Malignant bowel obstruction: natural history of a heterogeneous patient population followed prospectively over two years. J Pain Symptom Manage 2011; 41(2):412–20. [DOI] [PubMed] [Google Scholar]
- 3.Feuer DJ, Broadley KE, Shepherd JH, et al. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000(4):CD002764. [DOI] [PubMed] [Google Scholar]
- 4.Paul Olson TJ, Pinkerton C, Brasel KJ, et al. Palliative surgery for malignant bowel obstruction from carcinomatosis: a systematic review. JAMA Surg 2014; 149(4):383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badgwell BD. Palliative Surgical Research: Challenges and Solutions. J Palliative Care Med 2012; S2(e001):1–2. [Google Scholar]
- 6.Wright FC, Chakraborty A, Helyer L, et al. Predictors of survival in patients with non-curative stage IV cancer and malignant bowel obstruction. J Surg Oncol 2010; 101(5):425–9. [DOI] [PubMed] [Google Scholar]
- 7.Mooney SJ, Winner M, Hershman DL, et al. Bowel obstruction in elderly ovarian cancer patients: a population-based study. Gynecol Oncol 2013; 129(1):107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winner M, Mooney SJ, Hershman DL, et al. Management and outcomes of bowel obstruction in patients with stage IV colon cancer: a population-based cohort study. Dis Colon Rectum 2013; 56(7):834–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96(2):330–4. [DOI] [PubMed] [Google Scholar]
- 10.Shinoda M, Kojima M, Fukase T, et al. Percutaneous transgastric intestinal decompression: the management of malignant bowel obstruction without nasal intubation. Surg Today 1994; 24(10):937–9. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine Committee on Approaching Death. Dying in America: Addressing Key End of Life Issues. Institute of Medicine; 2014. [Google Scholar]
- 12.Lilley EJ, Cauley CE, Cooper Z. Using a palliative care framework for seriously ill surgical patients: the example of malignant bowel obstruction. JAMA Surgery 2016; In press. [DOI] [PubMed] [Google Scholar]
- 13.Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16(6):520–6. [DOI] [PubMed] [Google Scholar]
- 14.Campagnutta E, Cannizzaro R, Gallo A, et al. Palliative treatment of upper intestinal obstruction by gynecological malignancy: the usefulness of percutaneous endoscopic gastrostomy. Gynecol Oncol 1996; 62(1):103–5. [DOI] [PubMed] [Google Scholar]
- 15.Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44(8):1105–15. [DOI] [PubMed] [Google Scholar]
- 16.Badgwell B, Krouse R, Klimberg SV, et al. Outcome measures other than morbidity and mortality for patients with incurable cancer and gastrointestinal obstruction. J Palliat Med 2014; 17(1):18–26. [DOI] [PubMed] [Google Scholar]
- 17.Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage 2014; 48(1):75–91. [DOI] [PubMed] [Google Scholar]
- 18.Schwarze ML, Brasel KJ, Mosenthal AC. Beyond 30-day mortality: aligning surgical quality with outcomes that patients value. JAMA Surg 2014; 149(7):631–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miner TJ, Jaques DP, Tavaf-Motamen H, et al. Decision making on surgical palliation based on patient outcome data. Am J Surg 1999; 177(2):150–4. [DOI] [PubMed] [Google Scholar]
- 20.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002; 40(8 Supp1):IV-3-18. [DOI] [PubMed] [Google Scholar]
- 21.Surveillance, Epidemiology, and End Results; National Cancer Institute. Documentation for the Patient Entitilement and Diagnosis Summary File (PEDSF) 2014. Available at: http://healthcaredelivery.cancer.gov/seermedicare/aboutdata/PEDSF.pdf. Accessed March 2, 2015.
- 22.Winner M, Mooney SJ, Hershman DL, et al. Incidence and predictors of bowel obstruction in elderly patients with stage IV colon cancer: a population-based cohort study. JAMA Surg 2013; 148(8):715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 24.Kwok AC, Semel ME, Lipsitz SR, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet 2011; 378(9800):1408–13. [DOI] [PubMed] [Google Scholar]
- 25.Wright AA, Keating NL, Ayanian JZ, et al. Family Perspectives on Aggressive Cancer Care Near the End of Life. JAMA 2016; 315(3):284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care 2007; 45(5):386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 2003; 21(6):1133–8. [DOI] [PubMed] [Google Scholar]
- 28.National Quality Forum. NQF-Endorsed Palliative Care and End-of-Life Care Endorsement Maintenance Standards 2012. Available at: http://www.qualityforum.org/Publications/2012/04/Palliative_Final_Report.aspx. Accessed March 16, 2015.
- 29.Centers for Medicare and Medicaid Services. Quality Payment Program 2016. Available at: https://qpp.cms.gov/measures/quality. Accessed November 23, 2016.
- 30.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 2013; 309(5):470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 1999; 94:496–509. [Google Scholar]
- 32.Berry SD, Ngo L, Samelson EJ, et al. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc 2010; 58(4):783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haller B, Schmidt G, Ulm K. Applying competing risks regression models: an overview. Lifetime Data Anal 2013; 19(1):33–58. [DOI] [PubMed] [Google Scholar]
- 34.Cannizzaro R, Bortoluzzi F, Valentini M, et al. Percutaneous endoscopic gastrostomy as a decompressive technique in bowel obstruction due to abdominal carcinomatosis. Endoscopy 1995; 27(4):317–20. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham MJ, Bromberg C, Kredentser DC, et al. Percutaneous gastrostomy for decompression in patients with advanced gynecologic malignancies. Gynecol Oncol 1995; 59(2):273–6. [DOI] [PubMed] [Google Scholar]
- 36.Herman LL, Hoskins WJ, Shike M. Percutaneous endoscopic gastrostomy for decompression of the stomach and small bowel. Gastrointest Endosc 1992; 38(3):314–8. [DOI] [PubMed] [Google Scholar]
- 37.Issaka RB, Shapiro DM, Parikh ND, et al. Palliative venting percutaneous endoscopic gastrostomy tube is safe and effective in patients with malignant obstruction. Surg Endosc 2014; 28(5):1668–73. [DOI] [PubMed] [Google Scholar]
- 38.Kawata N, Kakushima N, Tanaka M, et al. Percutaneous endoscopic gastrostomy for decompression of malignant bowel obstruction. Dig Endosc 2014; 26(2):208–13. [DOI] [PubMed] [Google Scholar]
- 39.Meyer L, Pothuri B. Decompressive percutaneous gastrostomy tube use in gynecologic malignancies. Curr Treat Options Oncol 2006; 7(2):111–20. [DOI] [PubMed] [Google Scholar]
- 40.Teriaky A, Gregor J, Chande N. Percutaneous endoscopic gastrostomy tube placement for end-stage palliation of malignant gastrointestinal obstructions. Saudi J Gastroenterol 2012; 18(2):95–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gemlo B, Rayner AA, Lewis B, et al. Home support of patients with end-stage malignant bowel obstruction using hydration and venting gastrostomy. Am J Surg 1986; 152(1):100–4. [DOI] [PubMed] [Google Scholar]
- 42.Mack JW, Cronin A, Keating NL, et al. Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. J Clin Oncol 2012; 30(35):4387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012; 367(17):1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang ST, Liu TW, Chow JM, et al. Associations between accurate prognostic understanding and end-of-life care preferences and its correlates among Taiwanese terminally ill cancer patients surveyed in 2011-2012. Psychooncology 2014; 23(7):780–7. [DOI] [PubMed] [Google Scholar]
- 45.Meier EA, Gallegos JV, Thomas LP, et al. Defining a Good Death (Successful Dying): Literature Review and a Call for Research and Public Dialogue. Am J Geriatr Psychiatry 2016; 24(4):261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anthony T, Baron T, Mercadante S, et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage 2007; 34(1 Suppl):S49–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


