Abstract
Cancer treatments are often more successful when the disease is detected early. We evaluated the feasibility and safety of multi-cancer blood testing coupled with PET-CT imaging to detect cancer in a prospective, interventional study of 10,006 women not previously known to have cancer. Positive blood tests were independently confirmed by a diagnostic PET-CT, which also localized the cancer. Twenty-six cancers were detected by blood testing. Of these, 15 underwent PET-CT imaging and nine (60%) were surgically excised. Twenty-four additional cancers were detected by standard-of-care screening and 46 by neither approach. 1.0% of participants underwent PET-CT imaging based on false positive blood tests, and 0.22% underwent a futile invasive diagnostic procedure. These data demonstrate that multi-cancer blood testing combined with PET-CT can be safely incorporated into routine clinical care, in some cases leading to surgery with intent to cure.
Introduction
Most individuals who die from cancer are not diagnosed until they have disseminated disease (1–3). Nevertheless, all metastatic cancers are localized at some point in their natural history, providing an opportunity for them to be detected and treated at an earlier stage (4, 5). In addition to the potential for surgery, radiation, and adjuvant therapy to cure localized disease, conventional as well as newer therapeutic agents, including immunotherapy, are more effective when the tumor burden is low (6–8). Moreover, large studies have shown that deaths from colorectal cancers – even late-stage cases – are considerably less common when the cancers are detected by any form of screening than when detected by symptoms (9). The goal of earlier detection is to identify the presence of cancer at a stage when a treatment is more likely to be successful, thereby offering a better chance of long-term survival (3–9).
Screening modalities like colonoscopy, mammography, low-dose computed tomography (LDCT), and Pap smears have been shown to decrease mortality from colon, breast, lung, and cervical cancers, respectively (10–13). However, adherence to standard-of-care (SOC) screening varies widely (14, 15) and screening is not recommended for average-risk individuals for any other cancer types. There is thus a need and an opportunity for minimally-invasive, multi-cancer screening tests to reduce morbidity and mortality from these diseases. However, there are several risks posed by such tests. A positive result can cause anxiety, and false positive tests can lead to unneeded procedures with associated risks. Moreover, some cancers detected early may never progress to clinically meaningful states, a phenomenon referred to as “overdiagnosis” (16). Because current SOC approaches have proven to be effective, a new multi-cancer test should not supplant or discourage individuals from undergoing SOC cancer screening. Optimally, such a test should be employed in a complementary way to increase cancer detection rates when applied to a population adherent to SOC screening.
The ability to identify cancers through blood testing is one of the most exciting advances in cancer diagnostics (17–20). To date, studies of blood-based multi-cancer tests have recruited individuals who were already known to have cancer at the time of testing (17, 18). Cancers studied in this manner are likely to be larger and more advanced than undiagnosed prevalent cancers because they are often identified following the onset of symptoms. The sensitivity of detecting cancers in prospective studies of patients not already known to have cancer is therefore likely to be lower than reported in studies of patients already diagnosed with cancer (3, 16, 21). Additionally, the controls used in retrospective studies often do not include individuals with all relevant co-morbidities, potentially overestimating specificity. Finally, an interventional study, as opposed to an observational study that does not report results to participants, is required to evaluate the impact of the test on patient management. Otherwise, the potential for harm due to unnecessary invasive procedures consequent to the test cannot be evaluated (16).
To address these challenging issues, we have performed an exploratory study to evaluate four critical issues confronting all multi-cancer blood tests for screening purposes:
Can a multi-cancer blood test prospectively detect cancer in individuals whose cancer was not previously detected by other means?
Can such a test be used to intervene in the cancer progression process, leading to therapy with intent to cure?
Can such a test be incorporated into routine clinical care and not discourage participants from engaging in SOC screening?
Can such a test be performed safely, without incurring a large number of futile, invasive follow-up tests based on the test results?
We did not attempt to address other important questions that can best be addressed in registration trials designed for regulatory approval of a test. These questions include clinical validity, risk vs. benefit to the screened population, and cost-effectiveness.
Study design
We performed an exploratory prospective, interventional study, called DETECT-A (Detecting cancers Earlier Through Elective mutation-based blood Collection and Testing) to evaluate an early version of a multi-analyte blood test incorporating DNA and protein biomarkers. Our goal was to enroll 10,000 women 65 to 75 years of age with no personal history of cancer from a population with high adherence to SOC screening (fig. S1). We chose to enroll only women to enrich for ovarian cancer, a malignancy that lacks SOC screening and typically has a favorable prognosis only when detected early. The age demographic was chosen to focus on individuals who were at greater risk for cancer due to their seniority but had sufficient life expectancy to derive benefit from an earlier cancer diagnosis (1). In addition to the increased prevalence of cancer, individuals of this age often have multiple comorbidities (22) which could decrease the specificity of the test. Thus, this age group allows a rigorous evaluation for detecting cancer despite a variety of other medical conditions. The only exclusion criterion for study entry was a current or previously known cancer. All cancer types were included in our analysis except for skin, central nervous system and leukemias, which have very low likelihood of being detected by a blood-based screening test such as that used here (23). All participants were enrolled through the Geisinger Health System, a large integrated health services organization. This enabled access to the electronic medical records (EMR) of participating individuals and minimizing loss to follow-up (24).
Given that there was no precedent for an interventional study of this nature, the most important issues we attempted to address were feasibility and safety. Accordingly, several safety features were incorporated to maximize safety to participants (Fig. 1). In particular, DETECT-A incorporated three steps prior to initiating a diagnostic work-up for cancer (Fig. 1A). First, a peripheral blood sample was evaluated with the baseline test described below. Individuals with abnormal values for at least one of the biomarkers evaluated in this test were invited back to provide a second blood sample. This second blood sample was used for a distinct confirmation blood test, described below, to determine whether the identical biomarker was persistently abnormal as well as to rigorously exclude mutations due to clonal hematopoiesis of indeterminate potential (CHIP) ((25) and Methods). The design of this two-step test was similar to that of Lo and colleagues, who studied Epstein-Barr virus DNA in the plasma of patients with nasopharyngeal cancers (26). Our goals were to achieve moderate (~95%) specificity with the first step and high specificity (~99%) with the combination of the baseline and confirmation tests. If the biomarker was reproducibly abnormal in the confirmation test and CHIP was excluded, the blood test was considered positive. Participants with a positive blood test were reviewed by a Multidisciplinary Review Committee (MRC) composed of experts from several relevant fields (Supplementary Materials). The committee then reviewed the medical history of participants to rule out a potential non-cancer-related cause for any abnormal result. If no such cause was found, the individual was invited to undergo the third component of testing, a full-body diagnostic positron emission tomography-computed tomography (PET-CT) scan with contrast, using fluorodeoxyglugose (FDG) as the tracer. Diagnostic PET-CT imaging was used to confirm the results of blood testing and localize the potential cancer in a safe and minimally-invasive manner (27).
Fig. 1. DETECT-A process and rationale.
(A) Three-step testing process for DETECT-A. (B) Safety rationale for study design.
Diagnostic PET-CT is an FDA cleared test that is routinely used in clinical practice to aid in to detecting, localizing, and diagnosing tumors. A large and growing body of clinical evidence supports its high sensitivity for early-stage cancers of multiple different organs (28–30). Patients with findings of concern on diagnostic PET-CT were referred to cancer specialists for further evaluation; subsequent work-up and management was performed according to standard clinical practice and was outside the scope of the study. Similarly, some participants with a positive baseline test had developed signs or symptoms of cancer before their confirmation blood test or before the MRC made their recommendation, and these participants were managed by their primary physicians. Patients with cancer were defined as those with biopsy-proven cancer or other undisputed clinical evidence of disease (see Methods). This definition was conservative and excluded patients who had benign tumors, including non-invasive pre-cancerous lesions.
Another concern of this study was the potential anxiety that cancer testing might cause to participants (Fig. 1B). To mitigate this anxiety, the consenting process for all participants included a clear discussion of the implications of a positive and negative test. Moreover, all participants were informed at the time of enrollment that they might be called back randomly to provide a second peripheral blood sample. The participants with a positive result in the baseline test were invited to provide an additional blood sample for a distinct confirmation test, as noted above. A similar number of participants with a negative baseline test result were also invited to provide an additional blood sample. In these “study controls”, a repeat baseline test was performed rather than a distinct confirmation test, allowing an evaluation of the reproducibility of the baseline test. Neither the participants invited back, nor the Geisinger professional extending the invitations, knew the group to which the participant belonged (i.e., this component was double-blinded). We hoped that this process would reduce anxiety in the patients invited back for a confirmation test. In addition, study genetic counselors were available to patients who had concerns at any stage of the study.
Communication of the test results to patients was done in a careful and prescribed manner (Fig. 1B). Patients with a positive blood test were not told they had cancer. Rather, the study health care professional noted they had an increased risk of harboring a cancer compared to the general population based on their blood testing results. Patients were thereby encouraged to undergo the diagnostic PET-CT. They were informed that this imaging would indicate whether any follow-up for a potential cancer was needed. The diagnostic PET-CT component was therefore an integral part of the screening process. The cost of the diagnostic PET-CT was included in the study budget, as was an initial visit with a specialist for patients with abnormalities found upon this imaging.
A further concern in introducing a new screening test is that a negative test result could cause harm by providing a false sense of security that an individual did not harbor a cancer. Careful counselling was therefore given to participants about the need to undergo recommended SOC cancer screening and to practice primary cancer prevention measures. This recommendation was provided at several stages of the testing process and follow-up (Fig. 1B).
Finally, a concern for any new screening test is the potential for incidental findings in participants without cancer that lead to unnecessary follow-up tests or procedures. Although not performed as part of the DETECT-A study protocol, all additional procedures that were performed in patients whose blood test was positive were carefully documented.
The DETECT-A blood test
The DETECT-A blood test incorporated baseline and confirmation test components that have the potential to detect cancer in many organs. The baseline test component represented an early version of a multi-analyte test, called CancerSEEK (21). It did not employ the machine learning methods described in (21) to increase sensitivity and specificity. Rather, it used pre-defined thresholds for each DNA and protein biomarker and a confirmation test to enhance specificity. The advances described in (21) and elsewhere (31) to increase sensitivity while maintaining specificity were made only after design and IRB-approval of the DETECT-A study, which occurred in 2016 and 2017, respectively.
The baseline test component employed a sequencing error reduction technology (32) to assess whether mutations in defined regions of 16 genes, represented by 61 amplicons of ~75 bp each, are present in cell-free plasma DNA (cfDNA, table S1). The baseline test component also assessed nine highly validated protein biomarkers (table S2) (21). To ensure high specificity, the selected thresholds for these biomarkers were considerably higher than thresholds conventionally considered abnormal. For example, the CA125 level required for positivity in the DETECT-A blood test was >16x as high as that commonly considered the upper limit of normal (577 vs 35 U/mL, respectively; see table S2) (33). This resulted in per-protein specificity of 99.8–100.0% (table S3).
The confirmation test component was performed only on participants whose baseline test was positive. It employed the same sequencing error reduction technology (32) and the same high thresholds for protein biomarkers used in the baseline test, but assessed only particular DNA mutations or proteins that were abnormal in the baseline test. It also rigorously excluded CHIP through more thorough examination of a larger amount of white blood cell (WBC) DNA than used in the baseline test. Technical details of the baseline and confirmation test components are provided in the Methods.
Study participants
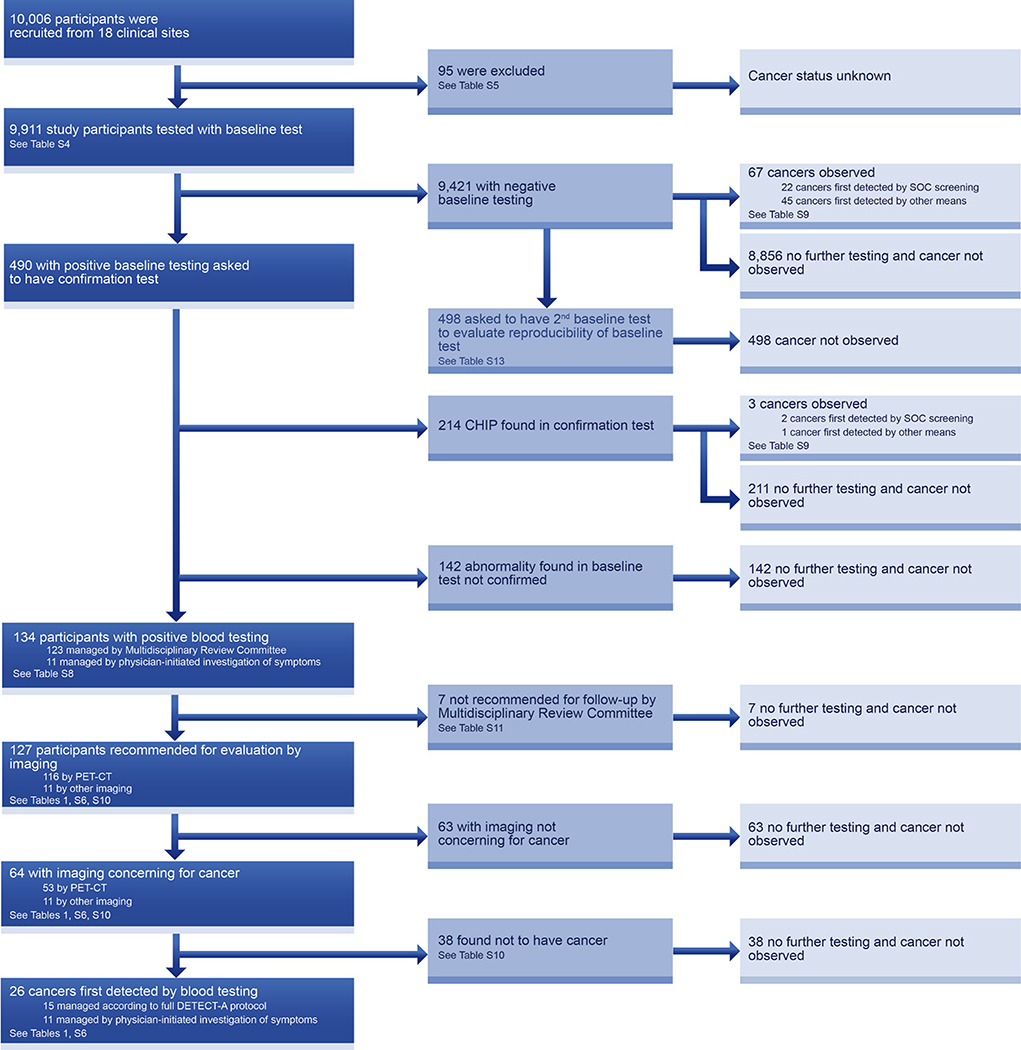
Between September 2017 and May 2019, 10,006 participants were enrolled in the DETECT-A study at 18 clinical sites across the Geisinger catchment area (Fig. 2, table S4). Of these, 73 withdrew from the study or were lost to follow up, 12 were later found from medical records to have actually had a history of cancer prior to enrollment and were excluded, and ten did not have a complete clinical work-up (table S5). The remaining 9,911 individuals (99.1% of the enrollees) were the participants assessed in this study. Surveys and reviews of medical records to assess status were scheduled to be performed 12 months after enrollment. To date, 12-month follow-up surveys have been completed for 6,874 of 7,366 (93.3%) eligible participants. For participants followed for less than 12 months, median follow-up time since enrollment was 9.7 months (inter-quartile range 8.7–10.8 months). Details on the process through which cancer status was ascertained are described in the Methods.
Fig. 2. Flowchart describing the DETECT-A study.
The DETECT-A diagnostic path (dark blue) indicates how cancers first detected by blood testing were identified. Each box indicates both the number of individuals proceeding through each step as well as the relevant table that provides additional detail.
Blood test results
The diagnostic procedures performed in the DETECT-A study are presented in the flow diagram of Fig. 2. Of the 9,911 participants, 490 (4.9%) scored positive in the baseline test component of the blood test, consistent with the 5% expected from the Study Design (see above). Of these, 134 tests (1.35% of the 9,911 participants) were confirmed in the confirmation test component, also similar to the fraction anticipated during Study Design. Sixty percent of those not confirmed were due to CHIP, which was analyzed more rigorously in the confirmation test than in the baseline test (Methods and Fig. 2). Of the 134 participants with positive blood testing, 127 (95%) were evaluated by imaging. Sixty-four (50%) of these 127 participants had imaging concerning for cancer. And of these 64 patients, 26 (41%) were subsequently shown to have cancer through biopsy (25 of 26 cases, 96%) or other unequivocal evidence (one of 26 cases, 4%; Supplementary Materials).
Results were presented to the MRC an average of 7.0 months (IQR 5.7 to 8.3) following the first blood draw. The timeline for the process is described in fig. S2. During this relatively long interval, eleven of the participants with a positive baseline test presented signs or symptoms concerning for cancer and sought clinical care from their primary physicians (Fig. 2 and Table 1). Though the blood test results had not yet been disclosed to them, six of these eleven participants had already been invited for a second blood draw before they contacted their physicians. We do not know whether these invitations prompted the participants to contact their physicians or impacted their physicians’ subsequent management. Diagnostic procedures in these eleven participants occurred at a median of 4.3 months (IQR 1.7 to 6.2 months) after their baseline test, and imaging other than diagnostic PET-CT was performed to localize disease (Fig. 2 and Table 1). These eleven participants with cancer tended to have more advanced disease than the 15 whose blood test follow-up was directed by the MRC (Table 1 and table S6). In four of the eleven, it was not possible to obtain a second blood sample prior to surgical excision of the tumors or because of advanced disease (Table 1 and table S6). In these four cases, the original baseline test was confirmed through analysis of an independent aliquot of blood obtained during the first visit. This need for independent confirmation in participants who developed symptoms during the study was recognized prior to the onset of the trial, and we attempted to obtain sufficient blood for two independent tests from all participants at baseline. All 26 participants with cancer first detected by blood testing, whether or not they were imaged by PET-CT, were reviewed by the MRC and reported to the IRB and Data Safety Monitoring Board. The diagnostic evaluations and clinical courses of these cancer patients are detailed in Table 1 and table S6.
Table 1.
Details on cancers detected by blood testing.
| Subject ID | Baseline test | Confirmation test | Time between blood tests (months) | Time between baseline blood test and imaging (months) | Imaging performed | Reason for imaging | Primary cancer organ | Stage | Cancer treatment | Months since biopsy-proven diagnosis of cancer | Status | Time from blood test to biopsy proven cancer (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 904337 | CA19-9 | CA19-9 | 5 | 6 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Ovary | I | Surgery | 11 | In remission | 7 |
| 905454 | CA15-3 | CA15-3 | 6 | 10 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Lung | I | Radiotherapy | 4 | Undergoing treatment | 12 |
| 906031 | TP53 | TP53 | 3 | 6 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Uterine | I | Surgery; Chemotherapy | 6 | Undergoing treatment | 9 |
| 906063 | CEA | CEA | 3 | 5 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Thyroid | I | Surgery | 5 | In remission | 10 |
| 901350 | BRAF | BRAF | 7 | 10 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Colorectal | II | Surgery | 14 | In remission | 10 |
| 902382 | CEA | CEA | 8 | 11 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Lung | II | Surgery | 10 | In remission | 12 |
| 906365 | HGF | HGF | 3 | 5 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Lung | II | Surgery | 9 | In remission | 6 |
| 902655 | BRAF;TP53 | BRAF;TP53 | 6 | 9 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Colorectal | III | Surgery; Chemotherapy | 13 | In remission | 9 |
| 903784 | PIK3CA;TP53 | PIK3CA;TP53 | 6 | 10 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Breast | III | Surgery; Chemotherapy | 9 | In remission | 11 |
| 907389 | TP53 | TP53 | 3 | 6 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Lung | III | Radiotherapy | 5 | Undergoing treatment | 7 |
| 904709 | CEA;HGF | HGF | 4 | 6 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Lymphoma | III | Surgery; Chemotherapy | 7 | In remission | 10 |
| 904006 | KRAS;TP53 | KRAS;TP53 | 5 | 9 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Lung | IV | Chemoradiotherapy | 11 | No progression | 9 |
| 904345 | CA125;CA15-3 | CA125;CA15-3 | 9 | 10 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Ovary | IV | Unknown (care provided outside of GHS)2 | 8 | Unknown (care provided outside of GHS)2 | 11 |
| 905645 | CA15-3 | CA15-3 | 2 | 5 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Ovary | IV | Surgery; Chemotherapy | 10 | No progression | 5 |
| 909448 | TP53 | TP53 | 4 | 6 | Diagnostic PET-CT | Multidisciplinary Review Committee Recommended | Ovary | IV | Chemotherapy | 23 | Undergoing treatment | 7 |
| 902959 | CA19-9 | CA19-9 | N/A1 | 6 | Ultrasound | Physician-initiated due to post-menopausal bleeding | Uterine | I | Surgery; Radiotherapy | 15 | In remission | 6 |
| 907351 | TP53 | TP53 | N/A1 | 0 | CT | Physician-initiated due to change in bowel patterns | Ovary | III | Surgery; Chemotherapy | 10 | In remission | 3 |
| 901500 | NRAS | NRAS | 6 | 11 | CT | Physician-initiated due to fever, fatigue, and weight loss | Lymphoma | III | Chemotherapy | 13 | Undergoing treatment | 11 |
| 900956 | KRAS | KRAS | 6 | 6 | MRI | Physician-initiated due to hematuria | Kidney | III | Surgery | 18 | In remission | 7 |
| 909646 | PIK3CA;TP53 | PIK3CA;TP53 | 4 | 3 | PET-CT | Physician-initiated due to shortness of breath | Lung | III | Chemotherapy | 6 | Palliative care | 3 |
| 907789 | TP53 | TP53 | N/A1 | 4 | CT | Physician-initiated due to abdominal bloating | Carcinoma of unknown primary | IV | Chemotherapy | 7 | Undergoing treatment | 5 |
| 900031 | CA15-3 | CA15-3 | 4 | 5 | PET-CT | Physician-initiated due to cough and shortness of breath | Lung | IV | Unknown (care provided outside of GHS)2 | 3 | Deceased | 5 |
| 903886 | EGFR;TP53 | EGFR;TP53 | 6 | 4 | PET-CT | Physician-initiated due to lower back pain | Lung | IV | Chemotherapy | 6 | Deceased | 4 |
| 903909 | PIK3CA;TP53 | PIK3CA;TP53 | N/A1 | 1 | CT | Physician-initiated due to rib pain | Lung | IV | Unknown (care provided outside of GHS)2 | 11 | Deceased | 1 |
| 909383 | TP53;CA125 | TP53;CA125 | 5 | 1 | CT | Physician-initiated due to shortness of breath | Ovary | IV | Chemotherapy | 8 | Undergoing treatment | 1 |
| 905879 | CEA | CEA | 12 | 9 | CT | Physician-initiated due to abdominal distenstion | Appendix | Pending surgery | Pending Surgery | 5 | Recently diagnosed | 9 |
Result was confirmed using an independent aliquot of the initial blood sample.
GHS = Geisinger Health System
In case 909448, unequivocal evidence of metastatic cancer was obtained and biopsy confirmation was deemed clinically inappropriate
As expected, the specificity of testing increased, and the sensitivity decreased, during the three-step process (Table 2). There were 29 patients with cancer whose baseline test was positive, but in three of these, the confirmation test showed that the positivity was due to CHIP. These three cases were considered DETECT-A blood test negative, and their exclusion decreased the sensitivity of blood testing from 30% to 27%. Conversely, in those participants with confirmed baseline blood tests (i.e., DETECT-A blood test positive), the specificity was 98.9%, compared to 95.3% in participants with positive baseline tests in the absence of confirmation. The positive predictive value (PPV) of testing concomitantly increased from 5.9% to 19.4%. The specificity and PPV of testing further increased with imaging, rising to 99.6% and 40.6%, respectively (Table 2). If only the 15 participants who received diagnostic PET-CT imaging were considered, the sensitivity was 16%, the specificity was 99.6%, and the PPV was 28%. The negative predictive values and number of individuals needed to screen to detect a cancer at each stage of the process are also listed in Table 2. For comparison, metrics for SOC screening and blood testing combined with SOC screening are provided in table S7.
Table 2.
Performance at different steps of the testing process. Brackets indicate 95% confidence interval. TP = true positive; FP = false positive; TN = true negative; FN = true negative. Numbers in parentheses indicate underlying calculations: PPV = TP/(TP+FP); specificity = TN/(TN+FP); NPV = TN/(TN+FN); NNTS = (TP+FP+TN+FN)/TP; sensitivity = TP/(TP+FN).
| Metric | Blood test without confirmation | Blood test1 | Blood test + diagnostic PET-CT2 | Blood test + any form of imaging3 | |
|---|---|---|---|---|---|
| Positive predictive value (PPV) (%) | 5.9% [4.0–8.4] (29/490) | 19.4% [13.1–27.1] (26/134) | 28.3% [16.8–42.3] (15/53) | 40.6% [28.5–53.6] (26/64) | |
| Specificity (%) | 95.3% [94.9–95.7] (9,354/9,815) | 98.9% [98.7–99.1] (9,707/9,815) | 99.6% [99.5–99.7] (9,777/9,815) | 99.6% [99.5–99.7] (9,777/9,815) | |
| Negative predictive value (NPV) (%) | 99.3% [99.1–99.4] (9,354/9,421) | 99.3% [99.1–99.4] (9,707/9,777) | 99.1% [99.0–99.3] (9,777/9858) | 99.3% [99.1–99.4] (9,777/9,847) | |
| Number needed to screen (NNTS) to detect one cancer (N) | 342 [238–510] (9,911/29) | 381 [260–583] (9,911/26) | 661 [401–1,180] (9,911/15) | 381 [260–583] (9,911/26) | |
| Sensitivity (%) | All included cancer types | 30.2% [21.3–40.4] (29/96) | 27.1% [18.5–37.1] (26/96) | 15.6% [9.0–24.5] (15/96) | 27.1% [18.5–37.1] (26/96) |
| Cancer types with recommended SOC screening available | 27.5% [15.9–41.7] (14/51) | 23.5% [12.8–37.5] (12/51) | 15.7% [7.0–28.6] (8/51) | 23.5% [12.8–37.5] (12/51) | |
| Cancer types with no recommended SOC screening available | 33.3% [20.0–49.0] (15/45) | 31.1% [18.2–46.6] (14/45) | 15.6% [6.5–29.5] (7/45) | 31.1% [18.2–46.6] (14/45) | |
As noted in the text, participants who had positive blood tests were defined as those in which the abnormalities found in the baseline test were validated, and CHIP rigorously excluded, by the confirmation test.
The 11 cancer patients with positive blood tests who did not receive a diagnostic PET-CT because they were managed by their physicians were included as FN in the calculations in this column (see main text).
The 11 cancer patients with positive blood tests who did not receive a diagnostic PET-CT because they were managed by their physicians were included as TP in the calculations in this column (see main text).
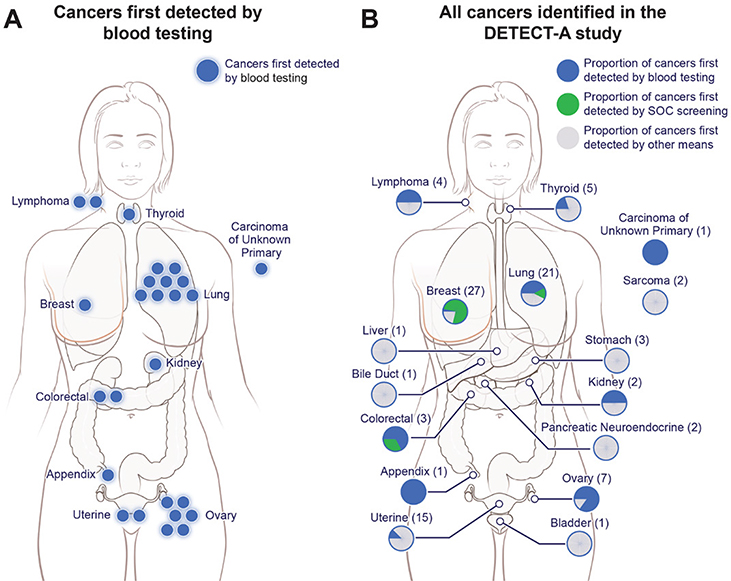
We considered the 26 cancer patients with positive blood testing to be “first detected by blood testing”. These included nine lung cancers, six ovarian cancers, and two colorectal cancers (Fig. 3A–B, fig. S3). Seventeen (65%) of the 26 cancers were localized or regional, including five patients with Stage I tumors (fig. S3 and S3A, table S6). The single participant with cancer of unknown stage did not have evidence of metastatic disease by CT. Eleven patients are in remission and nine are undergoing treatment or have stable disease (median follow-up for all 26 cancer patients was 8.4 months following definitive diagnosis, table S6). Expected survival of these patients is listed in table S6.
Fig. 3. Overview of cancers incident during the DETECT-A study.
(A) Twenty-six cancers (blue) in 10 organs were first detected by blood testing. (B) Ninety-six cancers were identified in the study (see Supplementary Materials). The location, and number of those first detected by blood testing (blue), standard-of-care screening (green) or by other means (grey) are shown.
Of the 26 cancers, 14 were blood test positive by virtue of mutations in circulating tumor DNA (ctDNA), 11 by virtue of elevated levels of protein biomarkers, and one by both a mutation and an elevated protein level (fig. S4B, Table 1). In participants with confirmed cancers, TP53 was the most frequently mutated gene (11 participants), followed by PIK3CA (three participants), KRAS and BRAF (two participants each), and EGFR and NRAS (one participant each) (fig. S4C, Table 1). These mutations were generally found in cancers expected to harbor the mutated genes – for example, EGFR in a lung cancer and BRAF in a colon cancer. In the 15 cancers detected by ctDNA mutations, the median mutant allele frequency (MAF) was 0.395% and ranged from 0.009% to 33.9% (table S8).
The most commonly elevated protein biomarkers in participants with cancer were CA15–3 (four participants) and CEA (three participants), while CA19–9, CA125, and HGF were elevated in two participants each (fig. S4D, table S3, Table 1). Interestingly, highly elevated levels of some proteins were found in patients with cancer types not usually associated with those markers, such as CEA in a lung cancer and CA19–9 in an ovarian cancer (Table 1). This is consistent with studies showing that circulating levels of several glycoproteins are often elevated in many tumor types (21, 33). Importantly, above-threshold elevations of the protein biomarkers in the panel were extremely rare in individuals without cancer (99.4% specificity, i.e., 0.6% false-positive rate; table S3), even though many comorbid diseases were present in the study cohort as measured by the Charlson Comorbidity Index (figs. S5 and S6, table S9) (34).
Standard-of-care screening
Ninety-six cancers among the 9,911 participants were identified within 12 months of enrollment (fig. S3, table S9). This number (0.97%) is consistent with the number of incident cancers expected from Centers for Disease Control (CDC) Surveillance, Epidemiology, and End Results (SEER) data during the achieved follow-up period in a well-screened population (2). Twenty-four of these cancers were detected through SOC screening during the follow-up period. These were verified by biopsy or other unequivocal clinical evidence. These patients were considered “first detected by SOC screening”. These cases did not overlap with the 26 first detected by blood testing. The remaining 46 cancers were not first detected by either blood testing or by SOC screening. In most of these cases, diagnostic tests were initiated on the basis of patient symptoms (table S9).
Twenty breast cancers, three lung cancers, and one colorectal cancer were first detected by SOC screening (fig. S3). Twenty-two (91.7%) of the 24 SOC-detected cancers were localized or regional, including 16 Stage I breast cancers (fig. S3). Interestingly, twelve cancers of these three organs were first detected by blood testing rather than by SOC screening, boosting the sensitivity of screening-based detection from 47% for SOC screening alone to 71% with SOC screening plus blood testing in these three cancer types (fig. S3, table S7). Fourteen (31%) of 45 cancers in seven organs for which no SOC screening test is available were first detected by blood testing (fig. S3, table S9). The 46 cancers not first detected by either blood testing or SOC screening included ten patients with Stage I uterine cancer; these cancers are often first detected at early stages by post-menopausal bleeding (fig. S3). Thirty-eight of these 46 cancers were localized or regional, and 26 were Stage I (fig. S3).
Feasibility and safety
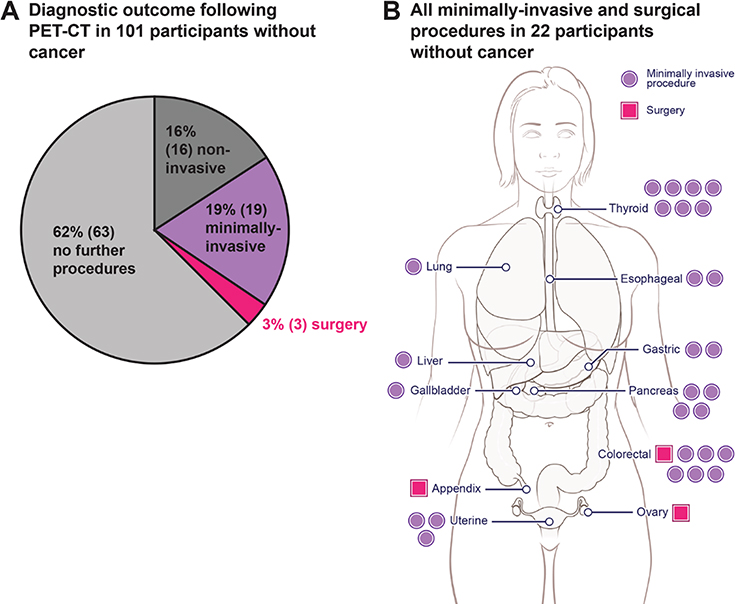
There were no serious adverse events resulting from the procedures dictated by the study protocol itself (i.e., venipuncture and diagnostic PET-CT). Therefore, safety risk was concentrated in the 108 participants with positive blood testing but no cancer. Seven of these participants were not recommended for follow-up by the MRC (Table 2). The remaining 101 participants were recommended for evaluation by diagnostic PET-CT. Prior to the study, a concern about safety was the potential number of incidental findings that could arise from PET-CT testing, potentially leading to unnecessary procedures in individuals without cancer. We found that diagnostic PET-CT efficiently guided follow-up: in 63 of 101 (62%) participants without cancer who received a diagnostic PET-CT, no additional follow-up was performed (Fig. 4A, tables S10 and S11). There were only 38 participants without cancer who had any procedure subsequent to their PET-CT findings. Sixteen (42%) of these 38 participants received only non-invasive testing, and in 19 (50%), minimally-invasive procedures were suitable to conclude that a cancer was not present (Figs. 4A–B, tables S10–S12). Furthermore, diagnostic PET-CT led to a clinically relevant diagnosis in 15 of the 38 cases (e.g., a common bile duct stone or a pre-cancerous lesion requiring surveillance, table S10). In only three of the 38 cases was surgery performed (Fig. 4B). One of these three patients had large colonic polyps with high-grade dysplasia which could not be removed endoscopically, a second had an in situ carcinoma of the appendix, while the third had a 10 cm ovarian lesion that was ultimately found to be a mucinous cystadenoma. Although lesions with high-grade dysplasia are considered to be pre-cancerous, we conservatively counted them as false positive results in the performance statistics, as blood tests for circulating tumor DNA generally do not detect precancerous lesions.
Fig. 4. Risk exposure in participants without cancer but with positive DETECT-A blood tests.
(A) 101 participants with positive DETECT-A blood tests underwent a diagnostic PET-CT but had no cancer identified. Risk stratifications are defined in table S12. Only the most invasive procedure a participant underwent is shown; for example, a participant who received minimally-invasive follow-up (e.g., a bronchoscopy) may also have first received non-invasive follow-up (e.g., a follow-up chest CT) (table S10). (B) Anatomical locations of minimally-invasive or surgical procedures following diagnostic PET-CT in the 22 participants with positive PET-CT imaging but without cancer. Each dot or square indicates a single procedure; seven participants had more than one minimally-invasive or surgical procedure, which explains why there are 30 procedures depicted in the 22 patients.
An independent way of assessing specificity was through the control group of individuals who had two sequential baseline tests (rather than a baseline test followed by a distinct confirmation test). As noted above in the Study Design section, 498 participants with a negative baseline test result were invited to provide a second peripheral blood sample in a double-blinded fashion. One purpose of this component of the study was to reduce potential anxiety, as participants did not know whether the invitation to provide a second blood sample was based on a positive baseline test result vs. selection as a study control (Fig. 1). A second purpose was to assess reproducibility of the baseline test. Only four of the 498 study controls scored positive in the second blood sample, indicating a specificity of 99.2% (95% confidence interval 98.0% to 99.8%); none of these four patients developed cancer within the study period (table S13).
Another source of potential harm associated with the DETECT-A protocol was the radiation exposure from diagnostic PET-CT and follow-up imaging tests in the participants without cancer. We compared the futile radiation exposure attributable to DETECT-A to that arising from other medical imaging tests either during the three years before enrollment (all participants) or during the study period after enrollment (true negative participants; fig. S7, tables S14 and S15). This comparison showed that 13% of true negative participants reported imaging tests associated with more than 10 mSv dose, such as that from CT scans, in the year after enrolling in DETECT-A. In comparison, only 101 (1%) of participants without cancer underwent futile imaging tests associated with >10 mSv dose as a result of DETECT-A. Diagnostic PET-CT confers a radiation exposure of ~25 mSv, ~15 to 18 mSV more than a standard CT (table S14).
A potential danger of a multi-cancer blood test is that patients might interpret a negative test result as meaning they are cancer-free, decreasing adherence to SOC screening. To measure this possible effect, we evaluated whether the participants in the study had fewer mammograms after the baseline test than they had in the years prior to the test. The recommended interval for mammography for normal-risk participants in our cohort was every two years. At the follow-up survey, more participants reported at least one mammogram within the last year than participants who reported more than one mammogram within three years prior to enrollment (fig. S8A). Of the 3,295 participants with available health insurance claims data, an orthogonal measure of screening rates, the percentage of participants that had a mammogram within one year prior to enrollment was 30.5%, while 28.7% of participants had a mammogram within one year after enrollment (McNemar χ2 = 2.4, p = 0.12; fig. S8B).
It was also of interest to query patients about their impressions of the study after they had completed their participation in it. A survey that included a measure of decisional regret (35) was administered to all participants at 12 months post-enrollment. 6,874 of 7,366 participants (93.3%) who had reached 12 months post-enrollment responded. Only 0.3% of respondents felt that they had made the wrong decision by participating in the study (fig. S9A). The responses were similar in patients who received accurate final blood testing results (i.e., true positives or true negatives) and who received inaccurate results (false positives or false negatives; p = 0.294, two-sided two-sample proportion test; fig. S9B–C). Similarly, only 1.0% of respondents stated they would not join a similar, subsequent study if it were offered to them again (fig. S10A–C).
With respect to technical specificity, DNA-based tests that rely on mutation, as opposed to other, less specific properties of cell-free plasma DNA, have the advantage that the precise mutation detected in the plasma can be validated in a completely orthogonal manner through evaluation of DNA from the primary tumor. Though the collection of tumor material was not mandated in the DETECT-A study protocol, we were able to acquire formalin-fixed, paraffin-embedded (FFPE) blocks of tumors from four patients whose cancers were first detected by blood testing on the basis of a DNA mutation. There was a total of six mutations found in these patients’ plasma DNA samples, all of which (100%) were found to be present in the patients’ corresponding tumors (table S16). We were also able to acquire FFPE blocks of tumors from 36 patients who did not score positively in the DNA component of the blood test. We asked whether these tumors contained mutations in the 1,933 bp that were queried by the mutation panel used in the baseline test component. We found that 78% of these cases had mutations in their tumors within the queried 1,933 bp (table S16). This suggested that the majority of the false negative results were due to an amount of cancer-derived ctDNA too small to be detected by the DETECT-A blood test rather than to the absence of relevant mutations in the cancers.
Discussion
In the DETECT-A study, we attempted to answer four fundamental questions that confront all multi-cancer screening tests, as noted in the introduction. The answer to all four questions was affirmative:
Blood testing makes it possible to detect cancers, including early cancers, in individuals without any history of the disease.
It is possible to intervene on the basis of blood testing, leading to surgery with intent to cure.
Blood testing can be incorporated into routine medical care without discouraging patients from engaging in other forms of screening.
Such testing can be performed in a safe manner without incurring a large number of futile, invasive follow-up tests.
One of the key safety features of DETECT-A was the diagnostic PET-CT for confirmation of blood testing and localization of suspected cancers. The cost of PET-CT was only a small component of the total cost of screening because only 1.2% of the 9,911 tested individuals underwent PET-CT. We and others have suggested that a blood test itself, through molecular tissue-of-origin prediction, could localize primary tumors to some degree (21, 36–41). Though this may be possible in the future, no multi-cancer blood test yet described has the specificity required to adequately interpret a positive test result. For example, suppose the PPV of a new test is as high as 50% and molecular tissue-of-origin prediction has >90% accuracy. The majority of test-positive participants, including 100% of those with false positive tests, would thereby receive an incorrect tissue of origin result rather than a definitive confirmation or rejection of the presence of a cancer. This uncertainty could lead to a diagnostic odyssey whose outcomes and risks are unknown. On the other hand, diagnostic PET-CT provides a uniform way to accurately determine tissue-of-origin while minimizing the risk for an unnecessary diagnostic odyssey. Importantly, diagnostic PET-CT is also precise in a way that a molecular tissue-of-origin predictor cannot be. For example, it reveals whether a presumptive cancer is in the right vs. left kidney, its size and location within that kidney, and the presence or absence of possible metastatic lesions. This information is immediately actionable, enabling physicians to move forward with histopathological assessment and development of a timely and informed treatment strategy.
Note that diagnostic PET-CT is not well-suited to be a primary screening modality for the general population, in part because of low disease prevalence and a relatively high rate of incidental findings in that setting. In contrast, we expected that its use exclusively in individuals who already had a positive blood test would result in much more favorable performance. Indeed, the feasibility and safety of utilizing diagnostic PET-CT for disease localization was clearly demonstrated in the DETECT-A study: the median number of procedures following diagnostic PET-CT required to conclude that a participant did not have cancer was zero (inter-quartile range 0 to 1) (Fig. 4 and table S10). The positive predictive value of any multi-cancer blood test will be lower in individuals younger than 65 years of age due to their lower prevalence of cancer, making definitive conclusions about positive tests from subsequent imaging studies even more important.
The DETECT-A study design included a second test to confirm the baseline test. Given the lack of precedent for such a prospective, interventional study of patients not known to have cancer, we considered this confirmation essential for the safety of the participants. This two-step process took a relatively long time to complete (fig. S2) and is not optimal for widespread testing outside a research study. In clinical practice, a participant would optimally be quickly directed to diagnostic PET-CT following a positive test result. The DETECT-A blood test was created in 2016, and new generations of it (21, 31), have been shown to have higher sensitivity and specificity. As such, newer tests with specificity >99% would be expected to have similar performance without a confirmation test.
Another of our safety concerns was that blood testing would result in decreased adherence with SOC screening, particularly mammography. It was therefore important that there was continued mammogram utilization following blood testing in the DETECT-A cohort (fig. S7). We attribute part of this adherence to the consistent communication about SOC screening to participants. The high degree of participant satisfaction (figs. S8 and S9) may similarly have been due to the pre-defined method of communicating test expectations and results to minimize anxiety.
How can secondary prevention measures such as those constituting the DETECT-A study be improved? In the cohort studied, 96 cancer diagnoses were made during the study interval. Of these, 26 were first detected by blood testing and an additional 24 were first detected by SOC screening. For cancers for which SOC screening is already effective, there is no need to replace SOC screening with a multi-cancer blood test. In addition to their relatively high sensitivity for malignant lesions, SOC screening procedures can detect high-risk pre-malignant lesions, such as adenomas, which are not generally detectable by blood tests (42). Nonetheless, blood testing can complement SOC screening, as documented here (table S7). On the other hand, there were 46 cancers not first detected by either blood testing or SOC screening. The challenge is to detect these other 46 cancers, plus any additional cancers in this cohort that have not yet been diagnosed, while maintaining high specificity. It has already been demonstrated that the sensitivity of the blood test can be substantially improved, without compromising specificity (21, 31). Other multi-cancer tests, based on epigenetic changes in DNA or chromatin, have also been shown to have high sensitivity for cancer detection (36–41, 43, 44). Further improvements in and combinations of these technologies will undoubtedly improve sensitivity even further.
There are several limitations of our study. First, our analysis only considered the initial follow-up after the baseline test. Longer follow-up will undoubtedly reveal more undetected cancers, thereby increasing the number of false negatives. It could also reveal patients with cancer with positive blood testing but in whom diagnostic PET-CT failed to identify a cancer, thereby decreasing the number of false positives. In the next phase of studying this cohort, longitudinal follow-up for up to five years is planned. Another limitation of our study is that not all enrolled individuals completed the prescribed course (Fig. 2, tables S5 and S6). And finally, future studies will need to include races and ethnicities other than those highly represented in the Geisinger Health System, as well as men (table S4).
The DETECT-A study was able to address several fundamental issues about multi-cancer blood tests but was not designed for regulatory approval of a specific test. Larger trials, including formal registration trials, will be necessary to accurately dissect benefit vs. risk, and to further evaluate the clinical validity and utility of such testing. At present, we cannot be certain that the DETECT-A blood test used in our study helped any participant. It is possible that none of the cancers first detected by this test would ever have caused symptoms or led to death. The same is true for participants whose cancers were first detected by SOC screening in the DETECT-A study. Though theoretically possible, this hypothesis is not readily congruent with the fact that every late stage, metastatic cancer starts out as a Stage I cancer. What is not known is the fraction of localized or regional cancers that progress to clinically significant or metastatic disease in the absence of treatment. All that we can confidently conclude at present is that a minimally invasive blood test can be safely used to detect several types of cancers in patients not previously known to have cancer, enabling treatment with intent to cure in at least a subset of individuals. This advance will facilitate future randomized, interventional trials to assess the ability of minimally-invasive blood tests to improve the effectiveness of cancer screening.
Supplementary Material
ACKNOWLEDGMENTS
We thank our DETECT-A participants for their courage and generosity. We thank the DETECT-A study staff for conducting a participant-centered study. We thank Pinaki Bose, Bryan Chesnick, Phil Darden, Callie Delehunt, Ahmed Farshori, Hadassa Guttman, Jasmine Kennedy, Martha Kimos, Angela Koenig, Edgardo Lopez, Kellie McCreesh, Bailey Mincemoyer, Derrick Murphy, Nicole Parks, Callen Riggins, Mark Roberts, Ayana Robinson, Naomi Sengamalay, Joanna Shoenfelt, and Min Tun for technical assistance with this study. We thank Matthew A. Facktor and Eduardo V.C. Moroni for clinical assistance with this study. We thank Elizabeth Cook for art assistance. We thank Cherie Blair, Lisa Dobbyn, Maria Popoli, Janine Ptak, Joy Schaefer, Natalie Sillman, Christopher Thoburn, and Howie Kaufman for assistance with this study. We thank Yali Li and Geoff Otto for helpful comments on the manuscript.
Funding: This work was supported by The Marcus Foundation; Lustgarten Foundation for Pancreatic Cancer Research; The Virginia and D.K. Ludwig Fund for Cancer Research; The Sol Goldman Center for Pancreatic Cancer Research; Susan Wojcicki and Dennis Troper; the Rolfe Foundation; The Conrad R. Hilton Foundation; The John Templeton Foundation; Burroughs Wellcome Career Award For Medical Scientists; ancillary support to investigators was provided by National Institutes of Health grants and contracts CA06973, U01-CA152753, U01-CA230691, P50-CA62924, R44CA203350, R37CA230400, T32-GM007309, and HHSN261201600034C.
Footnotes
Competing interests: B.V., K.W.K., N.P., I.K., and C.L. are founders of and hold equity in Thrive Earlier Detection. K.W.K., N.P., and C.L. are consultants to and are on the Board of Directors of, Thrive Earlier Detection. C.L. is an officer of Thrive Earlier Detection. B.V., K.W.K., N.P. & S.Z. is a founder of, holds equity in, and serves as a consultant to Personal Genome Diagnostics. S.Z. holds equity in Thrive Earlier Detection, and has a research agreement with BioMed Valley Discoveries, Inc. K.W.K. & B.V. are consultants to Sysmex, Eisai, and CAGE Pharma and hold equity in CAGE Pharma. B.V. is also a consultant to Nexus, and K.W.K., B.V., S.Z., and N.P. are consultants to and hold equity in NeoPhore. N.P. is a consultant to and hold equity in CAGE. C.B. is a consultant to Depuy-Synthes and Bionaut Pharmaceuticals. F.S. is a consultant to The Marcus Foundation. C.T. is a consultant to Thrive Earlier Detection, Bayer, and Johnson & Johnson. D.H.L. is a consultant to Clear Genetics, Inc. (now InVitae, Inc.), Natera, Inc., and X-Therma, Inc. The companies named above, as well as other companies, have licensed previously described technologies related to the work described in this paper from Johns Hopkins University. J.D.C., C.T., C.D., C.B., B.V., K.W.K., I.K., S.Z., R.H.H., A.M.L, and N.P. are inventors on some of these technologies; relevant filings include PCT/US2018/045669 and US9476095B2. Licenses to these technologies are or will be associated with equity or royalty payments to the inventors as well as to Johns Hopkins University. Patent applications on the work described in this paper have or may be filed by Johns Hopkins University. The terms of all these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies. C.D. and A.W. hold equity in and are consultants to Thrive Earlier Detection. A.W. holds equity in Glympse Bio. A.T.C., B.U., H.J.H., L.N.H., L.K., A.P., and N.M. hold equity and/or stock options in Thrive Earlier Detection. N.M. is employed by Genosity. Geisinger has an equity stake in Thrive; no Geisinger authors have financial interests in Thrive.
Data and materials availability: Sequencing data have been deposited in the European Genome-phenome Archive (EGAS00001004372). Protein biomarker data have been deposited in the European Genome-phenome Archive in the phenotype data section (EGAS00001004372). Patient sequencing and phenotype data can be released only under a Data Transfer Agreement with Johns Hopkins University School of Medicine. All other data needed to evaluate the conclusions in the paper are present in the paper and supplementary materials.
REFERENCES AND NOTES
- 1.Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020). doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Howlader N et al. , Eds., SEER Cancer Statistics Review, 1975–2014, National Cancer Institute, Bethesda, MD: (2017). [Google Scholar]
- 3.Ahlquist DA, Universal cancer screening: Revolutionary, rational, and realizable. NPJ Precis Oncol 2, 23 (2018). doi: 10.1038/s41698-018-0066-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldblum JR, Lamps LW, McKenney J, Myers JL, Surgical Pathology. (Elsevier, 2018). [Google Scholar]
- 5.Singhi AD, Koay EJ, Chari ST, Maitra A, Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 156, 2024–2040 (2019). doi: 10.1053/j.gastro.2019.01.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.André T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand A, Scriva A, Hickish T, Tabernero J, Van Laethem JL, Banzi M, Maartense E, Shmueli E, Carlsson GU, Scheithauer W, Papamichael D, Möehler M, Landolfi S, Demetter P, Colote S, Tournigand C, Louvet C, Duval A, Fléjou J-F, de Gramont A, Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J. Clin. Oncol. 33, 4176–4187 (2015). doi: 10.1200/JCO.2015.63.4238 [DOI] [PubMed] [Google Scholar]
- 7.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D’Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ, T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017). doi: 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M, Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 378, 449–459 (2018). doi: 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner H, Jansen L, Ulrich A, Chang-Claude J, Hoffmeister M, Survival of patients with symptom- and screening-detected colorectal cancer. Oncotarget 7, 44695–44704 (2016). doi: 10.18632/oncotarget.9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD; National Lung Screening Trial Research Team, Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 365, 395–409 (2011). doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabar L, Fagerberg G, Chen H-H, Duffy SW, Smart CR, Gad A, Smith RA, Efficacy of breast cancer screening by age. New results from the Swedish Two-County Trial. Cancer 75, 2507–2517 (1995). doi: [DOI] [PubMed] [Google Scholar]
- 12.Vesco KK, Whitlock EP, Eder M, Burda BU, Senger CA, Lutz K, Risk factors and other epidemiologic considerations for cervical cancer screening: A narrative review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 155, 698–705, W216 (2011). doi: 10.7326/0003-4819-155-10-201111150-00377 [DOI] [PubMed] [Google Scholar]
- 13.Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, Smith N, Whitlock EP, Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 315, 2576–2594 (2016). doi: 10.1001/jama.2016.3332 [DOI] [PubMed] [Google Scholar]
- 14.Liang PS, Wheat CL, Abhat A, Brenner AT, Fagerlin A, Hayward RA, Thomas JP, Vijan S, Inadomi JM, Adherence to Competing Strategies for Colorectal Cancer Screening Over 3 Years. Am. J. Gastroenterol. 111, 105–114 (2016). doi: 10.1038/ajg.2015.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall IJ, Tangka FKL, Sabatino SA, Thompson TD, Graubard BI, Breen N, Patterns and Trends in Cancer Screening in the United States. Prev. Chronic Dis. 15, E97 (2018). doi: 10.5888/pcd15.170465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava S, Koay EJ, Borowsky AD, De Marzo AM, Ghosh S, Wagner PD, Kramer BS, Cancer overdiagnosis: A biological challenge and clinical dilemma. Nat. Rev. Cancer 19, 349–358 (2019). doi: 10.1038/s41568-019-0142-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cescon DW, Bratman SV, Chan SM, Siu LL, Circulating tumor DNA and liquid biopsy in oncology. Nature Cancer. 2020. [DOI] [PubMed] [Google Scholar]
- 18.Mattox AK, Bettegowda C, Zhou S, Papadopoulos N, Kinzler KW, Vogelstein B, Applications of liquid biopsies for cancer. Sci. Transl. Med. 11, eaay1984 (2019). doi: 10.1126/scitranslmed.aay1984 [DOI] [PubMed] [Google Scholar]
- 19.Berger MF, Mardis ER, The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 15, 353–365 (2018). doi: 10.1038/s41571-018-0002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz LA Jr., A. Bardelli, Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 32, 579–586 (2014). doi: 10.1200/JCO.2012.45.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, Hruban RH, Wolfgang CL, Goggins MG, Dal Molin M, Wang T-L, Roden R, Klein AP, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Vogelstein JT, Browne JD, Schoen RE, Brand RE, Tie J, Gibbs P, Wong H-L, Mansfield AS, Jen J, Hanash SM, Falconi M, Allen PJ, Zhou S, Bettegowda C, Diaz LA Jr., Tomasetti C, Kinzler KW, Vogelstein B, Lennon AM, N. Papadopoulos, Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018). doi: 10.1126/science.aar3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Divo MJ, Martinez CH, Mannino DM, Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 44, 1055–1068 (2014). doi: 10.1183/09031936.00059814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, Antonarakis ES, Azad NS, Bardelli A, Brem H, Cameron JL, Lee CC, Fecher LA, Gallia GL, Gibbs P, Le D, Giuntoli RL, Goggins M, Hogarty MD, Holdhoff M, Hong S-M, Jiao Y, Juhl HH, Kim JJ, Siravegna G, Laheru DA, Lauricella C, Lim M, Lipson EJ, Marie SKN, Netto GJ, Oliner KS, Olivi A, Olsson L, Riggins GJ, Sartore-Bianchi A, Schmidt K, Shih M, Oba-Shinjo SM, Siena S, Theodorescu D, Tie J, Harkins TT, Veronese S, Wang T-L, Weingart JD, Wolfgang CL, Wood LD, Xing D, Hruban RH, Wu J, Allen PJ, Schmidt CM, Choti MA, Velculescu VE, Kinzler KW, Vogelstein B, Papadopoulos N, Diaz LA Jr., Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224ra24 (2014). doi: 10.1126/scitranslmed.3007094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood GC, Chu X, Manney C, Strodel W, Petrick A, Gabrielsen J, Seiler J, Carey D, Argyropoulos G, Benotti P, Still CD, Gerhard GS, An electronic health record-enabled obesity database. BMC Med. Inform. Decis. Mak. 12, 45 (2012). doi: 10.1186/1472-6947-12-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shumilov E, Flach J, Pabst T, Fiedler M, Angelillo-Scherrer A, Trümper L, Joncourt R, Kohlmann A, Bacher U, Genetic alterations crossing the borders of distinct hematopoetic lineages and solid tumors: Diagnostic challenges in the era of high-throughput sequencing in hemato-oncology. Crit. Rev. Oncol. Hematol. 126, 64–79 (2018). doi: 10.1016/j.critrevonc.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 26.Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, Chu SWI, Mak C, Tse IOL, Leung SYM, Chan G, Hui EP, Ma BBY, Chiu RWK, Leung S-F, van Hasselt AC, Chan ATC, Lo YMD, Analysis of Plasma Epstein-Barr Virus DNA to Screen for Nasopharyngeal Cancer. N. Engl. J. Med. 377, 513–522 (2017). doi: 10.1056/NEJMoa1701717 [DOI] [PubMed] [Google Scholar]
- 27.Czernin J, Allen-Auerbach M, Nathanson D, Herrmann K, PET/CT in Oncology: Current Status and Perspectives. Curr. Radiol. Rep. 1, 177–190 (2013). doi: 10.1007/s40134-013-0016-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrevens L, Lorent N, Dooms C, Vansteenkiste J, The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist 9, 633–643 (2004). doi: 10.1634/theoncologist.9-6-633 [DOI] [PubMed] [Google Scholar]
- 29.Schöder H, Gönen M, Screening for cancer with PET and PET/CT: Potential and limitations. J. Nucl. Med. 48 (Suppl 1), 4S–18S (2007). [PubMed] [Google Scholar]
- 30.Sachelarie I, Kerr K, Ghesani M, Blum RH, Integrated PET-CT: Evidence-based review of oncology indications. Oncology 19, 481–490, discussion 490–492, 495–496 (2005). [PubMed] [Google Scholar]
- 31.Douville C, Cohen JD, Ptak J, Popoli M, Schaefer J, Silliman N, Dobbyn L, Schoen RE, Tie J, Gibbs P, Goggins M, Wolfgang CL, Wang T-L, Shih I-M, Karchin R, Lennon AM, Hruban RH, Tomasetti C, Bettegowda C, Kinzler KW, Papadopoulos N, Vogelstein B, Assessing aneuploidy with repetitive element sequencing. Proc. Natl. Acad. Sci. U.S.A. 117, 4858–4863 (2020). doi: 10.1073/pnas.1910041117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B, Detection and quantification of rare mutations with massively parallel sequencing. Proc. Natl. Acad. Sci. U.S.A. 108, 9530–9535 (2011). doi: 10.1073/pnas.1105422108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamakoshi K, Kikkawa F, Shibata K, Tomoda K, Obata NH, Wakahara F, Tokuhashi Y, Ishikawa H, Kawai M, Tomoda Y, Clinical value of CA125, CA19–9, CEA, CA72–4, and TPA in borderline ovarian tumor. Gynecol. Oncol. 62, 67–72 (1996). doi: 10.1006/gyno.1996.0191 [DOI] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, MacKenzie CR, A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383 (1987). doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 35.Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, Feldman-Stewart D, Validation of a decision regret scale. Med. Decis. Making 23, 281–292 (2003). doi: 10.1177/0272989X03256005 [DOI] [PubMed] [Google Scholar]
- 36.Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, Cummings SR, Absalan F, Alexander G, Allen B, Amini H, Aravanis AM, Bagaria S, Bazargan L, Beausang JF, Berman J, Betts C, Blocker A, Bredno J, Calef R, Cann G, Carter J, Chang C, Chawla H, Chen X, Chien TC, Civello D, Davydov K, Demas V, Desai M, Dong Z, Fayzullina S, Fields AP, Filippova D, Freese P, Fung ET, Gnerre S, Gross S, Halks-Miller M, Hall MP, Hartman A-R, Hou C, Hubbell E, Hunkapiller N, Jagadeesh K, Jamshidi A, Jiang R, Jung B, Kim TH, Klausner RD, Kurtzman KN, Lee M, Lin W, Lipson J, Liu H, Liu Q, Lopatin M, Maddala T, Maher MC, Melton C, Mich A, Nautiyal S, Newman J, Newman J, Nicula V, Nicolaou C, Nikolic O, Pan W, Patel S, Prins SA, Rava R, Ronaghi N, Sakarya O, Satya RV, Schellenberger J, Scott E, Sehnert AJ, Shaknovich R, Shanmugam A, Shashidhar KC, Shen L, Shenoy A, Shojaee S, Singh P, Steffen KK, Tang S, Toung JM, Valouev A, Venn O, Williams RT, Wu T, Xu HH, Yakym C, Yang X, Yecies J, Yip AS, Youngren J, Yue J, Zhang J, Zhang L, Zhang LQ, Zhang N, Curtis C, Berry DA, Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. (2020). doi: 10.1016/j.annonc.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J, Cell-free DNA Comprises an In Vivo Nucleosome Footprint that Informs Its Tissues-Of-Origin. Cell 164, 57–68 (2016). doi: 10.1016/j.cell.2015.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, Jensen SØ, Medina JE, Hruban C, White JR, Palsgrove DN, Niknafs N, Anagnostou V, Forde P, Naidoo J, Marrone K, Brahmer J, Woodward BD, Husain H, van Rooijen KL, Ørntoft MW, Madsen AH, van de Velde CJH, Verheij M, Cats A, Punt CJA, Vink GR, van Grieken NCT, Koopman M, Fijneman RJA, Johansen JS, Nielsen HJ, Meijer GA, Andersen CL, Scharpf RB, Velculescu VE, Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 570, 385–389 (2019). doi: 10.1038/s41586-019-1272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razavi P, Li BT, Brown DN, Jung B, Hubbell E, Shen R, Abida W, Juluru K, De Bruijn I, Hou C, Venn O, Lim R, Anand A, Maddala T, Gnerre S, Vijaya Satya R, Liu Q, Shen L, Eattock N, Yue J, Blocker AW, Lee M, Sehnert A, Xu H, Hall MP, Santiago-Zayas A, Novotny WF, Isbell JM, Rusch VW, Plitas G, Heerdt AS, Ladanyi M, Hyman DM, Jones DR, Morrow M, Riely GJ, Scher HI, Rudin CM, Robson ME, Diaz LA Jr., Solit DB, Aravanis AM, Reis-Filho JS, High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat. Med. 25, 1928–1937 (2019). doi: 10.1038/s41591-019-0652-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann-Werman R, Neiman D, Zemmour H, Moss J, Magenheim J, Vaknin-Dembinsky A, Rubertsson S, Nellgård B, Blennow K, Zetterberg H, Spalding K, Haller MJ, Wasserfall CH, Schatz DA, Greenbaum CJ, Dorrell C, Grompe M, Zick A, Hubert A, Maoz M, Fendrich V, Bartsch DK, Golan T, Ben Sasson SA, Zamir G, Razin A, Cedar H, Shapiro AMJ, Glaser B, Shemer R, Dor Y, Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc. Natl. Acad. Sci. U.S.A. 113, E1826–E1834 (2016). doi: 10.1073/pnas.1519286113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang P, Sun K, Peng W, Cheng SH, Ni M, Yeung PC, Heung MMS, Xie T, Shang H, Zhou Z, Chan RWY, Wong J, Wong VWS, Poon LC, Leung TY, Lam WKJ, Chan JYK, Chan HLY, Chan KCA, Chiu RWK, Lo YMD, Plasma DNA End-Motif Profiling as a Fragmentomic Marker in Cancer, Pregnancy, and Transplantation. Cancer Discov. CD-19–0622 (2020). doi: 10.1158/2159-8290.CD-19-0622 [DOI] [PubMed] [Google Scholar]
- 42.Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, Diaz LA Jr., Goodman SN, David KA, Juhl H, Kinzler KW, Vogelstein B, Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. U.S.A. 102, 16368–16373 (2005). doi: 10.1073/pnas.0507904102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mouliere F, Chandrananda D, Piskorz AM, Moore EK, Morris J, Ahlborn LB, Mair R, Goranova T, Marass F, Heider K, Wan JCM, Supernat A, Hudecova I, Gounaris I, Ros S, Jimenez-Linan M, Garcia-Corbacho J, Patel K, Østrup O, Murphy S, Eldridge MD, Gale D, Stewart GD, Burge J, Cooper WN, van der Heijden MS, Massie CE, Watts C, Corrie P, Pacey S, Brindle KM, Baird RD, Mau-Sørensen M, Parkinson CA, Smith CG, Brenton JD, Rosenfeld N, Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 10, eaat4921 (2018). doi: 10.1126/scitranslmed.aat4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao X, Luo H, Krawczyk M, Wei W, Wang W, Wang J, Flagg K, Hou J, Zhang H, Yi S, Jafari M, Lin D, Chung C, Caughey BA, Li G, Dhar D, Shi W, Zheng L, Hou R, Zhu J, Zhao L, Fu X, Zhang E, Zhang C, Zhu J-K, Karin M, Xu R-H, Zhang K, DNA methylation markers for diagnosis and prognosis of common cancers. Proc. Natl. Acad. Sci. U.S.A. 114, 7414–7419 (2017). doi: 10.1073/pnas.1703577114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization, International Agency for Research on Cancer, WHO Classification of Tumours of the Digestive System, Bosman FT et al. , Eds., World Health Organization Classification of Tumours, vol. 3 (International Agency for Research on Cancer, Lyon, ed. 4, 2010). [Google Scholar]
- 46.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP, The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67, 93–99 (2017). doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 47.Rohatiner A, d’Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M, Somers R, Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann. Oncol. 5, 397–400 (1994). doi: 10.1093/oxfordjournals.annonc.a058869 [DOI] [PubMed] [Google Scholar]
- 48.Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, Cole CG, Ward S, Dawson E, Ponting L, Stefancsik R, Harsha B, Kok CY, Jia M, Jubb H, Sondka Z, Thompson S, De T, Campbell PJ, COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 45, D777–D783 (2017). doi: 10.1093/nar/gkw1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, Silliman N, Tacey M, Wong H-L, Christie M, Kosmider S, Skinner I, Wong R, Steel M, Tran B, Desai J, Jones I, Haydon A, Hayes T, Price TJ, Strausberg RL, Diaz LA Jr., Papadopoulos N, Kinzler KW, Vogelstein B, Gibbs P, Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 8, 346ra92 (2016). doi: 10.1126/scitranslmed.aaf6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Do H, Dobrovic A, Sequence artifacts in DNA from formalin-fixed tissues: Causes and strategies for minimization. Clin. Chem. 61, 64–71 (2015). doi: 10.1373/clinchem.2014.223040 [DOI] [PubMed] [Google Scholar]
- 51.Sacks D, McClenny TE, Cardella JF, Lewis CA, Society of Interventional Radiology clinical practice guidelines. J. Vasc. Interv. Radiol. 14, S199–S202 (2003). doi: 10.1097/01.RVI.0000094584.83406.3e [DOI] [PubMed] [Google Scholar]
- 52.CDC, Behavioral Risk Factor Surveillance System Survey Data (U.S. Department of Health and Human Services, Center for Disease Control and Prevention, Atlanta, GA, 2018). [Google Scholar]
- 53.Zahnd WE, Eberth JM, Lung Cancer Screening Utilization: A Behavioral Risk Factor Surveillance System Analysis. Am. J. Prev. Med. 57, 250–255 (2019). doi: 10.1016/j.amepre.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 54.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG, Screening for lung cancer with low-dose computed tomography: A systematic review to update the US Preventive services task force recommendation. Ann. Intern. Med. 159, 411–420 (2013). doi: 10.7326/0003-4819-159-6-201309170-00690 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.