Abstract
Solitary extramedullary plasmacytoma (SEP) of the larynx is a rare haematological malignancy and an infrequent cause of persisting dysphonia. We present the case of a 54-year-old woman with a long-standing history of dysphonia. While clinical examination showed a rather inconspicuous prominent right vestibular fold, an MRI revealed a laryngeal mass with erosion of the thyroid cartilage. A biopsy taken during rigid endoscopy demonstrated plasma cell infiltration with light chain restriction amidst amyloid deposits. After exclusion of systemic involvement, the diagnosis of an SEP of the larynx with secondary amyloidosis was made. The patient received primary radiation therapy. Another biopsy taken 3 months after the end of therapy did not show any signs of ongoing neoplastic plasma cell disease. The patient was therefore considered to be in remission. She is currently receiving regular follow-up and has not shown signs of persistent or progressive disease for the past 18 months.
Keywords: ear, nose and throat/otolaryngology; haematology (incl blood transfusion); radiotherapy
Background
While laryngitis and benign or cancerous laryngeal neoplasms are more common causes of persistent dysphonia,1 solitary extramedullary plasmacytoma (SEP) of the larynx is very rare. It accounts for less than 0.2% of laryngeal malignancies and should be considered if there is seemingly no explanation in a patient with chronic dysphonia. This contribution highlights workup and the involved treatment modalities of this uncommon disease.
Case presentation
An otherwise healthy 54-year-old woman was referred to our institution by a local speech therapist with a complaint of hoarseness, which had initially begun after an upper respiratory tract infection. She had initially been seen by an ear, nose and throat specialist at another clinic, who had not found any obvious pathologies on flexible endoscopy at the time. Stroboscopy was not performed. However, vocal therapy was prescribed, which was carried out rigorously under supervision and at home. Despite those efforts, symptoms had now been increasing over a period of 8 months. The patient denied any history of smoking or alcohol abuse. Laryngostroboscopy showed normal true vocal folds with full closure at phonation and symmetrical movement. The false vocal folds were unusually prominent with intact mucosa and hesitant movement on the right side (figure 1). Voice quality was rated R2B0H0. However, no extralaryngeal symptoms of functional dysphonia were present. No cervical masses could be palpated.
Figure 1.
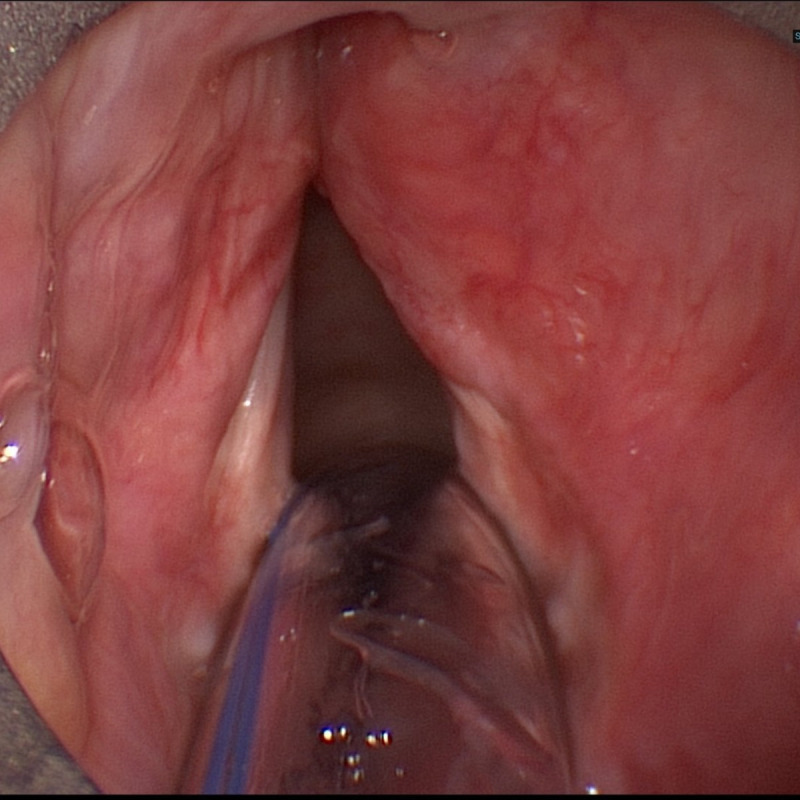
Initial presentation of the false vocals fold on the right without apparent ulcerations or tumorous lesions.
Investigations
Additional diagnostic measures included MRI (figure 2) and CT (figure 3) imaging of the neck, which displayed a unilateral infiltrating mass of the right vestibular fold. Its size was measured at 19×10×19 mm and it eroded the thyroid cartilage and the thyrohyoid membrane. Rigid endoscopy and biopsy were performed. Histological specimens demonstrated immunoglobulin light chain restricted plasma cell infiltration (characteristic of plasmacytoma) as well as amyloid deposits (figure 4). An extensive workup was done by the haematology department, including comprehensive urine and blood analysis, bone marrow biopsy, positron emission tomography (PET; figure 5) and full-body MRI. There was no evidence of systemic disease. Therefore, the diagnosis of a solitary extramedullary plasmacytoma of the larynx with secondary amyloidosis was established.
Figure 2.
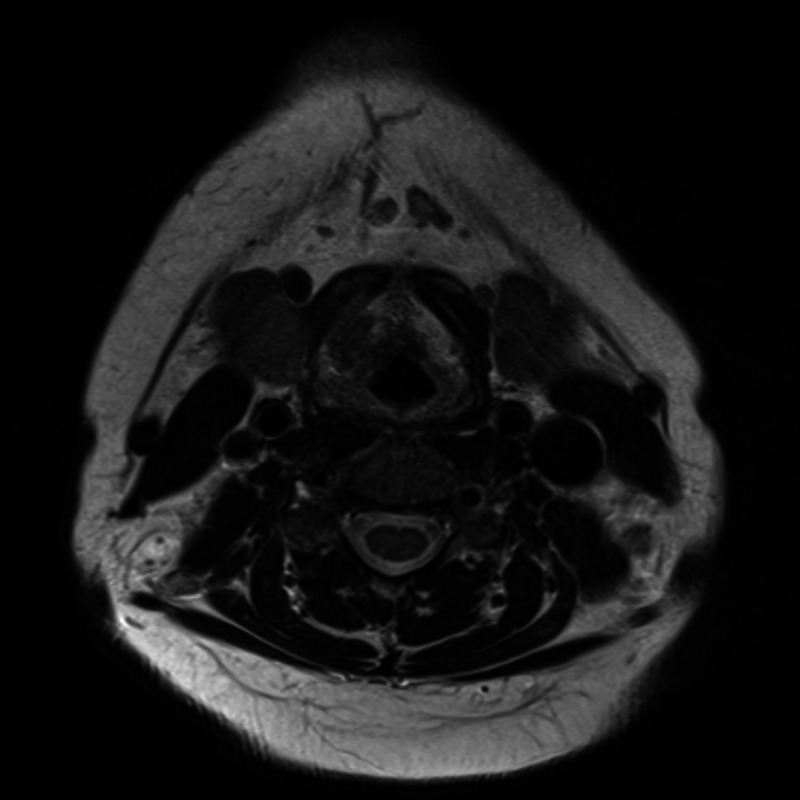
An MRI taken prior to therapy shows an infiltrating laryngeal mass.
Figure 3.
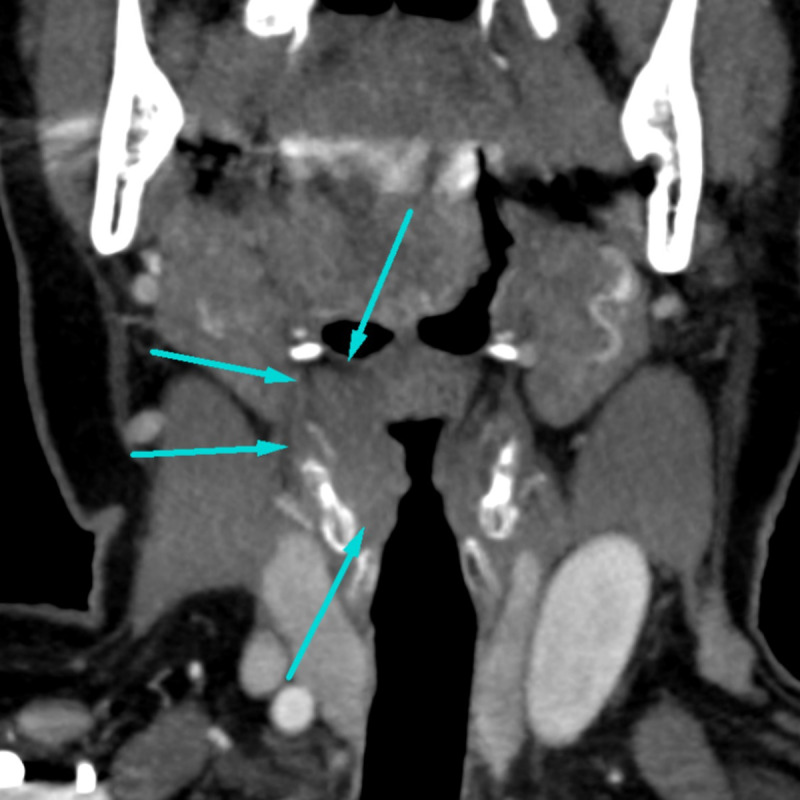
Coronal CT imaging displays a mass eroding through the thyroid cartilage on the right.
Figure 4.
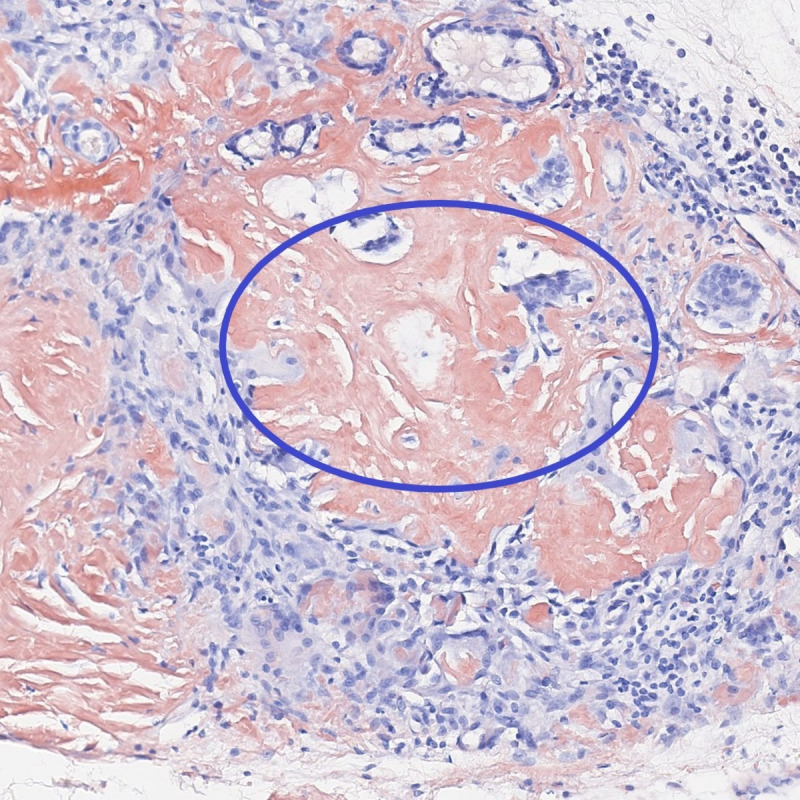
Histological specimens demonstrate plasma cell infiltration next to amyloid deposits in characteristic “Congo red” staining (circle).
Figure 5.
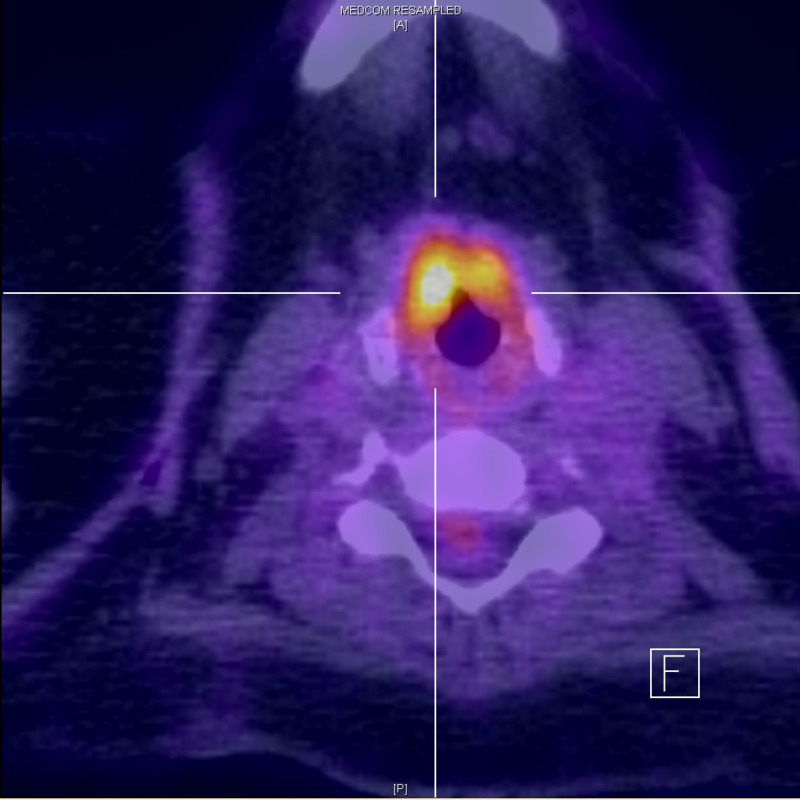
Positron emission tomography CT demonstrates metabolic activity in the right false vocals fold.
Differential diagnosis
Differential diagnosis in this setting must invariably include the most common malignancy in this area—squamous cell carcinoma, especially in the context of a malignant mass eroding through the thyroid cartilage. Amyloid deposits in this area without evidence of plasma cell neoplasia may represent another entity called primary amyloidosis. Although uncommon in general, the larynx represents one of the—if not the—most common locations for this disease.
Treatment
The case was presented at our interdisciplinary tumour board, where primary radiation therapy in curative intention was determined. Treatment was executed at the Department of Radiation Oncology with a dosing of 50 Grey (25×200 cGy) over 5 weeks’ time.
Outcome and follow-up
Three months after therapy, the patient underwent re-staging consisting of an MRI (figure 6) and another endoscopy with biopsy (figure 7). Radiological studies demonstrated a persistent mass in the previously described area, histology showed persistent amyloid deposits but did not reveal evidence of ongoing plasma cell neoplasia. Therefore, the patient is considered to be in remission. She is now in our clinic for follow-up once every 3 months, where she is evaluated by haematologists and otorhinolaryngologists clinically and with comprehensive blood and urine analysis. Radiological follow-up consists of a yearly MRI of the neck. Her last endoscopic and laboratory evaluation, 18 months after therapy showed no signs of persistent or progressive disease.
Figure 6.
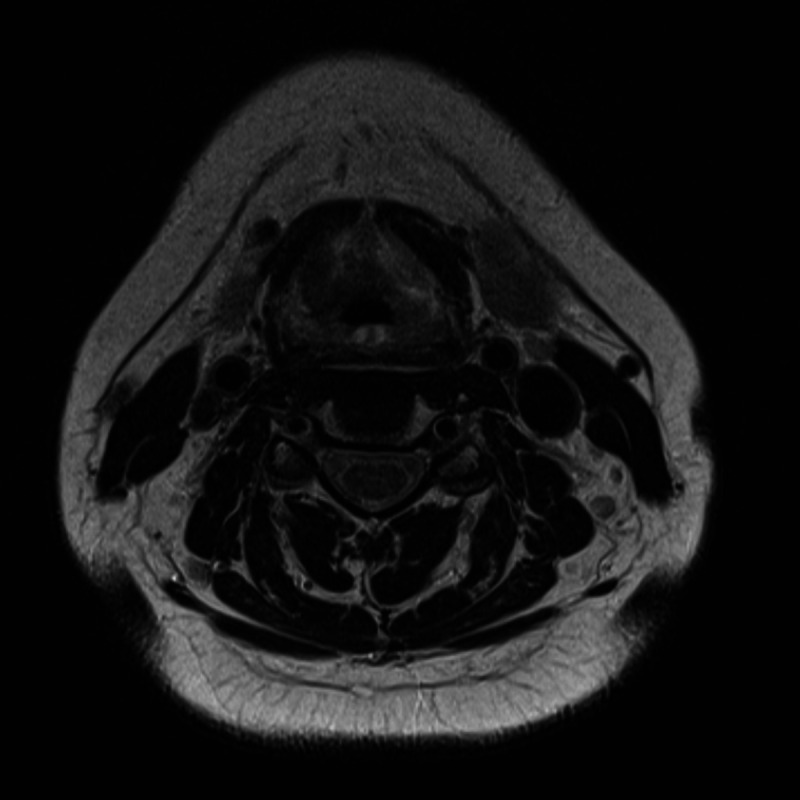
An MRI after therapy does not show significant changes in the area of the plasmacytoma.
Figure 7.
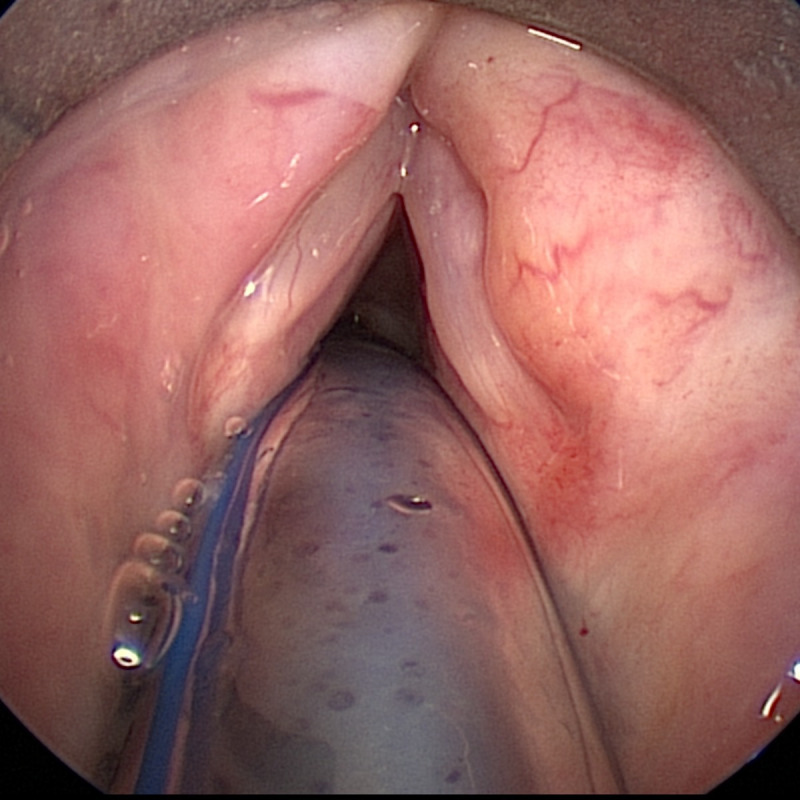
The post-therapeutic presentation of the false vocals fold on the right.
Discussion
Plasma cell malignancies are a group of hematological neoplasms, characterised by abnormal clonal proliferation of plasma cells.2 Depending on the level of dissemination, it is possible to distinguish between multiple myeloma (MM) and plasmacytoma. MM is comparatively common, accounting for around 10% of all hematological malignancies and 1% of all cancers.3 4 It can be seen as a disseminated plasma cell dyscrasia with systemic involvement.3 The incidence of MM is around 2 to 5 per 100 000 people.
Plasmacytoma, however, is defined as a solitary lesion either in the bone marrow (termed a solitary bone plasmacytoma (SBP)) or the soft tissue (termed an SEP).5 SBPs are far less common. A report from 2009 gives an estimate of around three new cases per million per year. SBP seems to have a higher prevalence than SEP.2
The entities described have a close relationship, as the prognosis of plasmacytoma is essentially dependent on the progression to MM.
The larynx represents a common location among SEPs, as 70%–85% of these neoplasms are discovered in the head and neck mucosa,6 7 primarily in the nasal cavity and paranasal sinuses.8 A recent review has reported a total of 99 cases since 1948.9 Clinical symptoms closely relate to the location of the tumour and the infiltration of laryngeal structures. Clinical presentation of SEP of the larynx may vary. Findings on flexible endoscopy can show a polypoid mass10 or a rather inconspicuous swelling of the paraglottic soft tissue.11
Definitive diagnosis of electromagnetic pulse (EMP) is based on histological and immunohistochemical evaluation and the exclusion of a systemic proliferative plasma cell disorder.12 The role of PET may increase the diagnostic accuracy by detecting or excluding additional lesions not diagnosed by other radiological studies.13 Secondary amyloid deposits are often spotted in the proximity of SEPs.11 14
There are no specific treatment guidelines for laryngeal SEP. In general, radiotherapy is the first-line treatment for all cases of solitary plasmacytoma.6 15 Surgical resection represents a favourable therapeutic option for small and easily accessible EMP of the head and neck region and may still be combined with adjuvant radiotherapy.9 11
As mentioned above, the prognosis of plasmacytoma depends on its progression to MM, and about 10% of solitary plasmacytoma progress to MM within 3 years.4 Prognosis of SBP is far worse than that of SEP.5 7 16 In general, prognosis of SEP seems favourable, with a reported 5-year survival rate of over 80%.9 Moreover, SEP located in the head and neck region seem to have an even better prognosis compared with other locations of the body.17 Overall survival of 10 years is estimated to exceed 70%. However, negative prognostic factors include cervical lymphadenopathy and the involvement of multiple sites9; the latter excluding the pathology from the group of SEP.
Because patients may present with the systemic disease even years after local curative therapy, long-term follow-up is indicated.18
Learning points.
Consider radiographical evaluations in a patient with chronic dysphonia and ‘normal’ flexible endoscopy.
In case biopsy of a laryngeal mass reveals plasma cell neoplasia, hematological investigations are warranted as guidance towards the correct diagnosis.
Radiation therapy is the therapy of choice in solitary extramedullary plasmacytoma, even though easily accessible or exposed locations may render surgery a favourable therapeutic option.
Long-term follow-up after definitive therapy is mandatory in SEP, as patients may present with the systemic disease even years after local curative therapy.
Footnotes
Contributors: SJB made initial contact in her phoniatric clinic and signed patient up for further testing. NT performed first operation and initiated therapy. HHB performed second operation and wrote first draft of case report. It was then thoroughly reviewed by NT and SJB.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cohen SM, Kim J, Roy N, et al. . Prevalence and causes of dysphonia in a large treatment-seeking population. Laryngoscope 2012;122:343–8. 10.1002/lary.22426 [DOI] [PubMed] [Google Scholar]
- 2.Dores GM, Landgren O, McGlynn KA, et al. . Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol 2009;144:86–94. 10.1111/j.1365-2141.2008.07421.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016;91:719–34. 10.1002/ajh.24402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. . International myeloma Working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–48. 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 5.Grammatico S, Scalzulli E, Petrucci MT. Solitary plasmacytoma. Mediterr J Hematol Infect Dis 2017;9:e2017052. 10.4084/mjhid.2017.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohana N, Rouvio O, Nalbandyan K, et al. . Classification of solitary plasmacytoma, is it more intricate than presently suggested? A commentary. J Cancer 2018;9:3894–7. 10.7150/jca.26854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finsinger P, Grammatico S, Chisini M, et al. . Clinical features and prognostic factors in solitary plasmacytoma. Br J Haematol 2016;172:554–60. 10.1111/bjh.13870 [DOI] [PubMed] [Google Scholar]
- 8.Liebross RH, Ha CS, Cox JD, et al. . Clinical course of solitary extramedullary plasmacytoma. Radiother Oncol 1999;52:245–9. 10.1016/S0167-8140(99)00114-0 [DOI] [PubMed] [Google Scholar]
- 9.Ge S, Zhu G, Yi Y. Extramedullary plasmacytoma of the larynx: literature review and report of a case who subsequently developed acute myeloid leukemia. Oncol Lett 2018;16:2995–3004. 10.3892/ol.2018.8992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratibha CB, Sreenivas V, Babu MK, et al. . Plasmacytoma of larynx--a case report. J Voice 2009;23:735–8. 10.1016/j.jvoice.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 11.Loyo M, Baras A, Akst LM. Plasmacytoma of the larynx. Am J Otolaryngol 2013;34:172–5. 10.1016/j.amjoto.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Hamilos G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr Treat Options Oncol 2002;3:255–9. 10.1007/s11864-002-0015-2 [DOI] [PubMed] [Google Scholar]
- 13.Schirrmeister H, Buck AK, Bergmann L, et al. . Positron emission tomography (PET) for staging of solitary plasmacytoma. Cancer Biother Radiopharm 2003;18:841–5. 10.1089/108497803770418382 [DOI] [PubMed] [Google Scholar]
- 14.Agrawal R. Soft tissue amyloidoma in association with plasmacytoma. Indian J Pathol Microbiol 2016;59:444. 10.4103/0377-4929.191752 [DOI] [PubMed] [Google Scholar]
- 15.Tsang RW, Campbell BA, Goda JS, et al. . Radiation therapy for solitary plasmacytoma and multiple myeloma: guidelines from the International lymphoma radiation Oncology Group. Int J Radiat Oncol Biol Phys 2018;101:794–808. 10.1016/j.ijrobp.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 16.El-Fattah MA, Aboelmagd M, Elhamouly M. Clinical risk factors of plasmacytoma mortality: a US population-based study. Br J Haematol 2017;179:161–2. 10.1111/bjh.14189 [DOI] [PubMed] [Google Scholar]
- 17.Gerry D, Lentsch EJ. Epidemiologic evidence of superior outcomes for extramedullary plasmacytoma of the head and neck. Otolaryngol Head Neck Surg 2013;148:974–81. 10.1177/0194599813481334 [DOI] [PubMed] [Google Scholar]
- 18.Alexiou C, Kau RJ, Dietzfelbinger H, et al. . Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer 1999;85:2305–14. [PubMed] [Google Scholar]


