Abstract
Objectives
In developed countries, the incidence of colorectal cancer (CRC) has declined in the over 50 years age group but increased in younger people. We studied CRC incidence by age and the influence of screening uptake.
Design
Age-standardised and sex-standardised incidences for CRC from 1997 to 2017 were obtained from the Scottish Cancer Registry (SCR). In addition, linkage between the Scottish Bowel Screening Database and the SCR allowed investigation of any association between screening participation and CRC incidence.
Setting
Scotland and the Scottish Bowel Screening Programme, in which guaiac faecal occult blood test screening was piloted from March 2000 and fully rolled by December 2009.
Participants
From the introduction of screening in 2000 through to 2017, 2 395 172 were invited to participate, of whom 1 487 999 participated at least once.
Main outcome measures
Incidence of CRC.
Results
In the screening age range (50–74 years), CRC incidence peaked at 156.5 cases per 100 000 in 2010 after full roll-out of screening across Scotland but fell to 123.9 per 100 000 in 2017. However, under 50 years, there was a rise from 5.3 cases per 100 000 in 2000 to 6.8 per 100 000 in 2017. When CRC incidence was examined in those who had been offered screening, incidence fell in the participant group more than in the non-participant group after roll-out of screening was complete. Analysis of cumulative incidence demonstrated that CRC incidence in the participant group remained consistently below that of the non-participant from around 7 years of follow-up.
Conclusions
The incidence of CRC in Scotland has declined in the over 50 years age group but increased in younger people. It is likely that population screening has contributed to the reduction in CRC incidence in the over 50 years age group.
Keywords: adenoma, colorectal cancer, faecal occult blood test, incidence, mortality, screening
Strengths and limitations of this study.
We examine age-standardised and sex-standardised incidence data for colorectal cancer (CRC) for the years 1997–2017 obtained from the Scottish Cancer Registry and linkage between the Registry and the Scottish Bowel Screening Database.
We are able to show previously unreported changes in the incidence of CRC in Scotland in all age groups.
We are able to examine in detail, for the first time, if population-based screening using faecal occult blood test is associated with incidence of CRC.
Associations between incidence and plausible reasons for the changes over time will help the development of future interventions.
Although this was a large study, we did not examine incidence by yearly age, only in three large groups.
Introduction
Colorectal cancer (CRC) is a major health problem worldwide. In Scotland, it is currently the third most commonly diagnosed non-cutaneous cancer and the second most common cause of cancer-related deaths.1 However, the incidence of CRC varies widely across the world and it is estimated that 60% of CRC deaths occur in those countries with a high or very high Human Development Index (HDI).2 Western lifestyle factors, including obesity, lack of physical exercise, a diet rich in red and processed meat, smoking and alcohol, have all been linked to the risk of developing CRC,3 and marked increases in both incidence and mortality have been observed in many medium–high HDI countries, especially in Asia, South America and Eastern Europe.2 However, both incidence and mortality have either stabilised or declined in countries with the highest HDI, for example, Australia, New Zealand, the USA and several western European countries.4 These reductions in mortality are likely to be attributable to improved access to high-quality treatment and earlier diagnosis through both screening and through prompt investigation of those presenting with symptoms.
The declines in incidence are not so easy to explain, especially since the frequency of obesity continues to rise in all high HDI countries.5 However, there is now good evidence, largely from the USA and particularly from the Surveillance, Epidemiology and End Results Database that, while incidence has been falling since around 1985 in those aged 50 years and over, it has been rising in those under 50 years since the mid-1990s.3 In a recent analysis of incidence and mortality databases from 39 countries, it was confirmed that countries with the highest HDI had a decrease in CRC incidence, but that incidence of colon and rectal cancers has continued to increase in countries with medium–high HDI, and in younger populations.6 It has been postulated that screening may be responsible for the reduction in incidence in the group aged over 50 years, especially where this is associated with high rates of large bowel endoscopy, which facilitates the diagnosis and removal of pre-malignant adenomas.3 Since there is now robust evidence from randomised trials of endoscopic screening that removal of adenoma leads to a reduction in CRC incidence,7 this is an attractive hypothesis, but one that is difficult to test.
In Scotland, CRC screening, initially using biennial guaiac faecal occult blood tests (gFOBT), was rolled out to the whole of the population aged 50–74 years in 2007 after a three screening rounds pilot which started in 2000.8 9 In addition, Scotland, along with the rest of the UK, is ranked as having a very high HDI,2 and although the incidence has fallen by 18.6% from 2007 to 2017,1 it still has a high incidence of CRC,1 and lifestyle factors associated with CRC are prevalent in the population.10 We therefore examined the incidence of CRC between 1997 and 2017 in the 50–74 years screening age range, the post-screening age range and the pre-screening age range. In addition, the effect of screening participation on CRC incidence was assessed.
Methods
In Scotland, screening for CRC using biennial gFOBT, offered to everyone in the 50–69 years age range and registered with a general practitioner, commenced in March 2000 with a pilot involving three of the fourteen National Health Service (NHS) Boards responsible for routine healthcare. Roll-out to the rest of Scotland began in July 2007 and was completed by December 2009. The age range was also extended up to age 74 years for the whole of Scotland during roll-out. Details of the pilot, roll-out and descriptions of the screening algorithms have been published previously.8 9 Data are collected centrally by the Information Services Division of NHS National Services Scotland and held in the Scottish Bowel Screening Database (SBSD).
To assess changes in the incidence of CRC in different age ranges around the time of the introduction of screening, data were obtained from the Scottish Cancer Registry (SCR) for the years 1997–2017. Crude incidence rates were calculated by sex and 5-year age group using midyear population estimates from the National Records of Scotland (NRS). These rates were then directly standardised using the 2013 European Standard Population. Age–sex standardised rates were calculated separately for the screening (50–74 years), post-screening (75 years and higher) and pre-screening (under 50 years) age ranges. Age–sex standardised CRC mortality rates were also calculated for the screening (50–74 years) and pre-screening (under 50 years) age ranges using death registration data from NRS.
To investigate the impact of screening participation on incidence, linkage was carried out between the SBSD, the SCR and NRS deaths. The SBSD allowed identification of those invited for screening and those who participated. Participants who received a positive or negative screening test result at any point were included in the participant cohort. Those who did not receive a positive or negative test result, or never returned a completed test, were included in the non-participant cohort. Data were included from the pilot through national roll-out, with the data on invites available from March 2000. Linkage with the SCR allowed CRC incidence to be calculated for the participant and non-participant groups and linkage with the NRS deaths records allowed removal of participants from study at the point of death. Follow-up data were available on 31 December 2017. Age–sex standardised rates were calculated for participant and non-participant groups as described above.
The age structure of the screening population changed a great deal in the early years of the study period (see online supplemental table 1). The ageing of the original pilot cohort, in addition to the expansion of the age-range on national roll-out, influences the annual CRC incidence rate, despite adjustment through standardisation. In addition, any reduction seen in annual CRC incidence could be influenced by a shortening time to diagnosis. That is, since cancers are detected earlier, the years after roll-out see a reduction in incidence exclusively due to early detection rather than to prevention of disease. To better analyse these issues, time-to-event analysis was used in addition to the descriptive time-series analysis. This facilitated better understanding of the relationship between participation in screening and how it affects an individual’s risk over time. Cumulative incidence was estimated using the Kaplan-Meier method. Cox regression was also used to estimate the impact of screening participation on time from invite to CRC diagnosis, adjusting for age at first invite, sex and level of socioeconomic status as determined by the Scottish Index of Multiple Deprivation.
bmjopen-2020-037925supp004.pdf (34.7KB, pdf)
An underlying assumption of Cox regression is that of proportional hazards, that is, that the ratio of the hazards between treatment and non-treatment groups remains constant over time. This assumption was not met for the participation status variable, since the CRC hazard increases at biennial intervals for the participant group, consistent with screening participation. In consequence, an alternative analytical approach is also presented, with separate HRs reported for less than, and more than, 7 years of follow-up. Seven years was chosen as the cut-off because participant cumulative incidence is consistently lower than non-participant (and the proportional hazards assumption is met) from this point. All analyses were performed using R statistical software, V.3.5.1 and 95% CIs are shown as bars in the figures, when relevant.
Neither patients, participants in screening, nor the public were involved in any way in development of the research question, the design of the study, or any other aspect of this research. Dissemination to these groups is not possible nor applicable.
Results
A total of 77 262 CRCs were diagnosed in Scotland between 1997 and 2017. From the introduction of screening in 2000–2017, 2 395 172 individuals were invited to participate (409 255 in the pilot, 1 985 917 in the programme), of whom 1 487 999 participated at least once. There were 24 817 CRCs diagnosed within the population invited to screen (15 663 in participants, 9154 in non-participants) in the same period. These CRCs were detected through both screening and non-screening pathways.
In the 50–74 years (screening) age range, a slight drop in incidence was observed, from 154.4 cases per 100 000 in 2000, the first year of the demonstration pilot, to 137.8 in 2006. Then, coinciding with commencement of roll-out of screening across the country in 2007, an increase was noted, which peaked at 156.5 cases per 100 000 in 2010 and began to fall to levels well below those seen in the immediate pre-screening period, reaching 123.9 per 100 000 in 2017 (figure 1). In those aged 75 years and over (post-screening), a consistent drop in incidence was noted from 2009 (432.5 per 100 000) to 2017 (366.8 per 100 100) (figure 2) whereas, in those aged under 50 years (pre-screening) a rise throughout the study period from 5.3 cases per 100 000 in 2000 to 6.8 per 100 000 in 2017 was seen, although with fluctuations (figure 3).
Figure 1.
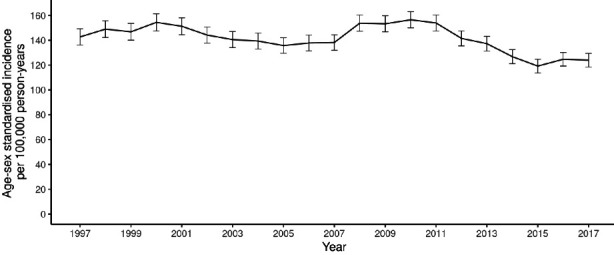
Age–sex standardised colorectal cancer incidence, ages 50–74 years, from 1997 to 2017 (95% CIs shown).
Figure 2.
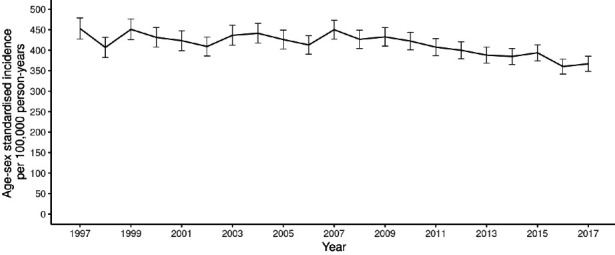
Age–sex standardised colorectal cancer incidence, ages 75 years and over, from 1997 to 2017 per 100 000 person-years (95% CIs shown).
Figure 3.
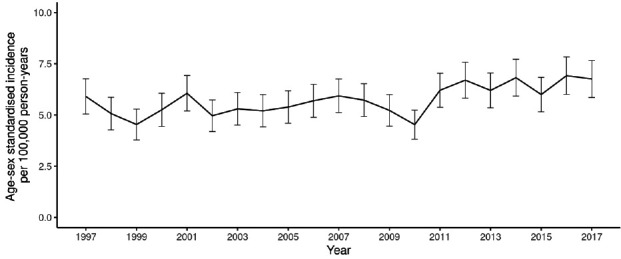
Age–sex standardised colorectal cancer incidence, ages less than 50 years, from 1997 to 2017 per 100 000 person-years (95% CIs shown).
When CRC incidence in the population who had been offered screening was examined, there was a distinct difference between those who had participated at least once and those who never participated. The data shown are age and sex standardised since these variables influence both CRC incidence and uptake of screening, with both uptake and incidence increasing with age, and with uptake being lower, but incidence higher, in men than in women.11 Figure 4 shows that incidence increased more in the participant group than in the non-participant group as national roll-out of screening started but that, after roll-out had been completed, incidence fell in the participant group to a greater extent than in the non-participant group, with participant incidence 13.9% below non-participant in 2017. The large increase in incidence in 2005 was due to there being no invitees in the over 75 years age range prior to this point (see online supplemental table 2). Since the CRC risk in the over 75 years age range is higher than in those aged below 75 years, the age-standardised rates are influenced by this ageing of the invited population.
Figure 4.
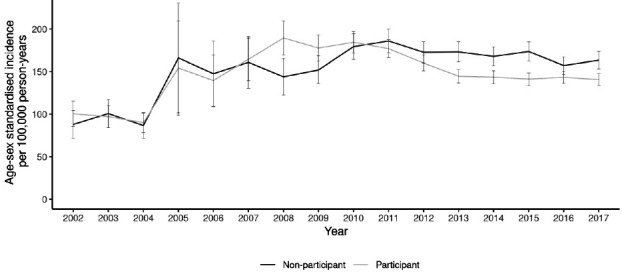
Age–sex standardised colorectal cancer incidence for the screening population per 100 000 person-years, by screening participation status (95% CIs shown).
bmjopen-2020-037925supp005.pdf (33.8KB, pdf)
Analysis of cumulative incidence shown in figure 5 demonstrates the risk of developing CRC over time. Fluctuations can be seen initially in the participant group, consistent with the biennial screening interval. The participant group then remains consistently below that of the non-participant group from around 7 years of follow-up. Cox regression analysis adjusted for age at first invite, sex and socioeconomic deprivation gave an HR of 0.92 for participants, relative to non-participants (95% CI: 0.90–0.95, p<0.001). The HRs, when separating the follow-up period at 7 years, were 0.95 (95% CI: 0.92–0.98, p<0.001) in the period up to 7 years and 0.87 (95% CI: 0.83–0.91, p<0.001) in the period 7 years or more. These data are shown separately for men and women in online supplemental figure 1.
Figure 5.
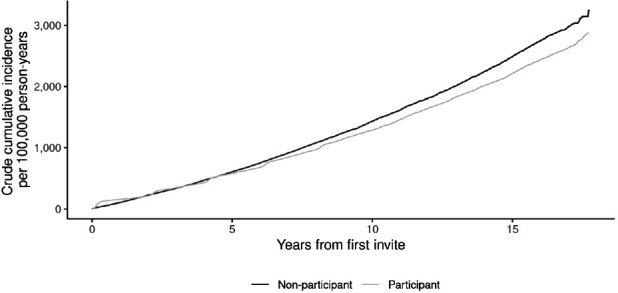
Cumulative colorectal cancer incidence, by screening participation status.
bmjopen-2020-037925supp001.pdf (44KB, pdf)
We also examined mortality in the 50–74 years (screening) and the under 50 years (pre-screening) age groups. These data, with 95% CI, are given as online supplemental figures 2 and 3, and show a substantial reduction in mortality since the introduction of screening in the 50–74 years range, but not in the under 50 years range.
bmjopen-2020-037925supp002.pdf (43.3KB, pdf)
bmjopen-2020-037925supp003.pdf (36.9KB, pdf)
Discussion
Statement of principal findings
The findings in this study have similarities with those reported in from other high-income countries,4 6 namely that the incidence of CRC is falling in older age groups but increasing in people under the age of 50 years. However, in this study, we were not only able to examine the changes in CRC incidence by age, but also by screening participation, and this demonstrated, for the first time to our knowledge, that the fall in incidence was more evident in those who had participated in screening.
Strengths and weaknesses in relation to other studies
The Minnesota randomised controlled trial (RCT), which employed mostly rehydrated Hemoccult II giving a positivity of 9.8 %, did demonstrate a modest reduction in the groups offered screening after 18 years of follow-up,12 but the Nottingham RCT, which used gFOBT in un-rehydrated form (the same approach that was adopted in Scotland) and reported a 2% positivity, showed no effect on CRC incidence after 11 years.13 Overall, previous studies of the effect of gFOBT screening on CRC incidence have not shown a substantial effect. In Scotland, from December 2009, biennial gFOBT screening was being offered to the whole of the eligible population. This resulted in a positivity of around 2%, so that, with an average uptake at this time of 55%, only around 1% of people being offered screening actually underwent colonoscopy.9 Of those that did, the average positive predictive value of gFOBT for CRC was 10% and 40% for adenoma,9 so that less than 0.5% of the population offered screening (the 50–74 years age range) would have had removal of adenoma. However, in the present study, the groups were much bigger than in the RCT and the reduction in incidence seen in the 50–74 years age range is likely to have been due, at least in part, to polypectomy following a positive screening test result. The rise in incidence immediately after roll-out and preceding the consistent fall is likely to have been due to the well-described screening effect caused by a combination of early and overdiagnosis.14 This would not explain the later fall in incidence, however, since the incidence of disease after the introduction of screening tends not to fall back to baseline because of overdiagnosis (ie, some people with screen-detected disease would have never presented clinically) as is the case in breast cancer screening.15
It could be argued that a fall in incidence would not necessarily translate into a fall in mortality, if only indolent cancers were being prevented. However, this is highly unlikely, given that the fall in incidence seen in the flexible sigmoidoscopy screening trials was accompanied by reductions in mortality.7 In addition, just as North America, Oceania and most European countries,6 CRC mortality in the 50–74 years age range in Scotland has fallen over time and it is likely that part of this effect can be attributed to early detection and prevention of disease as a result of screening.16 It is also interesting that we did not observe a fall in CRC mortality in the under 50 years age range, lending further strength to the argument that screening has contributed to this trend.
Meaning of the study
In November 2017, the Scottish Bowel Screening Programme changed the screening test from gFOBT to a quantitative faecal immunochemical test (FIT) at a threshold of 80 µg haemoglobin/g faeces. At this threshold, in the first year of screening with FIT, there was a 100% relative increase in the number of participants with adenomas identified,17 so that, going forward, screening using FIT can be expected to bring about a greater reduction in CRC incidence than has been seen to date and this will be examined when the data become available. The other very important consideration is the increase in CRC incidence seen in younger people. One approach to this could be to extend screening to those aged under 50 years, but it must be borne in mind that, under the age of 50 years, although incidence is increasing,18 it is still much lower than in the current screening age range.
Unanswered questions and future research
There is no objective evidence yet to support screening under the age of 50 years, and other approaches, including improved awareness of symptoms, increased use of FIT to triage patients presenting in primary care with symptoms19 and addressing lifestyle issues in the Scottish population must be part of the solution. The reasons underlying the marked increase in incidence in those aged under 50 years are not clear, but may relate to lifestyle factors, particularly around diet, body weight and physical activity, all of which are associated with increased risk of CRC.20 Rising rates of obesity in younger life (which are indicators of diet and physical activity) are of particular interest, because excess adiposity is now experienced by more people at earlier life stages and a recent study has demonstrated a relationship between body mass index in childhood and risk of adult CRC.21 We cannot necessarily screen our way out of this problem.
Observational data such as these cannot prove definitively that screening is the only cause of reduced incidence. Over the age of 50 years, individuals are much more likely to undergo colonoscopy because of lower bowel symptoms than those under 50 years, and this may explain at least part of the incidence reduction in those aged over 50 years. It is interesting that, in the over 75 years age range, a consistent decline in incidence was seen from 2009 onwards. Some of this cohort will have had the opportunity to return screening tests, but by no means all, and it is likely that colonoscopy for the investigation of symptoms is performed even more frequently in this age range.
However, the clear separation of yearly and cumulative incidence by participation in screening lends persuasive evidence to the hypothesis that screening is at least in part responsible for the observed incidence patterns in the population. It could still be argued that the people who participated in screening were healthier than those who did not, and that lifestyle factors were also responsible for this observation but, given the clear effect of removal of adenomas on CRC incidence,7 it is highly likely that screening played an important role.
Supplementary Material
Acknowledgments
We acknowledge the support of the Scottish government and the NHS National Services and Information Services Divisions.
Footnotes
Twitter: @anniescotta
Contributors: GRCC collected and analysed the data, participated in data interpretation and contributed to writing the paper. ASA contributed on dietary issues, participated in data interpretation and contributed to writing the paper. TGG assisted with analysing and validating the data, participated in data interpretation, and contributed to writing the paper. JAS directed the laboratories that analysed the faecal tests in the SBoSP from 2010, participated in data interpretation and contributed to writing the paper. CGF directed the SBoSP laboratories to 2010, participated in data interpretation and provided significant input into the writing of the paper. RJCS is the clinical director of the SBoSP, initiated the study, led the data interpretation, and wrote the first and final drafts of the paper, and is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The Scottish government is the funder of the study. Award/Grant number not applicable.
Disclaimer: The Scottish government played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare. CGF did consultancy for Immunostics Inc, Ocean, NJ, USA, and does for Hitachi Chemical Diagnostic Systems Co., Ltd, Tokyo, Japan. No other relationships or activities that could appear to have influenced the submitted work have been done.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Formal ethical approval for the study was not required because individual participants were not approached and only routinely collected population-based data were used.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All requests for data sharing should be discussed, in the first instance, with RJCS at r.j.c.steele@dundee.ac.uk.
References
- 1.NHS National Services Scotland Information services division, 2019. Available: https://www.isdscotland.org/Health-Topics/Cancer/Publications/2019-04-30/Cancer_in_Scotland_summary_m.pdf [Accessed 30 Apr 2020].
- 2.Arnold M, Sierra MS, Laversanne M, et al. . Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 3.Connell LC, Mota JM, Braghiroli MI, et al. . The rising incidence of younger patients with colorectal cancer: questions about screening, biology, and treatment. Curr Treat Options Oncol 2017;18:23. 10.1007/s11864-017-0463-3 [DOI] [PubMed] [Google Scholar]
- 4.Araghi M, Soerjomataram I, Bardot A, et al. . Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol 2019;4:511–8. 10.1016/S2468-1253(19)30147-5 [DOI] [PubMed] [Google Scholar]
- 5.GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong MCS, Huang J, Huang JLW, et al. . Global prevalence of colorectal neoplasia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18:e10:553–61. 10.1016/j.cgh.2019.07.016 [DOI] [PubMed] [Google Scholar]
- 7.Atkin W, Wooldrage K, Parkin DM, et al. . Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK flexible sigmoidoscopy screening randomised controlled trial. Lancet 2017;389:1299–311. 10.1016/S0140-6736(17)30396-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steele RJC, McClements PL, Libby G, et al. . Results from the first three rounds of the Scottish demonstration pilot of FOBT screening for colorectal cancer. Gut 2009;58:530–5. 10.1136/gut.2008.162883 [DOI] [PubMed] [Google Scholar]
- 9.Fraser CG, Digby J, McDonald PJ, et al. . Experience with a two-tier reflex gFOBT/FIT strategy in a national bowel screening programme. J Med Screen 2012;19:8–13. 10.1258/jms.2011.011098 [DOI] [PubMed] [Google Scholar]
- 10.Scottish Government Scottish health survey. Available: https://www.gov.scot/publications/scottish-health-survey-2018-volume-1-main-report/ [Accessed 30 Jan 2020].
- 11.Steele RJC, Kostourou I, McClements P, et al. . Effect of gender, age and deprivation on key performance indicators in a FOBT-based colorectal screening programme. J Med Screen 2010;17:68–74. 10.1258/jms.2010.009120 [DOI] [PubMed] [Google Scholar]
- 12.Mandel JS, Church TR, Bond JH, et al. . The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603–7. 10.1056/NEJM200011303432203 [DOI] [PubMed] [Google Scholar]
- 13.Scholefield JH, Moss SM, Mangham CM, et al. . Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut 2012;61:1036–40. 10.1136/gutjnl-2011-300774 [DOI] [PubMed] [Google Scholar]
- 14.Grey M, Raffle A. Evidence and practice. Oxford University Press, 2007. https://www.isdscotland.org/Health-Topics/Cancer/Publications/2019-08-06/2019-08-06-Bowel-Screening-KPI-Report.xlsx [Google Scholar]
- 15.Independent UK Panel on Breast Cancer Screening The benefits and harms of breast cancer screening: an independent review. Lancet 2012;380:1778–86. 10.1016/S0140-6736(12)61611-0 [DOI] [PubMed] [Google Scholar]
- 16.Malvezzi M, Carioli G, Bertuccio P, et al. . European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann Oncol 2018;29:1016–22. 10.1093/annonc/mdy033 [DOI] [PubMed] [Google Scholar]
- 17.Information Services Division (ISD) Scotland Bowel cancer screening. Available: https://www.isdscotland.org/Health-Topics/Cancer/Bowel-Screening/ [Accessed 30 Jan 2020].
- 18.Vuik FE, Nieuwenburg SA, Bardou M, et al. . Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019;68:1820–6. 10.1136/gutjnl-2018-317592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mowat C, Digby J, Strachan JA, et al. . Impact of introducing a faecal immunochemical test (fit) for haemoglobin into primary care on the outcome of patients with new bowel symptoms: a prospective cohort study. BMJ Open Gastroenterol 2019;6:e000293. 10.1136/bmjgast-2019-000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potter JD. Rising rates of colorectal cancer in younger adults. BMJ 2019;365:l4280. 10.1136/bmj.l4280 [DOI] [PubMed] [Google Scholar]
- 21.Jensen BW, Gamborg M, Gögenur I, et al. . Childhood body mass index and height in relation to site-specific risks of colorectal cancers in adult life. Eur J Epidemiol 2017;32:1097–106. 10.1007/s10654-017-0289-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037925supp004.pdf (34.7KB, pdf)
bmjopen-2020-037925supp005.pdf (33.8KB, pdf)
bmjopen-2020-037925supp001.pdf (44KB, pdf)
bmjopen-2020-037925supp002.pdf (43.3KB, pdf)
bmjopen-2020-037925supp003.pdf (36.9KB, pdf)


