Abstract
Aims
Intrarenal Aquaporin 5 (AQP5) is upregulated in patients with diabetic nephropathy. Here we investigate whether urinary AQP5 is independently associated with estimated glomerular filtration rate (eGFR) decline in patients with type 2 diabetes and nephropathy.
Methods
Baseline urine samples (n=997) from patients with type 2 diabetes and nephropathy of the sulodexide macroalbuminuria trial were measured for AQP5 through enzyme-linked immunosorbent assays. Pearson correlation and multiple linear regression between AQP5 with eGFR slope (calculated by ≥3 serum creatinine during follow-up) was performed, and association with fast renal function decline, defined as eGFR slope less than 3.0 mL/min/1.73m2/year, was determined by logistic regression.
Results
Follow-up eGFR data >1.4 years from n=700 were available for analyses. AQP5 was undetectable in 138 patients. Tertiles of AQP5 were 0.4 [0 – 2.2], 7.3 [5.9 – 9.1], and 16.0 [13.0 – 21.6] (ng/mL), respectively (p<0.01). Patients in the highest tertile of AQP5 had significantly higher total cholesterol, lower baseline eGFR, and higher levels of albuminuria compared to the lowest tertile. AQP5 was inversely correlated with eGFR slope (Pearson’s r = −0.12, p<0.001), and independent of clinical risk factors age, sex, race, and baseline systolic and diastolic blood pressure, hemoglobin A1c, total cholesterol, eGFR, and urine albumin-to-creatinine ratio (β = −0.05, p<0.004). Furthermore, AQP5 was significantly associated with fast eGFR decline (Odds Ratio = 1.03 (95% Confidence Interval 1.003 – 1.06), p<0.03).
Conclusion
Our data suggest that baseline AQP5 is independently associated with the progression of eGFR decline in patients with type 2 diabetes and nephropathy.
Keywords: water channels, Type 2 diabetes, nephropathy, urinary biomarker, eGFR slope
1. Introduction
About 25–40% of patients with diabetes develop diabetic nephropathy (DN) 1. Patients with DN often manifest with persistent albuminuria, hypertension, and progressive decline in the glomerular filtration rate (GFR). DN is associated with increased risk of cardiovascular morbidity and mortality in type 1 and in type 2 diabetic patients 2,3. DN has become the most common single cause of end-stage renal disease and one of the most significant long-term complications associated with diabetes 4. The onset and development of DN is typically characterized by subsequent transitions from normoalbuminuria to microalbuminuria and to macroalbuminuria. However, once patients with type 2 diabetes develop renal impairment and macroalbuminuria, predicting further progression of renal complications is limited.
Aquaporin 5 (AQP5) is a member of the water channel family and functions in the generation of saliva, tears, and pulmonary secretions 5. It is not detectable by immunoblotting in normal mouse and human kidneys 6,7, indicating that AQP5 plays little role in normal renal physiology. Global knockout of Aqp5 in mice does not have severe effects, even in lung, which strongly expresses Aqp5 8. AQP2 and AQP5 are most closely related, with 66% amino acid sequence identity. This offers the molecular basis for their interactions. Our previous studies showed that AQP5 coimmunoprecipitates with AQP2 from 293T cell lysates 7. AQP5 overexpression resulted in a decrease in AQP2 membrane localization in IMCD3 cells as evidenced by cell surface biotin assays 7, implying that AQP5 may interfere with AQP2 trafficking. AQP5 was upregulated in kidney biopsies from patients with DN. Consistently, AQP5 co-localized with AQP2. AQP2-AQP5 complexes dominantly resided at the peri-nuclear region rather than at the membrane 7. Such distribution of AQP2 may impair collecting duct to reabsorb water, contributing to polyuria. In fact, polyuria is the earliest clinical renal symptom in untreated or poorly controlled DN in addition to glucosuria. Polyuria causes dilatation of distal nephron segments, which is routinely seen in human biopsies and in histological sections of both experimental DN and obstructive nephropathy. The dilated tubules are the primary source for pro-inflammatory and pro-fibrogenic cytokines and regulators. Accordingly, polyuria is considered as a mechanistic determinant of tubulo-interstitial injury and progression of renal failure in DN (reviewed in9). In this regard, AQP5 may serve as a potential biomarker of tubular dysfunction and link to renal function decline. In a pilot study, we used an AQP5-specific enzyme-linked immunosorbent assay (ELISA) and determined serum and urine AQP5 in a test cohort (n=84) and in a validation cohort (n=44). We found that patients with DN had significantly elevated urine AQP5/creatinine, compared with normal controls and patients with diabetes mellitus. AQP5/creatinine improved the clinical models in distinguishing DN from other two groups (normal controls and diabetes mellitus) 10. However, if AQP5 is associated with fast estimated glomerular filtration rate (eGFR) decline remains unknown. In this study, we assess whether urinary baseline AQP5 is independently associated with eGFR decline in patients with type 2 diabetes and nephropathy that were enrolled in the randomized, double-blind, placebo-controlled, sulodexide macroalbuminuria (Sun-MACRO) trial 11.
2. Materials and Methods
2.1. Study population
Sun-MACRO was a large, randomized placebo controlled clinical trial investigating the effect of sulodexide in delaying the progression of kidney function decline in patients with type 2 diabetes and nephropathy (n=1248) (Clinical trial reg. no. NCT00130312, ClinicalTrials.gov). The study design and results of the trial have been previously published 11. The primary end point was a composite of a doubling of baseline serum creatinine, development of end-stage renal disease (ESRD), or serum creatinine ≥6.0 mg/dl. The Sun-MACRO trial was terminated early based on an interim analysis showing that there was no effect on the surrogate (proteinuria) and no effect on the hard outcome. In addition, sulodexide had no effect on change in kidney function. In short, patients with type 2 diabetes, renal impairment, and significant proteinuria (>900 mg/d) already receiving maximal therapy with angiotensin receptor blockers, were eligible for inclusion. The most important exclusion criteria were type 1 diabetes and non-diabetic kidney disease.
The institutional ethics committee of each center approved the Sun-MACRO trial, and all patients provided written informed consent. For this present analysis, Albany Medical College Institutional Review Board approved this study.
2.2. Clinical measurements
During the Sun-MACRO study, serum was collected from patients at baseline, and after 3, 6, 12, and 18 months of follow-up. Serum creatinine was routinely measured using a modified Jaffe, rate-blanked alkaline picrate method, incorporated on the automated Roche/Hitachi Modular System (Roche Diagnostics, IN, USA).
2.3. AQP5 measurements
For the present analysis, 997 baseline urine samples were available for AQP5 measurement. Baseline urine samples were stored at −80°C from the time of collection. AQP5 was measured using a human AQP5-specific ELISA kit, as we previously reported 10. For each patient, AQP5 was blindly measured in triplicates, averaged, and log2 transformed. In a sensitivity analysis, undetectable AQP5 levels were arbitrarily set to 0.0001.
2.4. Statistical analysis
Analyses were performed with SAS software (version 9.3; SAS Institute, Cary, NC) and SigmaPlot 11 (Systat Software). Data are presented as mean (standard deviation, SD) or median [1st, 3rd quartile] for skewed variables. Graphical techniques were used to detect outliers. P-values were two-tailed and statistical significance was set at p<0.05.
The outcome of interest was eGFR decline, defined as the within-patient annual eGFR slope expressed in ml/min/1.73m2 per year. The eGFR value at each time-point was estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) Study Equation. Renal function decline was calculated using a minimum of 3 serum creatinine measurements during follow-up using a mixed model repeated measures model (change in eGFR per year). Furthermore, renal and cardiovascular composite end points of the current study were identical to those of the Sun-MACRO study.
Baseline urinary AQP5 was stratified into tertiles. Differences in patient characters between tertiles were assessed by one-way ANOVA or Fischer Exact test, as appropriate. Pearson’s correlation coefficient was calculated between urinary AQP5 and clinical parameters. Simple and multivariable linear regressions were performed to assess the association between baseline urinary AQP5 and eGFR slope. Multivariable models were adjusted for baseline age, sex, race, and baseline: systolic blood pressure, diastolic blood pressure, hemoglobin A1c (HbA1c), total cholesterol (TotChol), eGFR, and the natural log of urine albumin-to-creatinine ratio (UACR). Finally, receiver operator curves were calculated to determine if baseline urinary AQP5 could discriminate fast renal function decline, defined as <−3 mL/min/1.73m2 per year. The threshold of −3 mL/min/1.73m2 was based on prior studies and its concurrence with the highest tertile of eGFR decline.
3. Results
3.1. Baseline AQP5 was differentially detected in patients with type 2 diabetes and nephropathy
The Sun Macro original study had n=1248 subjects. Among them, n=997 had available urine samples, n=905 had ≥3 serum creatinine measurements during follow-up, and n=859 had detectable AQP5. The following results are reported for n=700 patients who had both a urine sample for AQP5 measurement and at least ≥3 serum creatinine measurements during follow-up. The mean age of all patients was 64 ± 9 years. The average body mass index (BMI) was 32 kg/m2 and the mean HbA1c level was elevated at 8 ± 1.6% (64 ± 17 mmol/mol). All patients had macroalbuminuria, and baseline eGFR mean was 33.1 mL/min/1.73 m2, indicating moderate to severe renal impairment (chronic kidney disease class 3B).
Of the n=700 analyzed patients, n=138 patients had undetectable AQP5. The undetectable AQP5 levels were arbitrarily set to 0.0001, which were imputed in a sensitivity analysis (see below). The AQP5 levels (ng/ml) were 0.4 [95% CI: 0–2.2] in the 1st tertile (n=250). These numbers were increased to 7.3 [5.9 – 9.1] in the 2nd tertile (n=233) and 16.0 [13.0 – 21.6] in the 3rd tertile (n=217) (Table 1).
Table 1.
Patient characteristics by tertiles by AQP5
| 1st Tertile n=250 | 2nd Tertile n=233 | 3rd Tertile n=217 | p-value | |
|---|---|---|---|---|
| AGE (years) | 63.2 (9.2) | 63.0 (9.2) | 63.4 (62.2) | 0.913 |
| Male sex | 196 (78.4) | 191 (82.0) | 157 (72.3) | 0.047 |
| Caucasian | 153 (61.2) | 177 (76.0) | 164 (75.6) | 0.001 |
| Current or former smoker | 143 (57.2) | 145 (62.2) | 123 (56.7) | 0.530 |
| BMI kg/m2 | 33.3 (23.7) | 31.6 (6.5) | 32.0 (31.2) | 0.717 |
| SBP (mmHg) | 137.8 (14.3) | 138.8 (15.0) | 139.7 (137.9) | 0.321 |
| DBP (mmHg) | 72.4 (9.8) | 73.2 (10.3) | 75.0 (9.6) | 0.011 |
| HbA1c (%) | 7.9 (1.7) | 7.9 (1.5) | 8.1 (7.8) | 0.615 |
| TotChol (mg/dL) | 169.8 (45.6) | 180.1 (45.3) | 185.2 (177.0) | 0.025 |
| eGFR_0 (mL/min/1.73m2) | 31.6 (8.5) | 32.0 (8.3) | 30.0 (28.8) | 0.024 |
| eGFR_slope (mL/min/1.73m2/year) | −3.3 (2.8) | −4.1 (3.2) | −4.0 (2.9) | 0.011 |
| ACR (mg/gm) | 1144 [583 – 2272] | 1597 [763 – 2560] | 1237 [648 – 2346] | 0.006 |
| AQP5 (ng/ml) | 0.4 [0 – 2.2] | 7.3 [5.9 – 9.1] | 16.0 [13.0 – 21.6] | <.0001 |
AQP5 was measured in all patients with available urine samples (n=997). Data from patients who had both available urine samples and ≥3 serum creatinine measurements during follow-up (n=700) are presented as mean (SD), median (1st, 3rd quartile), or number (percent). Patients (n=138) with undetectable AQP5 levels arbitrarily set to 0.0001 were included.
Patient characteristics per tertile of baseline AQP5 are reported in Table 1. At baseline, patients in the highest tertile of AQP5 had significantly higher diastolic blood pressure (DBP), higher TotChol levels, higher UACR, and lower eGFR levels compared to the lowest tertile of AQP5 (Table 1).
3.2. AQP5 significantly correlated with race, TotChol and logUACR
Baseline urinary AQP5 significantly correlated with race (Pearson’s r= −0.14), TotChol (r= 0.08), and logUACR (r= 0.09). No significant correlation of AQP5 was found with other known risk parameters (age, gender, systolic blood pressure (SBP), DBP, eGFR, and HbA1c) (Supplemental Table S1).
3.3. AQP5 is independently associated with eGFR decline
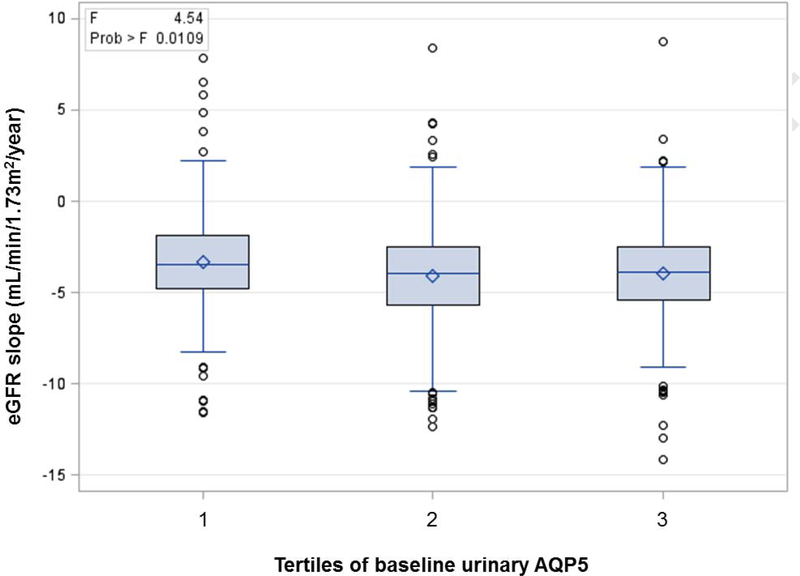
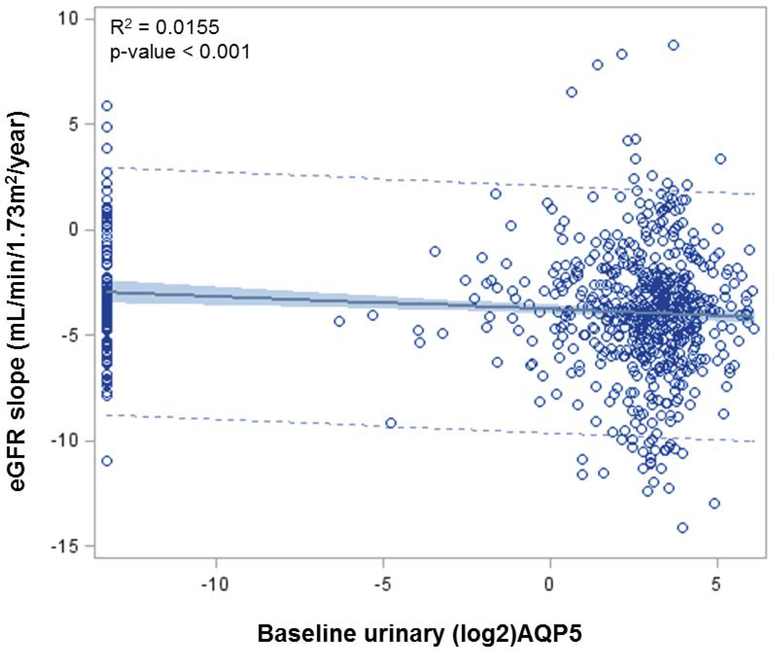
The mean ± SD of eGFR slope (mL/min/1.73m2/year) was −3.3 ± 2.8 in the 1st tertile. It was significantly changed to −4.1 ± 3.2 and −4.0 ± 2.9 in the 2nd and 3rd tertiles, respectively (Table 1 and Fig. 1). AQP5 was inversely correlated with eGFR slope (r = −0.12, p<0.001). Univariate analysis revealed a significant association of AQP5 with eGFP slope (β = −0.12, p<0.001, Table 2 and Fig. 2). Multivariable analysis unearthed that AQP5 was associated with eGFR slope, adjusted for baseline age, sex, race, SBP, DBP, HbA1c, TotChol, eGFR, and logUACR (β = −0.05, p<0.004, Table 2).
Fig. 1. Distribution of eGFR slope by Tertiles of AQP5.
Shown is the distribution of eGFR slope per tertiles of baseline urinary AQP5. Some patients (n=17) had eGFR slope increase >2ml/min/year (The highest increase was 8.8ml/min/year). They truly had diabetes since inclusion criteria of Sun Macro trial was type 2 diabetes. Per AQP5 tertile, for eGFR slope >2, there were 7, 6, and 4 for first, second, and third tertiles, respectively. Patients (n=138) with undetectable AQP5 levels arbitrarily set to 0.0001 were included.
Table 2.
AQP5 is associated with eGFR slope.
| β | S.E. | p-value | R-square | |
|---|---|---|---|---|
| Univariate | −0.06 | 0.02 | <.001 | 0.0155 |
| Multivariate* | −0.05 | 0.02 | 0.004 | N/A |
Note:
adjusted for baseline age, sex, race, SBP, DBP, HbA1c, TotChol, eGFR, logUACR. Patients (n=138) with undetectable AQP5 levels arbitrarily set to 0.0001 were included.
Fig. 2. Univariate analysis of log2_AQP5 association with eGFR slope.
Shown is the regression line of baseline AQP5 (mg/ml) with eGFR slope. The distribution of data points is also given. Patients (n=138) with undetectable AQP5 levels arbitrarily set to 0.0001 were included.
3.4. AQP5 is univariately associated with fast eGFR decline (−3ml/year)
AQP5 was strongly associated with fast eGFR decline (odds ratio (OR)= 1.03, 95% Confidence Interval (CI) = 1.003 to 1.055, p<0.029), but not with risk of ESRD and risk of cardiovascular (CV) event in univariate analyses, shown in Table 3. Receiver Operator Curve (ROC) analyses with baseline age, sex, race, SBP, DBP, HbA1c, TotChol, eGFR, and logUACR yielded a ROC of 0.7259 (95% CI = 0.69 – 0.76). While addition of AQP5 did not increase the area under the ROC curve (AUROC 0.7311 (p=0.432) on top of clinical predictors (Fig. S1), it may still be exploited as a urinary biomarker independently associated with eGFR decline in patients with type 2 diabetes and nephropathy.
Table 3.
Univariate analysis of AQP5 associations with fast eGFR decline
| OR or HR | 95%CI | p-value | |
|---|---|---|---|
| Fast eGFR decline* | 1.03 | 1.003 – 1.055 | 0.029 |
| Risk of ESRD# | 1.04 | 0.97 – 1.10 | 0.258 |
| Risk of CV event# | 1.02 | 0.98 – 1.06 | 0.322 |
Note
OR for fast decline was calculated with logistic regression.
HRs for ESRD and CV event were calculated with Cox regression. Patients (n=138) with undetectable AQP5 levels arbitrarily set to 0.0001 were included in the calculation of OR and HRs.
Finally, we performed a sensitivity analysis by comparing the results excluding the undetectable patients with the aforementioned results including the undetectable patients. There were no significant differences in the baseline characteristics of the 138 patients with undetectable AQP5 versus the remaining analyzed patients. Among these undetectable patients, 59 had fast eGFR decline. Their eGFR slope was −4.9 (SD 1.5, min −10.96 max −3.04). However, without the 59 undetectable patients, the OR was only slightly elevated by 0.05 to 1.08 (95%CI 0.98–1.19; p<0.13, calculated by logistic regression) and the ROC by 0.0011 to 0.727 (p<0.43, for Chi Square difference between models). This sensitivity analysis indicates that the missing values were imputed with an arbitrary value and that the results remained consistent (OR 1.08).
4. Discussion
Identification of patients with diabetes who will develop rapid decline in kidney function is critical. Currently, there are no clinical tests that accurately predict renal outcomes. The pathogenesis of kidney disease resulting from diabetes is multifactorial and complex. Emerging evidence suggests that tubular injury contributes to disease progression. In the present study, we analyzed the baseline urinary AQP5, a potential marker of tubular dysfunction, in a relatively large population of patients with type 2 diabetes and nephropathy enrolled in Sun-MACRO trial. Our data suggest that baseline urinary AQP5 is independently associated with both the progression of eGFR decline and fast eGFR decline in this particular cohort. These findings suggest that urinary AQP5 may serve as a new urinary prognostic biomarker of diabetic nephropathy.
The onset of DN is featured by an increase in albumin excretion rate (AER) and/or a transient rise in GFR (hyperfiltration). Without intervention AER rises exponentially and a linear decline in GFR after onset of overt nephropathy exists. In overt nephropathy, AER is considered as a predictor of GFR decline and the early AER response to antihypertensive therapy correlates with long-term GFR decline. Tubular expression of neutrophil gelatinase-associated lipocalin was independently associated with GFR decline slopes in 35 patients with confirmed DN 12. Examination of a type 1 diabetes mellitus cohort of 442 without and 458 with DN with up to 12 years of follow-up revealed increased high-sensitivity troponin T as an independent predictor of renal decline and cardiovascular events 13. The rate of GFR decline correlated with urinary CXCL9 mRNA level in a cohort comprising 26 patients with biopsy-proven DN, 15 with hypertensive nephrosclerosis and 10 healthy controls. The patients had a follow-up of about 3 years 14. A retrospective evaluation of 122 Type 2 Diabetes Mellitus (T2DM) patients with DN uncovered that proteinuria, hypoalbuminemia, anemia, and a change in systolic blood pressure were most effective in annual rate of GFR decline 15. Elevated soluble tumor necrosis factor receptor 2 was independently associated with an eGFR decline in 516 women with T2DM in the Nurses’ Health Study with an estimated GFR decline 25% being estimated over 11 years of follow up 16. Transforming growth factor and connective tissue growth factor are also potential markers of DN progression 17. However, none of the biomarkers described above have been reported to associate with fast GFR decline. Although epidermal growth factor (EGF), monocyte chemoattractant protein-1 and their ratio in urine showed significant predictive power for rapid renal progression after multivariate analysis with conventional factors (blood pressure, GFR and UACR), the cohort examined was very small, consisting of only 83 T2DM patients with DN who were followed up for 23 months 18. An unbiased renal biopsy transcriptome-driven screening also resulted in identification of EGF as a predictor of chronic kidney disease progression in patients with glomerular disease 19. In this study, urinary EGF levels were correlated positively with eGFR and reversely with interstitial fibrosis/tubular atrophy and rate of eGFR loss 19. Hence, patients with lower urinary EGF concentrations are more likely to be at risk of chronic kidney disease progression than those with higher urinary EGF measurements. In this regards, AQP5 may offer a complementary advantage (see below).
The urinary concentrating mechanism and glandular fluid secretion are the two primary roles assigned to water channel proteins. AQP5 dysregulation has been involved in several disorders, including Sjögren’s syndrome, cystic fibrosis, bronchitis 20,21, and even cancer 22,23. As the closest homolog of AQP2, AQP5 is undetectable in normal mouse and human kidneys. AQP5 silencing in the kidney is achieved partially via DOT1L-catalyzed histone H3 K79 hypermethylation at the AQP5 promoter. However, in kidneys from Dot1lAC mice or from patients with DN, abolition of H3 K79 methylation occurs, resulting in robust expression of AQP5 7. The detailed molecular mechanism linking AQP5 to GFR decline has not been well established. Whether AQP5 upregulation is the cause or the consequence of DN progression remains unclear. Nevertheless, several points can be speculated. First, the pathologically expressed AQP5 may execute a detrimental effect by forming a protein complex with AQP2. This protein complex is primarily located at the subnuclear region and impairs AQP2 apical localization 7, likely contributing to polyuria and polyuria-induced kidney injury. Secondly, the regulation of the cell volume in response to changes in osmolarity is crucial for cellular processes such as gene expression and cell proliferation 24. AQP5 interacts with osmosensing TRPV4 (transient receptor potential vanalloid 4) and controls regulatory volume decrease or increase in response to changes in the extracellular tonicity in salivary gland cells and 293T cells, respectively25,26. The abnormally expressed AQP5 may, therefore, cause deregulation of the collecting duct cell swelling or shrinkage in response to osmotic stress. Thirdly, AQP5 upregulation may result in enhanced Ca2+ entry 25, which also regulates cell volume and cell cycle. Dysfunction of collecting duct induced by abnormal AQP5 production may disrupt fluid, electrolyte, and acid-base homeostasis, which could trigger global effects inside and outside kidney and eventually lead to GFR decline. Hence, urine AQP5 may be correlated with the stage of CKD. Consistently, urine AQP5 was significantly higher in DN stage V than in stage III 10. Three possibilities can be speculated for the existence of AQP5 in urine: 1) secretion from tubular cells because of its small size (27 kD); 2) reduction in tubular reabsorption; and 3) shedding of AQP5-expressing tubular cells, as evidenced by the detection of detached AQP5+ cells in the lumen of the tubules 7.
Findings of this study have several implications. Urine AQP5 might be employed to identify high-risk T2DM patients with DN for clinical trials and/or for more aggressive intervention. For example, patients with diabetes possessing high urinary AQP5 and low EGF may be recruited to enrich clinical trials with subjects who are more likely to reach end points during the limited trial observation period. Our data also provide additional support for the pathological role of AQP5 in the DN progression. This is especially important as specific inhibitors of AQP5 may be developed for clinical studies with the potential to slow down GFR decline, particularly in DN patients with fast GFR decline. Because Aqp5 null mice had grossly normal appearance 27 and little or no renal AQP5 expression is detectable in healthy mice and humans 7, a low side-effect profile of potential AQP5 inhibitors is expected. It would be of interest to see if high urinary AQP5 could identify those who would mostly likely to respond to AQP5 blockade. To our knowledge, this is the first study to evaluate the association of urinary AQP5 with GFR decline and fast GFR decline in human patients. Since the cohort was followed up for only 1.4 years, validation of our findings in external cohorts with longer follow-up and/or nephropathy in other settings such as type 1 diabetes is necessary.
Limitations of this study include post hoc analysis of patients previously enrolled in a negative clinical trial. Although secondary analyses of clinical trials may be liable to misinterpretations, the current study used the original cohort of patients for a biomarker study at baseline. Treatment was included in all of our statistical analyses, and we found no associations between treatment and renal or cardiovascular risk. Therefore, our findings are unlikely to be influenced by the intervention. That is, use of maximal therapy with angiotensin receptor blockers and sulodexide during follow-up was unlikely to significantly affect our results and conclusions. Strengths of the current study include the large, homogeneous study cohort in which all patients were characterized by a well-defined phenotype of nephropathy and as a result a relatively high occurrence of renal and cardiovascular events.
In conclusion, our data suggest that baseline AQP5, a possible marker of tubular dysfunction, is independently associated with the progression of eGFR decline in patients with type 2 diabetes and nephropathy. Validation of these findings in an external cohort is necessary.
Supplementary Material
Significance statement.
Identification of new biomarkers is urgently needed to identify individuals at high risk of renal function decline. We show that baseline urinary AQP5 is associated with fast eGFR decline in patients with type 2 diabetes and nephropathy. Our data suggest that AQP5 may serve as a new, independent urinary prognostic biomarker to predict the fast decline in kidney function in patients with type 2 diabetes and nephropathy.
6. Acknowledgement
This work was supported by National Institutes of Health Grants R01DK080236A1 (to W.Z.Z.) and R21 DK104073 (to W.Z.Z). We greatly appreciate Michelle J. Pena and Hiddo L. Heerspin for kindly providing urine samples and assistance in data analyses and manuscript writing and editing.
Footnotes
5. Declaration of interest
The authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. Reference
- 1.Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. The New England journal of medicine. 1999;341(15):1127–1133. [DOI] [PubMed] [Google Scholar]
- 2.Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ. 1996;313(7060):779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 2000;160(8):1093–1100. [DOI] [PubMed] [Google Scholar]
- 4.Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S79–83. [DOI] [PubMed] [Google Scholar]
- 5.Da Silva N, Silberstein C, Beaulieu V, et al. Postnatal expression of aquaporins in epithelial cells of the rat epididymis. Biol Reprod. 2006;74(2):427–438. [DOI] [PubMed] [Google Scholar]
- 6.Krane CM, Towne JE, Menon AG. Cloning and characterization of murine Aqp5: evidence for a conserved aquaporin gene cluster. Mamm Genome. 1999;10(5):498–505. [DOI] [PubMed] [Google Scholar]
- 7.Wu H, Chen L, Zhang X, et al. Aqp5 is a new transcriptional target of dot1a and a regulator of aqp2. PloS one. 2013;8(1):e53342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verkman AS. Role of aquaporins in lung liquid physiology. Respir Physiol Neurobiol. 2007;159(3):324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Mitu GM, Hirschberg R. Osmotic polyuria: an overlooked mechanism in diabetic nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23(7):2167–2172. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Chen L, Zhao B, et al. Urine AQP5 is a potential novel biomarker of diabetic nephropathy. Journal of diabetes and its complications. 2016;30(5):819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packham DK, Wolfe R, Reutens AT, et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2012;23(1):123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang S, Park J, Kim J, et al. Tissue expression of tubular injury markers is associated with renal function decline in diabetic nephropathy. Journal of diabetes and its complications. 2017;31(12):1704–1709. [DOI] [PubMed] [Google Scholar]
- 13.Galsgaard J, Persson F, Hansen TW, et al. Plasma high-sensitivity troponin T predicts end-stage renal disease and cardiovascular and all-cause mortality in patients with type 1 diabetes and diabetic nephropathy. Kidney international. 2017;92(5):1242–1248. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Lai FM, Chow KM, et al. Urinary mRNA levels of ELR-negative CXC chemokine ligand and extracellular matrix in diabetic nephropathy. Diabetes/metabolism research and reviews. 2015;31(7):699–706. [DOI] [PubMed] [Google Scholar]
- 15.Unsal A, Koc Y, Basturk T, Akgun AO, Sakaci T, Ahbap E. Risk factors for progression of renal disease in patient with diabetic nephropathy. European review for medical and pharmacological sciences. 2012;16(7):878–883. [PubMed] [Google Scholar]
- 16.Lin J, Hu FB, Mantzoros C, Curhan GC. Lipid and inflammatory biomarkers and kidney function decline in type 2 diabetes. Diabetologia. 2010;53(2):263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerums G, Premaratne E, Panagiotopoulos S, Clarke S, Power DA, MacIsaac RJ. New and old markers of progression of diabetic nephropathy. Diabetes research and clinical practice. 2008;82 Suppl 1:S30–37. [DOI] [PubMed] [Google Scholar]
- 18.Satirapoj B, Dispan R, Radinahamed P, Kitiyakara C. Urinary epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as predictors for rapid loss of renal function in type 2 diabetic patients with diabetic kidney disease. BMC nephrology. 2018;19(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju W, Nair V, Smith S, et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Science translational medicine. 2015;7(316):316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsubota K, Hirai S, King LS, Agre P, Ishida N. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet. 2001;357(9257):688–689. [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. The Journal of biological chemistry. 2001;276(44):41288–41292. [DOI] [PubMed] [Google Scholar]
- 22.Verkman AS. Aquaporins in clinical medicine. Annu Rev Med. 2012;63:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verkman AS. Aquaporins at a glance. Journal of cell science. 2011;124(Pt 13):2107–2112. [DOI] [PubMed] [Google Scholar]
- 24.Okada Y, Maeno E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2001;130(3):377–383. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Bandyopadhyay BC, Nakamoto T, et al. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. The Journal of biological chemistry. 2006;281(22):15485–15495. [DOI] [PubMed] [Google Scholar]
- 26.Kitchen P, Oberg F, Sjohamn J, et al. Plasma Membrane Abundance of Human Aquaporin 5 Is Dynamically Regulated by Multiple Pathways. PloS one. 2015;10(11):e0143027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. The Journal of biological chemistry. 1999;274(29):20071–20074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.