Figure 2: Selenoxide elimination mechanism for reversing alkylation on Sec of TrxR.
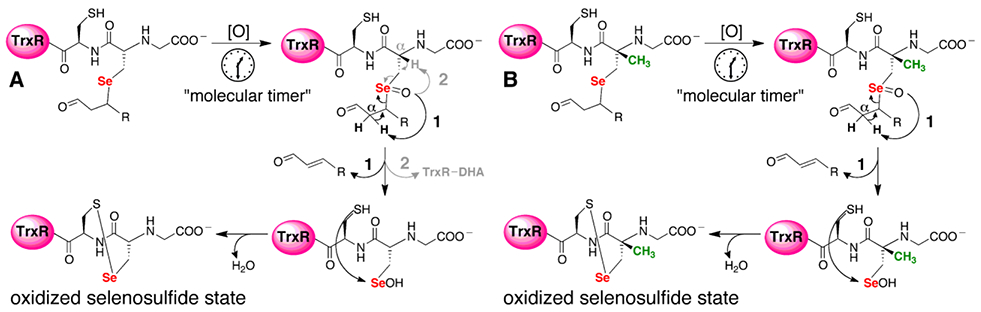
(A) Once Sec is alkylated by an electrophile, mTrx will produce H2O2, which can act as a type of “molecular timer”. Once the concentration of H2O2 is sufficient, the selenide will be converted to a selenoxide which can undergo rapid β-syn elimination via pathway 1 (bold pathway). Abstraction of the Cα-proton of the electrophile leads to elimination of the electrophile and restoration of TrxR back to its oxidized selenosulfide state. However, abstraction of the Cα-H from the enzyme backbone via pathway 2 (gray) results in elimination of selenium from the enzyme and the formation of dehydoalanine (DHA). Without Sec in the active site, enzyme activity cannot be restored. (B) In the mutant mTrxR, the backbone Cα-H is replaced with a Cα-CH3. This replacement means that the mutant can only utilize pathway 1 in the β-syn elimination mechanism. Thus, the mutant should be better able to reverse the electrophilic alkylation and restore Trx-reductase activity.
