Abstract
BACKGROUND
Eosinophilic granulomatosis polyangiitis (EGPA) is a small vessel necrotizing vasculitis that commonly presents as peripheral eosinophilia and asthma; however, it can rarely manifest with cardiac involvement such as pericarditis and cardiac tamponade. Isolated pericardial tamponade presenting as the initial symptom of EGPA is exceedingly rare. Early diagnosis and appropriate treatment are crucial to prevent life-threatening outcomes.
CASE SUMMARY
52-year-old woman with no past medical history presented with progressive dyspnea and dry cough. On physical exam she had a pericardial friction rub and bilateral rales. Vital signs were notable for tachycardia at 119 beats per minute and hypoxia with 89% oxygen saturation. On laboratory exam, she had 45% peripheral eosinophilia, troponin elevation of 1.1 ng/mL and N-terminal prohormone of brain natriuretic peptide of 2101 pg/mL. TTE confirmed a large pericardial effusion and tamponade physiology. She underwent urgent pericardial window procedure. Pericardial and lung biopsy demonstrated eosinophilic infiltration. Based on the American College of Radiology guidelines, the patient was diagnosed with EGPA which manifested in its rare form of cardiac tamponade. She was treated with steroid taper and mepolizumab.
CONCLUSION
This case highlights that when isolated pericardial involvement occurs in EGPA, diagnosis is recognized by performing pericardial biopsy demonstrating histopathologic evidence of eosinophilic infiltration.
Keywords: Eosinophilic granulomatosis polyangiitis, Cardiac tamponade, Pericardial effusion, Mepolizumab, Peripheral eosinophilia, Pericardial biopsy, Case report
Core Tip: (1) To be able to investigate the etiology of pericardial effusion and cardiac tamponade with eosinophilia which is rarely caused by eosinophilic granulomatosis polyangiitis (EGPA); (2) To be mindful that anti-neutrophil cytoplasmic antibody is negative in EGPA with cardiac involvement rather than pulmonary or renal involvement; (3) To be aware that when isolated pericardial involvement leading to cardiac tamponade occurs, diagnosis is recognized by performing pericardial biopsy demonstrating histopathologic evidence of eosinophilic infiltration; (4) To consider early diagnosis of EGPA with cardiac involvement is crucial because it carries a major burden of morbidity and mortality; (5) To initiate early treatment with corticosteroids when an isolated pericardial involvement is present whereas immunosuppressants are utilized with multiorgan involvement; and (6) To conduct close surveillance in the outpatient setting to monitor the response to treatment and maintenance medications such as steroids and monoclonal antibodies.
INTRODUCTION
Eosinophilic granulomatosis polyangiitis (EGPA) is a systemic necrotizing vasculitis with eosinophilic infiltrates of the small to medium vessels and extravascular granulomas, first described by Dr. Jacob Churg and Dr. Lotte Strauss in 1951[1]. EGPA is commonly revealed by late onset asthma and peripheral eosinophilia, however it can rarely present as isolated cardiac tamponade. Owing to the high mortality rate of EGPA presenting as isolated cardiac tamponade, rapid diagnosis and early treatment is critical and lifesaving. Other more common cardiac manifestation of EGPA includes pericarditis, myocarditis, valvulopathies, cardiomyopathy and coronary artery vasculitis.
CASE PRESENTATION
Chief complaints
52-year-old female presented to the hospital with chief complaints of progressive shortness of breath, orthopnea, chest pain, and dry cough.
History of present illness
The symptoms started one month ago and progressively worsened which prompted her to visit the hospital.
History of past illness
She had no significant prior personal or family history.
Physical examination
Vital signs were notable for blood pressure of 124/79 mmHg, sinus tachycardia with heart rate of 119 beats per minute, hypoxia with oxygen saturation of 89%, respiratory rate of 16 breaths per minute and afebrile temperature. On physical examination, she demonstrated sinus tachycardia, a pericardial friction rub, and rales in her bilateral lower lung fields. She did not demonstrate pulsus paradoxus. Review of system was notable for numbness and tingling of the right hand for the past month.
Laboratory examinations
Initial laboratory findings were notable normal white blood cell count of 11/nL, however with 45% peripheral eosinophilia. Troponin was mildly elevated at 1.1 ng/mL and N-terminal prohormone of brain natriuretic peptide (NT ProBNP) was elevated at 2101 pg/mL. D-Dimer was elevated at 3.66 µg/mL. Electrocardiogram revealed low voltage QRS complex with small ST-T wave changes (Figure 1). A chest radiograph showed cardiomegaly and bilateral opacities (Figure 2). At this stage, given the initial diagnostic information, differentials included congestive heart failure, pulmonary embolism, and/or eosinophilic pneumonia.
Figure 1.
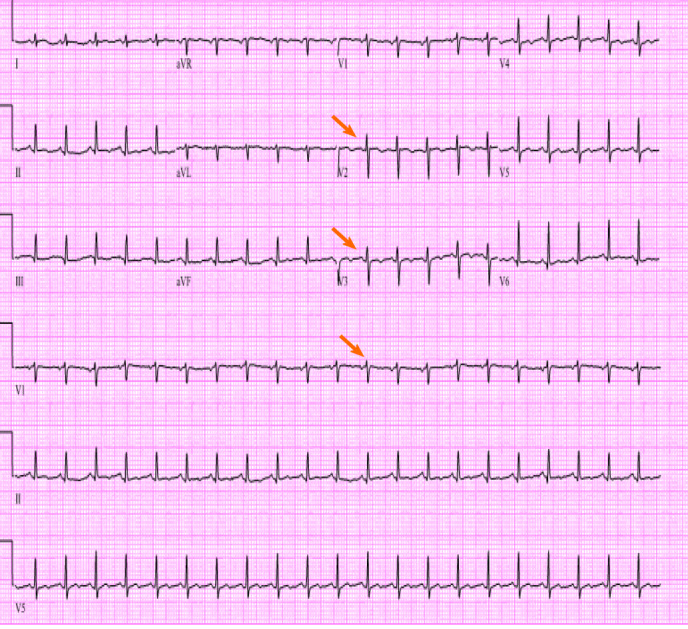
Electrocardiogram showed sinus tachycardia, low voltage QRS (arrows) with small ST-T wave changes.
Figure 2.
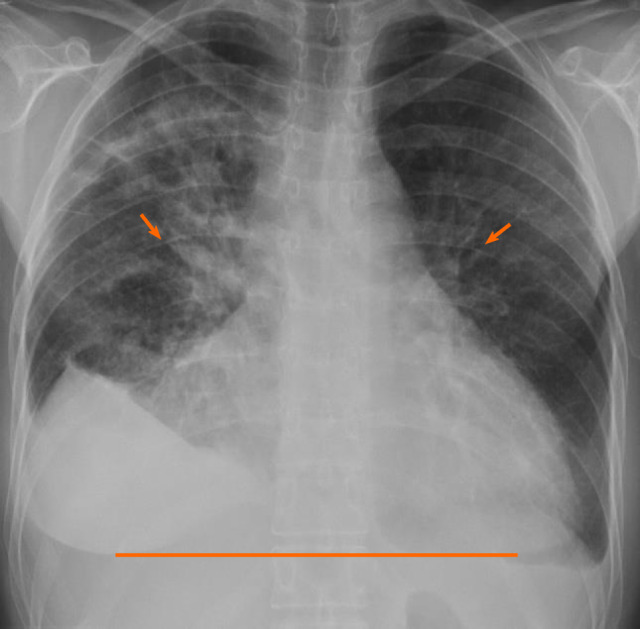
Chest radiograph revealed cardiomegaly (line) and bilateral opacities (arrows).
Imaging examinations
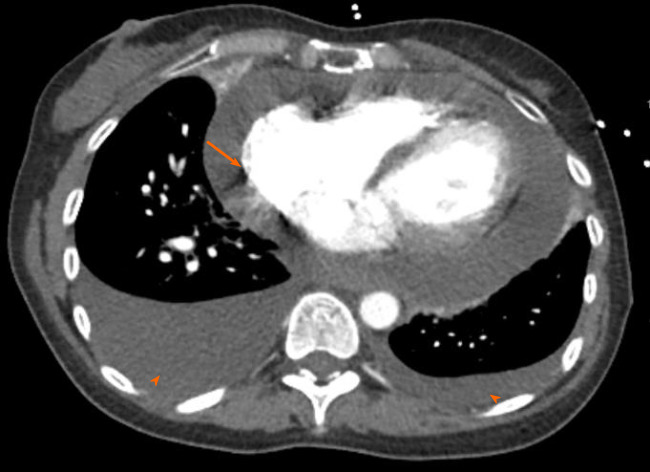
Computed tomography angiogram revealed a large pericardial effusion, moderate right and small left pleural effusions, and bilateral pulmonary infiltrates (Figure 3). This was followed with a transthoracic echocardiogram which demonstrated normal left ventricular cavity size, wall thickness and systolic function with estimated ejection fraction greater than 55%. No regional wall motion abnormalities were detected. Aortic and mitral valves were normal. There was trace tricuspid regurgitation. The right atrium was normal in size. The right ventricle collapsed in diastole. There was a large pericardial effusion (Figure 4). The diastolic compression of the right ventricle was suggestive of tamponade physiology.
Figure 3.
Chest computed tomography angiogram revealed a large pericardial effusion (arrow); moderate right and small left pleural effusion (arrowhead).
Figure 4.
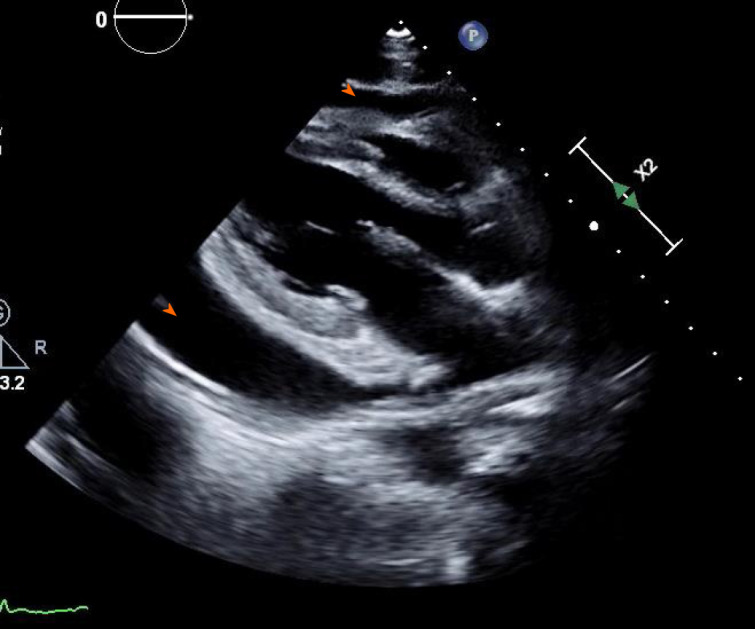
Transthoracic echocardiogram showed large pericardial effusion (arrowhead).
FINAL DIAGNOSIS
Based on the American College of Radiology (ACR) guidelines, the patient was diagnosed with EGPA which manifested in its rare form of cardiac tamponade.
TREATMENT
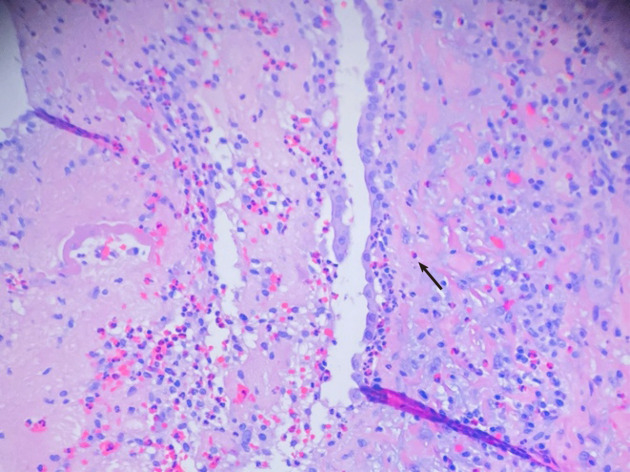
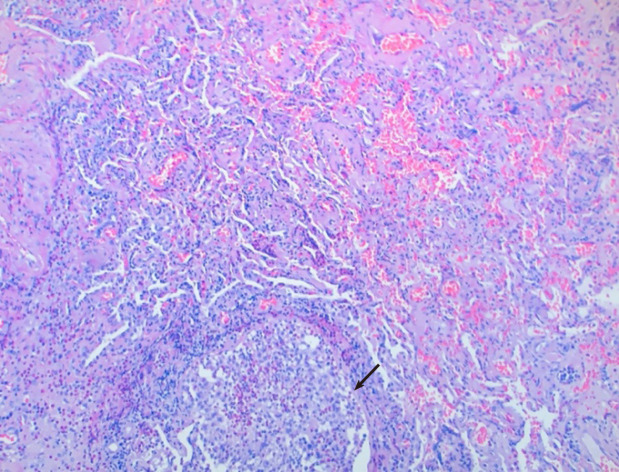
Cardiothoracic surgery was consulted, and she was taken to the operating room for right video-assisted thoracoscopic surgery pericardial window, right lower lobe lung wedge resection, and drainage of pericardial effusion. Pericardial fluid studies were notable for a cloudy, exudative effusion with white blood cell count of 3092 μL and 25% eosinophilia. Pericardial fluid cytology was negative for malignancy. Aerobic, anaerobic, and fungal cultures were negative for any growth. Pericardial biopsy demonstrated eosinophilic pericarditis (Figure 5). Lung tissue pathology demonstrated findings consistent with eosinophilic pneumonia vs EGPA (Figure 6).
Figure 5.
Cardiac biopsy revealed pericardial thickening with eosinophil predominant infiltrate (arrow).
Figure 6.
Lung biopsy revealed organizing inflammatory infiltrate, micro abscess (arrow), and eosinophil infiltrate.
Following the drainage of pericardial fluid, the patient demonstrated relief of her presenting symptoms. She denied any chest pain or shortness of breath. Repeat troponin levels were within normal limits. Other laboratory workup including respiratory viral panel, blood cultures, rheumatologic marker including antinuclear antibody, rheumatoid factor and anti-neutrophil cytoplasmic antibody (ANCA) were negative. Hypersensitivity pneumonitis panel was also negative. Repeat echocardiogram the following day showed normal biventricular function, valvular function, and wall motion. It did not demonstrate accumulation of new pericardial effusion. As such coronary catheterization was deferred.
The patient was started on oral prednisone 40 mg daily. A month later, mepolizumab was added to her treatment course and prednisone was concurrently tapered down. Eventually, she has been maintained on oral prednisone 7.5 mg daily as well as mepolizumab monthly with no recurrence of her symptoms.
OUTCOME AND FOLLOW-UP
At one-month interval, transthoracic echocardiogram, CT chest with contrast, and cardiac magnetic resonance imaging (MRI) with and without contrast were performed to monitor disease progression. Echocardiogram showed resolution of pericardial effusion and normal biventricular function. CT chest showed resolution of bilateral infiltrates. Cardiac MRI with and without contrast showed normal biventricular volume and systolic function. There were no areas of focal hyperenhancement, consistent with the absence of myocardial scarring or fibrosis. A post gadolinium LAVA sequence was acquired which demonstrated normal measurements of biventricular dimensions, volume and ejection fraction. At follow up office visits, she had no recurrence of cardiopulmonary symptoms, her mononeuritis had resolved, there was no evidence of eosinophilia on hemogram, and she tolerated the medication well.
DISCUSSION
EGPA is a rare systemic disease characterized by asthma, peripheral and tissue eosinophilia and systemic vasculitis. It is reported to have an annual incidence of 1.0 – 4.2 cases per million[2]. Cardiac involvement commonly includes eosinophilic endomyocarditis, coronary vasculitis, arrythmia, cardiomyopathy and pericarditis with small pericardial effusions[3]. EGPA presenting as cardiac tamponade is exceedingly rare[3,4]. When cardiac involvement is present, rapid diagnosis is necessary because it is an important predictor of morbidity and mortality.
According to the ACR, the diagnostic criteria for EGPA includes: (1) Asthma; (2) Eosinophilia > 10%; (3) Neuropathy; (4) Non-fixed pulmonary infiltrates; (5) Paranasal sinus abnormality; and (6) Extravascular eosinophils[5]. The ACR determined that having at least four of the six criteria yields a sensitivity of 85% and a specificity of 99.7% for EGPA[5]. Our patient met four of the six criteria for the diagnosis of EGPA including: peripheral eosinophilia of 45%, mononeuropathy of right hand, bilateral pulmonary infiltrates on CT angiogram and pericardial/Lung tissue biopsy with extravascular eosinophilia.
The French vasculitis study group demonstrated that EGPA constitutes of two separate clinical patterns which can be distinguished by the ANCA status; patients with cardiac eosinophilic tissue infiltration are usually ANCA negative, whereas patients with pulmonary and renal involvement are ANCA positive[6,7].
In a literature search on PubMed between January 1960 – March 2020 we found 14 cases of EGPA manifesting as cardiac tamponade (Table 1). Of the total 14 cases reviewed (including ours), age range included 12-73, with 50% male and 50% female patients. Myocardial involvement was detected in five of the fourteen EGPA cases with cardiac tamponade. ANCA status was only positive in one case, thus it is unlikely for ANCA to be related to pericardial involvement leading to cardiac tamponade in EGPA. Cytology from aspirated pericardial fluid did not provide diagnostic value as their presence were variable in the reported cytology results. The diagnosis of seven of the EGPA cases with cardiac tamponade were made from pericardial biopsy.
Table 1.
PubMed literature review of case reports of eosinophilic granulomatosis polyangiitis with cardiac tamponade
| Patient | Age | Sex | Histological diagnosis | Other cardiac involvement | Other organ involvement | Eosinophil in pericardial fluids | Treatment | ANCA | Ref. | |
| 1 | 66 | M | Pericardium | None | Lung | Numerous | Prednisolone | Negative | Agard et al[3] | |
| 2 | 73 | F | Pericardium | Myocarditis | Nerve | N/A | Prednisolone, cyclophosphamide | Negative | Agard et al[3] | |
| 3 | 73 | F | Skin | None | Skin, colon, nerve | Numerous | Prednisolone | Negative | Yano et al[4] | |
| 4 | 44 | F | Skin | Myocarditis | Lung, skin | None | Prednisolone, azathioprine | N/A | Hasley et al[11] | |
| 5 | 30 | M | Myocardium | Myocarditis | Nerve | Numerous | Prednisolone | Negative | Uren et al[12] | |
| 6 | 56 | F | Pericardium | None | Skin | N/A | Prednisolone | N/A | Sharma et al[13] | |
| 7 | 12 | M | Skin | Myocarditis | Lung, skin, nerve | N/A | Prednisolone | Positive | Wang et al[14] | |
| 8 | 50 | F | Pericardium | None | Lung, eye | 77% of white cells | Prednisolone | Negative | Keefe et al[15] | |
| 9 | 59 | M | Skin | None | Skin, ear, nerve | 0.4% of white cells | Prednisolone | Negative | Ovadia et al[16] | |
| 10 | 57 | M | N/A | None | Eye, nerve | 30% of white cells | Prednisolone | Negative | Suganuma et al[17] | |
| 11 | 31 | F | Pericardium | None | Gall bladder | Numerous | Prednisolone | Negative | Lenders et al[18] | |
| 12 | 57 | M | N/A | Myocarditis | N/A | Numerous | Prednisolone | N/A | Gerlach et al[19] | |
| 13 | 56 | M | Pericardium | None | None | N/A | Prednisolone | Negative | David et al[20] | |
| 14 | F | Pericardium, Lung | None | Lung, Nerve | 25% of white cells | Prednisone, mepolizumab | Negative | Our Case | ||
ANCA: Anti-neutrophil cytoplasmic antibody.
EGPA manifesting as isolated pericarditis with pericardial effusion may present as mild symptoms of chest pain, cough, and dyspnea to life threatening conditions such as hemodynamic instability. Chest radiograph and electrocardiogram can show initial abnormalities, while urgent echocardiogram confirms the diagnosis of pericardial effusion and tamponade. In such scenarios, pericardiocentesis is performed, and pericardial fluid analysis demonstrates exudative effusion with marked eosinophilia. Histopathology from pericardial biopsy demonstrates vasculitis with eosinophilic infiltration. When patients with EGPA exhibits tamponade without involvement of other visceral organs, the diagnosis is difficult to make without pericardial biopsy. Cardiac MRI is an important noninvasive tool in demonstrating extent of cardiac involvement and following disease progression[8].
The management and prognosis of EGPA depends on the number of organs involved and their severity. Isolated pericarditis can be treated with high dose steroids alone, whereas pericarditis with myocardial injury and other visceral organ involvement are treated with a combination of high dose steroids and immunosuppressive drugs[3,4]. Biologics such as mepolizumab, a humanized monoclonal anti-interleukin (IL)-5 antibody has been shown to maintain higher proportion of study participants in remission, as well as reduce glucocorticoid use in patients with EGPA[9,10]. IL-5 is a cytokine involved in eosinophil proliferation, maturation, and differentiation. IL-5 is found to be at increased levels in patients with EGPA[10]. Mepolizumab binds to IL-5 and prevents interaction with its receptor on the eosinophil surface, thus providing consistent reduction of peripheral eosinophilia and clinical improvement in patients with eosinophilic disorders such as severe eosinophilic asthma and EGPA[10].
CONCLUSION
In summary we have presented a patient with pericarditis leading to pericardial effusion and cardiac tamponade which lead to the diagnosis of EGPA. Pericardial fluid analysis revealed eosinophilic infiltrate and pericardial biopsy demonstrated eosinophilic tissue infiltration. Furthermore, cardiac MRI did not show any evidence of inflammation or scarring to indicate myocardial involvement. Thus, this is a rare case of EGPA presenting with isolated pericardial involvement which eventually lead to cardiac tamponade. The patient was initiated on high dose prednisone with good response over four weeks. Subsequently, the patient was started on a steroid taper and mepolizumab was added as an adjunct to reduce steroid requirement and maintain longer remission rates. On interval follow up the patient had excellent response, which indicates EGPA presenting as cardiac tamponade can be managed with mepolizumab as a steroid sparing agent.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare no potential financial interests.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: July 2, 2020
First decision: June 20, 2020
Article in press: September 1, 2020
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hamaoka T S-Editor: Zhang H L-Editor: A P-Editor: Li JH
Contributor Information
Loba Alam, Department of Medicine, Atlantic Health System-Overlook Medical Center, Summit, NJ 07901, United States. alamloba@gmail.com.
Glenmore Lasam, Department of Cardiology, Icahn School of Medicine, Mount Sinai Heart at Mount Sinai Morningside, New York, NY 10025, United States.
Robert Fishberg, Department of Cardiology, Atlantic Health System Overlook, Summit, NJ 07901, United States.
References
- 1.Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. 1951;27:277–301. [PMC free article] [PubMed] [Google Scholar]
- 2.Watts RA, Lane S, Scott DG. What is known about the epidemiology of the vasculitides? Best Pract Res Clin Rheumatol. 2005;19:191–207. doi: 10.1016/j.berh.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Agard C, Rendu E, Leguern V, Ponge T, Masseau A, Barrier JH, Trochu JN, Hamidou MA, Guillevin L. Churg-Strauss syndrome revealed by granulomatous acute pericarditis: two case reports and a review of the literature. Semin Arthritis Rheum. 2007;36:386–391. doi: 10.1016/j.semarthrit.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Yano T, Ishimura S, Furukawa T, Koyama M, Tanaka M, Shimoshige S, Hashimoto A, Miura T. Cardiac tamponade leading to the diagnosis of eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): a case report and review of the literature. Heart Vessels. 2015;30:841–844. doi: 10.1007/s00380-014-0556-x. [DOI] [PubMed] [Google Scholar]
- 5.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33:1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 6.Sablé-Fourtassou R, Cohen P, Mahr A, Pagnoux C, Mouthon L, Jayne D, Blockmans D, Cordier JF, Delaval P, Puechal X, Lauque D, Viallard JF, Zoulim A, Guillevin L French Vasculitis Study Group. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med. 2005;143:632–638. doi: 10.7326/0003-4819-143-9-200511010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, Maurier F, Jouneau S, Bienvenu B, Puéchal X, Aumaître O, Le Guenno G, Le Quellec A, Cevallos R, Fain O, Godeau B, Seror R, Dunogué B, Mahr A, Guilpain P, Cohen P, Aouba A, Mouthon L, Guillevin L French Vasculitis Study Group. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65:270–281. doi: 10.1002/art.37721. [DOI] [PubMed] [Google Scholar]
- 8.Eyler AE, Ahmad FA, Jahangir E. Magnetic resonance imaging of the cardiac manifestations of Churg-Strauss. JRSM Open. 2014;5:2054270414525370. doi: 10.1177/2054270414525370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:1336–1343. doi: 10.1016/j.jaci.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, Merkel PA, Moosig F, Specks U, Cid MC, Luqmani R, Brown J, Mallett S, Philipson R, Yancey SW, Steinfeld J, Weller PF, Gleich GJ EGPA Mepolizumab Study Team. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med. 2017;376:1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasley PB, Follansbee WP, Coulehan JL. Cardiac manifestations of Churg-Strauss syndrome: report of a case and review of the literature. Am Heart J. 1990;120:996–999. doi: 10.1016/0002-8703(90)90227-o. [DOI] [PubMed] [Google Scholar]
- 12.Uren NG, Hammond PJ. Myopericarditis in Churg-Strauss syndrome. Tex Heart Inst J. 1991;18:127–131. [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, De Varennes B, Sniderman AD. Churg-Strauss syndrome presenting with marked eosinophilia and pericardial effusion. Can J Cardiol. 1993;9:329–330. [PubMed] [Google Scholar]
- 14.Wang SJ, Yang YH, Lin YT, Tsai MJ, Chiang BL. Childhood Churg-Strauss syndrome: report of a case. J Microbiol Immunol Infect. 2000;33:263–266. [PubMed] [Google Scholar]
- 15.Keefe AC, Hymas JC, Emerson LL, Ryan JJ. An atypical presentation of cardiac tamponade and periorbital swelling in a patient with eosinophilic granulomatosis with polyangiitis: a case report. J Med Case Rep. 2017;11:271. doi: 10.1186/s13256-017-1434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ovadia S, Dror I, Zubkov T, Tanay A, Levy D, Zandman-Goddard G. Churg-Strauss syndrome: a rare presentation with otological and pericardial manifestations: case report and review of the literature. Clin Rheumatol. 2009;28 Suppl 1:S35–S38. doi: 10.1007/s10067-009-1119-x. [DOI] [PubMed] [Google Scholar]
- 17.Suganuma K, Hashimoto T, Sato H, Suzuki T, Sakurai S. Oculomotor Nerve Palsy following Cardiac Tamponade with Churg-Strauss Syndrome: A Case Report. Case Rep Neurol. 2011;3:274–277. doi: 10.1159/000334127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenders G, Goethals M, Verstreken S, Dierckx R, Vanderheyden M. Acalculous cholecystitis and tamponade: an unusual combination? Acta Cardiol. 2011;66:383–385. doi: 10.1080/ac.66.3.2114142. [DOI] [PubMed] [Google Scholar]
- 19.Gerlach RM, Saha TK, Allard RV, Tanzola RC. Unrecognized tamponade diagnosed pre-induction by focused echocardiography. Can J Anaesth. 2013;60:803–807. doi: 10.1007/s12630-013-9968-9. [DOI] [PubMed] [Google Scholar]
- 20.David C, Cazes A, Dossier A, Pasi N, Tadros VX, Papo T, Sacre K. A 56-Year-Old Man With Cardiac Tamponade and Eosinophilia. Chest. 2018;154:e173–e176. doi: 10.1016/j.chest.2018.06.031. [DOI] [PubMed] [Google Scholar]