Abstract
Cholangiocarcinoma (CCA) comprises of extra-hepatic cholangiocarcinoma and intrahepatic cholangiocarcinoma cancers as a result of inflammation of epithelium cell lining of the bile duct. The incidence rate is increasing dramatically worldwide with highest rates in Eastern and South Asian regions. Major risk factors involve chronic damage and inflammation of bile duct epithelium from primary sclerosing cholangitis, chronic hepatitis virus infection, gallstones and liver fluke infection. Various genetic variants have also been identified and as CCA develops on the background of biliary inflammation, diverse range of molecular mechanisms are involved in its progression. Among these, the Notch signalling pathway acts as a major driver of cholangiocarcinogenesis and its components (receptors, ligands and downstream signalling molecules) represent a promising therapeutic targets. Gamma-Secretase Inhibitors have been recognized in inhibiting the Notch pathway efficiently. A comprehensive knowledge of the molecular pathways activated by the Notch signalling cascade as well as its functional crosstalk with other signalling pathways provide better approach in developing innovative therapies against CCA.
Keywords: Cholangicarcinoma, Notch receptors, Therapeutic targets, Notch signalling pathway, Gamma secretase inhibitor, Cholangiocytes
Core Tip: In this review, we explore current findings on the Notch signalling pathway, its molecular components, its specific roles in the development and progression of cholangiocarcinma (CCA), the treatment approaches aimed at suppressing this signaling pathway, and discuss the encouraging results presented by basic science research and preclinical trials. The Notch signaling pathway is a key driver of cholangiocarcinogenesis and represents a promising therapeutic target in CCA. A wide and comprehensive understanding of the molecular mechanisms triggered by the pathway will help us explore novel therapies against CCA.
INTRODUCTION
Cholangiocarcinoma (CCA) is the most common biliary tract cancer and the second most common primary liver cancer, which is characterized by its late diagnosis and subsequent fatal outcome[1]. It is a heterogeneous group of malignancies that arise from the Canals of Hering to the primary bile duct. They are the rare tumours that comprise of around 3% of gastrointestinal tumours. The most common classification of CCA is based on its anatomical location. CCA are commonly staged into intrahepatic cholangiocarcinoma (IH-CCA) and extra-hepatic cholangiocarcinoma (EH-CCA) tumours. EH-CCA can further be subdivided into perihilar CCA, which are also known as a Klatskin, and distal tumours[2]. Over the past few decades, the incidence and mortality rates of CCA have increased dramatically. Globally, incidence and mortality rates of CCA show significant geographical variation. The incidence of CCA is considerably higher in the Eastern world comparing to the Western, with the substantial differences between areas of the same country too. In the United States, approximately 5000 new cases are diagnosed each year[3]. Age-adjusted rates are highest among Asians and Hispanics (2.8–3.3 per 100000) and lowest among non-Hispanic whites and blacks (2.1 per 100000). There has been gender disparity among male and female patients. Men appear to have a slightly greater mortality from CCA than women which is represented as 1.9 and 1.5 per 100000, respectively[4]. The incidence rate of intrahepatic cancer is reported more than that of the extrahepatic cancer in the United Kingdom, which is 3.64 vs 3.58 per 100000, respectively. Even globally, the current epidemiological reports indicate an increasing incidence of IH-CCA comparing to EH-CCA[5]. Speaking about the East Asian regions, Thailand has the highest incidence of CCA across all over the world. It is especially prevalent in the North-eastern part of Thailand where the prevalence of liver fluke infection is quite high. Age-adjusted rate shows the highest incidence rate in Khon Kaen region of Thailand that is around 84 per 100000 in males and 36 per 100000 in females[6]. The prognosis of CCA is poor, which reports about a median overall survival of just 18–30 mo after surgical resection. This poor prognosis is primarily in patients with poor prognostic pathological characteristics, such as involvement of regional surgical margins or lymph nodes. Hence, adjuvant chemotherapy plays a significant role in delaying cancer recurrence and prolonging life expectancy in cholangiocarcinomic patients[7]. Apparently, the variations in the incidence rates reflect the differences in geographical risk factors as well as genetic determinants. However, in addition to disparate risk factors, clinical presentations, pathophysiology, management and prognosis, each subtype of CCA show distinct epidemiological trends[8]. Among the various established risk factors are bile duct disorders particularly cysts of biliary tract, primary sclerosing cholangitis, hepatolothiasis, cholelithiasis and choledocholithiasis and many liver diseases like cirrhosis, hemochromatosis, viral hepatitis and Wilson’s disease. Digestive diseases like inflammatory bowel disease, chronic pancreatitis and duodenal or gastric ulcer are also among contributing factors. Many parasitic infections have also been involved. Metabolic and endocrine disorders like Type 2 diabetes, obesity, NASH have also been among the risk factors. Life-style also plays a major role in development of CCA such as alcohol consumption, smoking, and environmental toxin exposure. Host genetic polymorphisms have been reported to modulate risk of CCA. Combined hepatocellular and cholangiocarcinoma account for around 0.5% to 14% of primary liver cancers. They have been a mixture of parent phenotypic characteristics and are typically even more aggressive than hepatocellular carcinoma (HCC) or IH-CCA. Although less well studied, cholangiocarcinomas are postulated to arise from hepatic progenitor cells in the canals of Hering. It is perhaps not surprising that HCC and IH-CCA share several recurring risk factors with respect to chronic liver disease and its causes. Furthermore, despite improved understanding of CCA aetiologies, around 50% of cases are still diagnosed without any identifiable risk factor in Western countries. It is therefore presumable that other undefined etiologic factors are responsible for the current rise of CCA especially IH-CCA incidence globally and need to be explored further[1,8]. The pathologies of CCA can have many etiologies, including genetic, autoimmune, toxic and viral factors. All of them are characterized by a crucial inflammatory infiltrate that is associated with an excess of peri-portal fibrosis. The cell types that cause the regenerative response to liver damage belong to various lineages like inflammatory cells, mesenchymal cells, cholangiocytes. These cells interact with each other via different autocrine and paracrine signals. These signals/messengers can be morphogens (Notch, WNT/β-catenin, Hedgehog signalling cascades), pro-inflammatory cytokines and pro-fibrotic chemokines (IL-1, or 6; CXCL1, CXCL10 and CXCL12, and MCP-1), and ultimately growth factors (PDGF, VEGF, and TGFβ etc.)[9]. Molecular pathogenesis of cholangiocarcinoma is very complicated and involves multiple pathways; out of them Notch signalling pathway is significant because it is a highly conserved mechanism during development. This pathway is also critical for biliary cells (cholangiocytes) coordination and tubule formation[10]. The structure, homeostasis, and carcinogenesis of liver strictly rely on the Notch signalling pathway[11,12]. Furthermore, the Notch pathway plays an important part in many aspects of IH-CCA, that include the conversion of mature hepatocytes into malignant cholangiocytes, survival of tumour, proliferation and migration[13,14].
MOLECULAR MECHANISMS OF NOTCH SIGNALLING PATHWAY
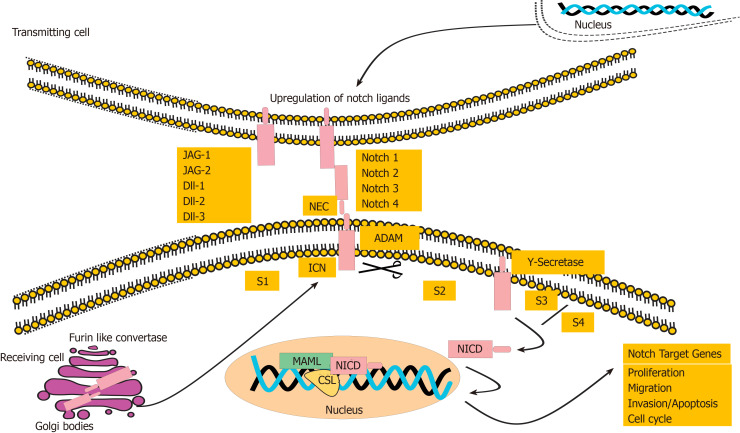
Our understanding of molecular mechanisms involved in pathogenesis of cholangiocarcinogenesis has improved lately, providing the basis for designing molecular targeted therapies. CCA tends to grow on the context of inflammation of bile duct and cholestasis[3]. Notch pathway, an evolutionary conserved signalling pathway, is mainly involved in regulating the embryonic development[15], homeostasis, differentiation, cardiac development, endocrine development, apoptosis, cell survival, stem cell function, angiogenesis and carcinogenesis[16-19]. Notch signalling is a unique cascade and comprises of four Notch receptors designated as Notch1, Notch2, Notch3 and Notch4. The ligands are named as Jagged-1 (JAG-1), JAG-2, delta like ligand 1 (Dll1), delta like Dll3 and delta-like Dll4. Notch receptors are type I transmembrane receptor molecules, which are coded by a single precursor receptor that becomes a non-covalently linked heterodimer. This non-covalently linked heterodimer comprises of N-terminal extracellular subunit and an intracellular (C-terminal transmembrane-intracellular subunit subunits[20]. These receptors and ligands, both, are the transmembrane proteins. The signalling pathway activates upon the direct cell-to-cell contact[20]. Upon activation, Notch receptors are cleaved from the membrane by the proteases, recognized as disintegrin and metalloproteinase family (ADAM10 or ADAM17), and a presenilin-dependent gamma secretase complex. As a result of cleavage, the Notch intracellular domain (NICD) translocate into the nucleus, where it binds to the inactive CBF1-Su(H)- LAG1, also known as a Recombination signal Binding Protein for immunoglobulin kappa J (RBP-J) transcription factor, recruits the coactivator protein mastermind-like 1 (MAML-1) and activates the downstream effector molecules as shown in Figure 1. The list of downstream target genes is very much dependent on type of the cell they are activating and include those genes, whose products are involved in fundamental aspects of cell biology, such as cell-cycle regulation[21,22], cellular differentiation, and metabolism[23]. Most of the downstream target genes include transcriptional repressors (Hairy Enhance of Split (Hes1, Hes5) and Hairy/Enhancer of Spit related with YRPW motif (Hey1), oncogenic pathways proteins (MYC and Nuclear Factor-Kappa B, cell cycle progression genes (CCND1/3), cell migration and metastasis gene (CCR7) and apoptotic inhibition genes (BCL2 and Survivin)[20], cell cycle regulation genes (p27cip1/waf1 and cyclinD1, p21), transcription factor in cancerous and transcriptional factor in Embryonic stem cells (Slug and Nanog, respectively)[24,25]. This pathway plays a critical oncogenic role in development of various cancers. It is genetically altered in a variety of haemopoietic and solid tumours[18], including T-cell leukaemia, prostate cancer, lung cancer, colorectal cancer, cancers of central nervous system. Furthermore, it has also been reported to be in association with the breast and oesophageal cancer stem cells. Targeting this pathway represent a reasonable approach for treating these cancer[24]. Pertaining to hepatic physiology, Notch pathway plays significant role in development of liver, and is also essential for biliary differentiation[26-28]. Mutations of genes involved in Notch pathway, particularly JAG-1 and Notch2, have been identified in patients of Alagille syndrome, which is a human genetic disorder illustrated by the scarcity of intrahepatic bile ducts, a condition that leads to the chronic cholestasis and hepatic failure[29,30]. In human IH-CCA, the Notch pathway is normally activated[31,32]. Notably, overexpression of the activated form of NICD1 facilitates development of IH-CCA[32,33], and plays an important role in accelerating the thioacetamide-induced cholangiocarcinogenesis in mice[34]. Notch pathway is understood to be a major signalling cascade stimulating IH-CCA[25,27]. In HCC development, components of Notch signalling pathway show complex features. Haplo-insufficient mutations in the ligands and receptors of Notch signalling pathway propose that Notch signalling play very level-dependent and context-dependent roles in liver tumours. It has been suggested that low levels of Notch associate with high activity of the Wnt signalling pathway, which is established as a major oncogenic pathway in HCC[35]. Besides that, higher levels of active Notch1 result in inhibition of the expansion of HCC cells[36]. It’s deletion in the liver of mice results in hyper- proliferative hepatocytes, that suggest a tumour-suppressive role of Notch in development of HCC[37]. Likewise, Notch signalling cascade has a tumour-suppressive effect in HCC, which is initiated by inactivation of the RB signalling pathway[38]. However, on the contrary, other studies in literature have given evidence that Notch signalling pathway is oncogenic in HCC[39,40], and might be important for the growth of tumours following hepatitis B virus infection[41]. In contrast to the complicated functions of Notch signalling pathway in development of HCC, gathering evidence supports a pro-tumourigenic function for Notch signalling pathway in CCA. Mutations of the Notch repressor, FBXW7, have been reported to be present in a subset of human tumours[42]. Similarly, like the disruption of bile ducts in Alagille syndrome patients, activation of Notch 2 pathway in hepatic progenitors and adult hepatocytes results in progression of biliary tubulogenesis[41]. Constitutive activation of Notch 1 receptor is proven to be sufficient to initiate the development of CCA in mice[31].
Figure 1.
The pathway activation of Notch signalling receptors and their downstream signalling molecules.
NOTCH SIGNALLING PATHWAY IN CHOLANGIOCARCINOMA
The Notch signalling is recognized to be vital for biliary fate and morphogenesis in the liver. Activation of Notch signalling pathway plays a significant role in ontogenesis of bile duct throughout the process of ductal plate tubulogenesis and remodeling[10,27,43-58]. As a consequence, to the signals received from periportal mesenchyme, Notch is involved in regulating the differentiation of hepatoblast cells to duct plate cells and produce branching tubules by induction of the HNF1-β, Hes-1, and sex-determining region Y (SRY)-box 9 (Sox-9) genes expression. Henceforth, Notch signalling mechanism is regarded as an important regulator for differentiation of cholangiocytes[10,43-45]. Additionally, various studies highlight that the Notch signalling is essential for the maintenance of homeostasis of liver tissue in the post-natal life, due to its involvement in the regulation of hepatic glucose and lipid content production[55]. During a chronic disease state, liver cells has been observed to be able of converting into biliary progenitor cells. Upon causing a chronic hepatic injury in mouse, induced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine, via Notch-RBPJ Hes1 signalling axis has revealed critical role of Notch signal activation[33,56]. In the meantime, the Notch pathway has been recognized as a significant biomarker as well as a key indicator of the progression and prognosis of cholangiocarcinoma. In IH-CCA, the Notch cascade has been established as the most vial pathway based on the nature of the overexpressed genes, compared with HCC, via microarray analysis[57]. Above all, a study has showed the association of abnormal expression of Notch 1 in IH-CCA with bigger tumour size, whereas the overexpression of Notch 4 has been found to be associated to poor overall rate of survival. This matched with higher serum levels of CA125, representing poor prognosis[59]. Another study conducted on specimens of EH-CCA has indicated that Notch 1, 2, 3, 4 and downstream effector molecule Hes-1 are expressed in, 56.1%, 50.0%, 6.1%, 81.8% and 42.2% of tumour samples. Also, Notch 1 and 3 levels have been found to be linked with poorer differentiation of histology[60]. Likewise, patients who overexpresses at least one among Notch 1, 2 and 3 receptors has showed a worse prognosis[60]. Besides, a recent analysis has revealed that in most cases the IH-CCA samples overexpresses the Notch 1 receptor[31]. Although studies investigating the clinical as well as pathological patterns of Notch receptors in human CCA are at an inadequate scale, a number of research projects on animal models and cell lines of CCA are in abundance. Particularly, the animal models are fundamental in determining the idea that the transformation of hepatic progenitor cells and even differentiated hepatocytes into biliary lineage cells through activation of Notch signalling can lead to the development of CCA[31]. Notch 1 receptor: Pathologically, the significance of Notch 1 receptor in development of CCA is evidenced via a number of studies conducted in experimental models and human specimens. For example, the overexpression of Notch 1 receptor in conjunction with the Notch ligand JAG-1 was observed in four human CCA cell lines (MzChA1, TFK1, SZ1and EgI1)[61]. Predominantly, it has been documented in the literature that stable unaided overexpression of the NICD1 in the mouse liver triggers the formation of cystic type cholangiocellular tumours, also recognized as cystoadenocarcinomas and cystoadenomas[32]. In addition, simultaneous activation of Notch signalling pathway and overexpression of v-akt murine thymoma viral oncogene homolog (AKT) proto-oncogene and inactivation of the p53 tumour suppressor has been found in eliciting the rapid transformation of normal hepatocytes into biliary cells, which ultimately becomes lethal IH-CCA[33,61]. In contrast to this data favouring the critical role of Notch 1 in IH-CCA formation, alternative study has showed that the treatment with an extremely specific anti-Notch1 antibody resulted in higher occurrence rate of IH-CCA like tumours, although drastically decreasing the growth of HCC like tumours in the mouse model manifested by the higher expression of activated neuroblastoma RAS viral oncogene homolog (NRas) and v-akt murine thymoma viral oncogene homolog (AKT) proto-oncogenes[62]. Another study proposes that the Notch 1 may primarily contribute to IH-CCA development instead of conversion of hepatocytes into biliary cells (cholangiocytes). The overexpression of Notch 1 has also been discovered to enhance IH-CCA cell migration due to the Rac1 activation. The atypical expression has also been supplemented by the up-regulation of Vimentin and α-SMA at the cost of the expression of E-cadherin, which results in an epithelial-mesenchymal transition (EMT) phenotype[63]. Notch 2 receptor: Notch 2 receptor holds significance in biliary cell fate specification along with the process of tubulogenesis during bile duct development[27,43-45,64]. In addition to JAG-1, Notch 2 mutations are recognized in Alagille syndrome patients, a genetic condition in humans characterized by the lack of intra-hepatic bile ducts[30]. Surprisingly, the higher expression of Notch 2 was noticed more frequently in well-established IH-CCA, therefore, suggests its part in differentiation of cancerous cell[59]. Besides, another research revealed that Notch 2 receptor is augmented in progenitor cells and significant reduction in the incidence of cholangiocellular tumours in PTEN-deleted mice have been reported as a result of its inhibition[65]. Similarly, Notch 2 signalling gets active in the AKT/Ras mouse model, where inhibition of Notch 2 impedes formation of tumour and extraordinarily decreases the tumour burden[62]. Notch2 has been found to regulate the hepatocyte-derived-CCA formation in mice[14]. It has been shown that subset of human ICCs originate from the mature hepatocytes. Wild-type and Notch2 flox/flox mice have been employed to investigate the role of canonical Notch signalling and Notch receptors in AKT/Yap-driven ICC formation. Human HCC and ICC cell lines have been transfected with small interfering ribose nucleic (siRNA) acid against Notch 2 to find whether Notch 2 regulates biliary marker expression in liver tumour cells and it has been observed that AKT/Yap-induced ICC formation is hepatocyte derived and this process is strictly dependent on the canonical Notch signalling pathway in vivo. Also, in vitro experiments on ICC and HCC cell lines has unveiled that Notch2 silencing down-regulates the expression of Sox9 and EpCAM, typical biliary markers. Hence, Notch2 is the major element of hepatocyte-derived ICC formation[14]. Notch 3 receptor: IH-CCA development was found to be associated with overexpression of the Notch 3 atypical receptor that promoted tumour cell survival through the PI3K-AKT cascade activation, instead of employing the canonical Notch-RBPJ mechanism[66]. Hepatocarcinogen thioacetamide (TAA) has been mentioned by the authors in inducing tumours in CK19CreERTeYFPp53 mouse model possessing constitutive deletion of the Notch 3, and established that the loss of single copy of Notch 3 gene was adequate in inhibiting intra-hepatic CCA development[66]. Noteworthy, the unique, non-canonical identified molecular mechanism downstream the Notch3 may propose the prospect in CCA to selectively aim Notch 3 effectors downstream (the PI3K/AKT pathway), evading the RBPJ-dependent toxicity[62]. Notch 4 receptor: The available research data on the Notch 4 receptor is quite limited in case of IH-CCA. As mentioned earlier, in IH-CCA human samples, the overexpression of Notch 4 is related to elevated levels of CA125 in serum, signifying poor prognosis[59]. More studies are required in order to completely comprehend the crucial function of Notch 4 in IH-CCA formation. JAG-1: Among the various ligands that have been found activated in the various types of cancers, JAG-1 has been studied in the course of IH-CCA. It is indeed a key entity of hepatic synthesis and is basically expressed in cholangiocytes and endothelial cells found in peri-portal mesenchymal cells and around the portal veins and bile ducts[26,67]. Expression of JAG-1 messenger ribose nucleic acid (mRNA) levels has been found significantly increased in IH-CCA patient samples. The overexpression of JAG-1 in disease form induce the expression of biliary lineage markers in neighbouring hepatocytes and hepatoblasts making them committed to transform into cholangiocytes and promotes the development of IH-CCA with AKT activation. In the mouse model of IH-CCA created by induction of TAA, JAG-1 expression has been observed in liver Kupffer cells which localize around the central vein transiently[16]. Afterward, the activation of Notch signalling pathway in peri-central hepatocytes leads to their transformation into cholangiocytes (biliary cells)[68]. This has been further confirmed through experiments that JAG-1 is expressed in myofibroblasts but not in hepatocytes, endothelial cells, hepatic stellate cells (ms) or myofibroblasts. Furthermore, it has been observed that the removal of Kupffer cells results in the apoptosis of pericentral hepatocytes that may occur due to the absence of Notch activation[68]. Another study confirms the role of JAG-1 in CCA pathogenesis by suppressing the JAG-1 that reduces the growth of human CCA cell lines (HUCCT1 and KKU-156) with subsequent down- regulation of downstream signalling molecules (Hes1 and Hes4)[16]. RBPJ: RBPJ, an important transcription factor downstream of Notch, plays a significant role in medication the effects of Notch signalling pathway activation. In embryogenesis it is a key component of hepatoblast transformation into biliary cells (cholangiocytes) and tubulogenesis highly depend on the RBPJ-driven effector transcription[26]. As RBPJ comprises the deoxyribonucleic acid binding site of Notch transcription site, its removal completely blocks the pathway activation[69]. Further, it has been documented that down-regulation of RBPJ protein in AKT/JAG-1 mouse model cause the complete inhibition of Notch signalling pathway with subsequent development of IH-CCA[16]. It has been well established that Notch 1 overexpresses in both nucleus and cytoplasm of CCA cells and plays its function via RBP-J-associated module (RAM) manner, Nongnuch and his colleagues found the role of Notch1 in development of CCA in RAM independent manner. They have found that Notch signalling activation enhanced the proliferation of CCA cell lines with cell survival via up regulation of pro survival protein factors Bcl-xL and Mcl-1. They have further demonstrated that Notch1 interacts with 14-3-3 theta and induce the CCA cell survival. Knockdown of 14-3-3 theta regulator proteins in RMCCA-1 cell lines that overexpress NICD1 has been found to abolish the pro-survival effects of NICD1 under gemcitabine antibiotic treatment. So, it has been concluded that Notch 1 also plays an important part in progression of CCA by promoting the cell survival and proliferation through the regulation of regulation of 14-3-3 theta in RAM independent manner[70]. Hence, together these all data suggest that RBPJ is a key component of Notch signalling pathway in CCA. It is of worth considering that Notch 1 exhibits its cancerous activity in assistance with its transcription factor, Sox-9, which is a well-established Notch target gene which is involved in biliary specification of hepatic progenitor cells[69,71]. Also, the continuous up-regulation in expression of Sox-9 correlates with the transformation of precancerous lesions in full-fledged CCA[71,72]. Hes1: Hes1 (Hairy and enhancer of split-1) is a basic helix-loop-helix transcription factor, which is one of the key regulators of organogenesis and target genes of the Notch signalling cascade, is involved in the development of IH-CCA. It has been found to be highly expressed by CCA-like tumours[62]. By studying the mice model, tamoxifen-inducible Alb CreERT2; R26RNotch/+ mice that expresses the intracellular fragment of mouse NICD1 and delivers constitutive Notch signalling activity in hepatocytes and Alb-CreERT2; Hes1fl/fl mice, in which the hepatocytes lack the gene encoding Hes1 has showed that on treatment with TAA, CK10 positive cells significantly have increased in hepatic lobules of Alb CreERT2; R26RNotch/+ and have decreased in Alb-CreERT2; Hes1fl/fl mice[73]. In addition to that, a rapid nodule formation has been observed in the livers of Alb CreERT2; R26RNotch/+ comparing to those of Alb-CreERT2; Hes1fl/fl[73]. This reveals that Hes1 is highly involved in pathogenesis of intrahepatic CCA and cell-fate conversion into biliary lineage cells. Inhibition of Hes1 can diminish this process[74] (Table 1 summarizes the main findings).
Table 1.
List of main representatives of Notch Signalling pathway and their current findings in development of cholangiocarcinoma
| Receptors, ligands and downstream signaling molecules | Type of model | Findings | Ref. |
| Notch 1 | Mice model | Overexpression of NICD1 in mice liver leads to the formation of cystoadenomas and cystoadenocarcinomas. IH-CCA cell migration is enhanced by overexpression of Notch 1. It further enhances the growth of IH-CCA as result of Rac1 activation. The atypical expression by the up-regulation of Vimentin and α-SMA at the expense of E-cadherin expression leads to an EMT | [32,63] |
| Human cell lines | Overexpression of Notch 1 receptor along with JAG-1 has been perceived in four human CCA cell lines (MzChA1, TFK1, SZ1and EgI1) | [61] | |
| Notch 2 | Wild-type and Notch2 flox/floxmice model | Notch2 is basically involved in regulating the hepatocyte derived CCA. Wild-type and Notch2 flox/floxmice models have been utilized for studying the Notch Signalling pathway in AKT/Yap-driven IH-CCA development and has been found that AKT/Yap-induced IH-CCA development is hepatocyte dependent and based on the canonical Notch signalling cascade in in vivo models | [14] |
| Human cell lines | HCC and ICC cell lines reveals the down-regulation in expression of Sox9 and EpCAM, typical biliary markers after Notch 2 silencing | [14] | |
| Notch 3 | Mice model | IH-CCA development and survival has been found to be associated with activation of PI3K-AKT cascade by overexpression of the Notch 3 atypical receptor rather than canonical Notch-RBPJ mechanism. Hepatocarcinogen Thioacetamide induces cancer in CK19CreERTeYFPp53 mouse model owing constitutive Notch3 deletion that establishes the IH-CCA inhibition after loss of single copy of Notch 3 gene | [66,62] |
| Notch 4 | Human samples | The overexpression of Notch 4 is associated with raised serum levels of CA125 that suggests the poor prognosis of IH-CCA | [59] |
| Jagged-1 | Human cell lines | The suppression of the Jagged-1 results in the reduction of growth of the human CCA cell lines (HUCCT1 and KKU-156) with consequent down-regulation of downstream signalling molecules (Hes1 and Hes4) | [16] |
| RBPJ | Mice model | The down-regulation of RBPJ protein in AKT/JAG-1 mouse model results in total inhibition of Notch signalling pathway following the development of IH-CCA. Notch 1 plays its role in advancement of CCA by enhancing the cell survival and proliferation through the regulation of 14-3-3 theta in RBP-J-associated module. Hence, it concludes that that RBPJ is a significant element in Notch signalling pathway in CCA | [16,70] |
| Hes-1 | Mice model | The tamoxifen-inducible Alb CreERT2; R26R Notch/+ mice expresses mouse NICD1 and delivers the constitutive Notch activity in hepatic cells. In Alb-CreERT2;Hes1fl/fl mice, these hepatocytes that lack the Hes1 gene have revealed that on treatment with TAA, CK10 positive cells significantly increases in lobules of Alb CreERT2; R26RNotch/+ and has been found to be reduced in Alb-CreERT2; Hes1fl/fl mice. Besides that, the rapid nodule formation has been found to be obvious in the livers of Alb CreERT2; R26RNotch/+ in contrast to those of Alb-CreERT2; Hes1fl/fl.. This observation confirms the fact that Hes1 is greatly involved in pathogenicity of IH-CCA. Further, this carcinogenesis can be blocked by inhibition of Hes1 | [73,74] |
CCA: Cholangiocarcinoma; TCC: Thioacetamide; IH-CCA: Intrahepatic cholangiocarcinoma; NICD: Notch intracellular domain; EMT: Epithelial-mesenchymal transition; RBP-J: Recombination signal Binding Protein for immunoglobulin kappa J; JAG: Jagged.
CROSSTALK BETWEEN NOTCH PATHWAY AND OTHER SIGNALLING CASCADES IN CHOLANGIOCARCINOMA
The outcome of activation of Notch signalling has been identified in enhancing discrete and frequent paradoxically contradictory effects, which are highly dependent on the cellular and/or tissue context[59]. Evidently, the Notch “net” pro-oncogenic or tumour suppressive function is a result of the interaction with other signalling pathways. The significance of the crosstalk between Notch and many other cascades, for instance Hippo, AKT/mTOR, VEGF, TGFβ, Hedgehog, EGFR, and Wnt/β-catenin is advancing and under deep investigation in the processes of development in addition to numerous tumour entities[60]. Sonic Hedgehog signalling pathway: The non-canonical Sonic Hedgehog signalling cascade contributes to the progression of CCA[75]. Fingas and his co-workers have explained the administration of cyclopamine (SMO inhibitor) as an agent to increase the apoptosis of CCA cells as well as their metastasis[76,77]. The Hedgehog and Notch pathways interact to control the fate of vital cell types involved in adult liver repair by modulating epithelialâ to mesenchymal–like or mesenchymal to EMT[78]. Blocking of Notch signalling in HSCs or myofibroblasts (MFs) suppress the hedgehog (Hh) activity and called a mesenchymal to EMT. Similarly, inhibition of the Hh pathway suppresses Notch signalling and induces a mesenchymal to EMT. Besides that, in mice, hepatic injury increases Notch activity in MFs and Hh responsive MF progeny, which are the HSCs and ductular cells. Manipulation of Hh signalling in MFs of bileâ duct–ligated mice results in inhibition of the Notch signalling and blocks the accumulation of both MFs and ductular cells and prevents hepatic fibrosis[78]. In CCA, the contents of knowledge explain the interaction between Notch signalling and further cascades are scant, but are promptly increasing with advancement in time. Hippo Signalling Pathway: The Hippo pathway plays an important regulatory role in the development of liver cancer. The function of this pathway has been demonstrated in mouse mixed HCC/ICC model, induced by overexpression of AKT and Ras (AKT/Ras) oncogenes. It has been found that Hippo inactivation in AKT/Ras hepatic tumors lead to the localization of TAZ and Yap in nucleus. Co-expression of AKT/Ras with Lats2, which activates Hippo results in removal of ICC-like lesions and delayed hepatocarcinogenesis in the liver. Subsequently, Notch 2 expression has been found to be downregulated by the Hippo pathway in liver tumors. Thus, this signifies the role of Hippo signaling pathway in in regulation of hepatic tumors[79]. Wnt Signalling Pathway: Abnormal Wnt and Notch signaling pathways are recognized as the key regulators of CCA. It has been observed that the transcription factor, the proline-rich homeodomain protein/hematopoietically expressed homeobox (PRH/HHEX), results in formation of a positive transcriptional feedback loop with receptor Notch 3, that is crucial in development of CCA. Expression of PRH/HHEX elevates in CCA and depletion of PRH diminishes the growth of CCA in a xenograft model. The study of the gene pathways regulated by Notch 3 and PRH shows that unlike Notch3, PRH is directly involved in activation of Wnt signaling pathway. Furthermore, new therapeutic strategies will be based on the dependence of specific Notch, Wnt and CDK4/6 inhibitors on the activity of PRH[80].
NOTCH SIGNALLING: A PROMISING THERAPEUTIC TARGET IN CHOLANGIOCARCINOMA
Gamma-Secretase Inhibitors (GSI): Numerous GSI have been developed and synthesized. These drugs play a significant role in blocking the proteolytic cleavage of Notch receptors in order to inhibit the downstream signalling process and are recognized as “Notch inhibitors”. They are reported to show the cytotoxicity in various types of cancer cells[81]. Though they have tested in clinical trials of various cancers, but not enough patients of CCA have been enrolled for these drugs tastings. So far, only NCT02784795 gamma secretase inhibitor of Notch pathway has been tested for Phase 1 trial on CCA patients. Another GSI, LY3039478, has only been studied in vitro to restrain Notch signalling and it’s downstream biologic properties. They will be further tested with anticancer mediators in patients of advanced CCA. A novel small-molecular inhibitor of GS, RO4929097, is in multiple phase 1 and phase 2 clinical trials of colorectal, pancreatic, and other solid tumours. It has shown anti-tumour activity with tolerated side effects[64,82]. There are many preclinical and in vitro investigations going on relating to the development and pathogenesis of CCA. The use of (DAPT), N-[N-(3,5-difluoro- phenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester, the potent GSI, abolishes the cleavage of Notch receptors and the Notch target genes mainly of Hes1 in human CCA cell lines (SZ1, MzChA1, EgI1 and TFK1)[30]. In addition to inhibiting the cleavage of Notch receptors, DAPT significantly reduces the growth of CCA xenografts, which is accompanied by the expression of p27, p53 and p21, and a downregulation of Cyclin E in tumour tissues[30]. GSI IX effectively impairs cell proliferation, cell migration, EMT and invasion in human CCA cell lines[61]. Though the GSI are being extensively studied in initial clinical trials of breast cancer, T-ALL and other solid tumours[83] but their application in clinical trial of CCA has not been extensively explored. So, there is a need on more clinical and experimental research on GSIs in CCA, as they appear to be effective inhibitory molecules of Notch signalling pathway and have great potential to suppress the CCA progression. MFAP5, a Miicrofibrillar-associated protein 5, has been detected as a diagnostic, prognostic and therapeutic marker of CCA. It facilitates the aggressiveness of IH-CAA by activating the Notch1 signalling pathway. A gamma secretase inhibitor, FLI-06, has been found to completely abolish the MFAP5- dependent Notch activation in CCA[84]. Crenigacestat is a LY3039478-specific inhibitor. It is a selective Notch 1 inhibitor that reduces the progression of IH-CCA by blocking VEGFA/Dll4/MMP13 signalling axis[85]. Reactivation of Notch signalling pathway has been observed in biliary tumours to conflicting degrees. Hindering prioritization of important therapeutic targets and identification of candidate responder patients for Notch-directed therapies has been documented in literature. Genomic data of 341 CCA patients has been analysed and Notch 1 has been found significantly overexpressed in a subgroup, which is characterized by distinct infiltration in stroma. Network-wide disturbance of the Notch signalling pathway has been observed in CCA that include the correlation of Notch 1 with Notch 3 and Notch pathway ligands. Considering the observed Notch receptor involvement, γ-secretase alteration has been rationalized as a therapeutic option. Certainly, subcutaneous transplantation of resistant and sensitive CCA cell lines pre-treated with a GSI cocktail has determined the antineoplastic effects of GSI in a subset of CCA and resulted in leading to the development of a 225-gene responder signature. Moreover, this signature has been found to be enriched in liver tumours initiated by hydrodynamic injections of activated-Notch comparing to the AKT-RAS-driven tumours[86]. Anti-Notch signalling Antibodies: As Gamma Secretase Inhibitors also inhibit the other signalling molecules and tend to have a high intestinal toxicity; anti- Notch antibodies specifically antagonize each receptor[81]. There are mainly two classes of anti-Notch antibodies; one that blocks the receptor conformational modification by binding to its negative regulator region, which is involved in ADAM protease cleavage, while the other one blocks the ligand binding domain with ligand competitors. Scientists support negative regulator region Abs more because ligand binding domain antibodies require higher concentrations to get the same effect. Lately, the antibody against Notch 1 (Brontictuzumab, OMP-52M51) and against Notch 2/3 (Tarextumab, OMP-59R5) are under clinical trial Phase 1 and Phase 2, however, Tarextumab has been more applied on small lung cancer patients the ones with CCA[87]. Some ligands inhibitors have been developed such as anti-JAG-1 and anti-Delta-like Dll4 antibodies (Demcizumab, OMP- 21M18). Demcizumab is a monoclonal antibody that specifically targets Dll4. Demcizumab has been reported to decrease the tumour growth in multiple tumour models and cause the defective cell fate differentiation, particularly, in human tumour xenografts[88,89]. The completed clinical trials have been mainly conducted in non-small cell lung cancer, ovarian cancer and pancreatic cancer patients; nevertheless, a Phase 1b trial of Demcizumab plus Pembrolizumab in metastatic solid tumours is recruiting (NCT02722954). A comprehensive understanding of Notch receptors and ligands is extremely significant before employing these antibodies, as preclinical studies have shown that the appearance of distinct phenotypes in response to Notch 1 and Notch 2 Abs treatment[62]. On treating AKT/Ras mice with the anti-JAG-1 or anti-Notch2 antibodies, a noticeable loss in tumour burden has been observed. Particularly, Notch2 signalling pathway has been impeded with the down regulation of Hes1 in tumour bearing livers, which is consistent with the inhibition of Notch 2 activation with Notch 2 antibody. Treatment with anti-JAG-1 or anti-Notch 2 antibodies in the start of tumourgensis approximately entirely has resulted in prevention of cholangio-carcinogenesis in AKT/Ras mouse model[62]. Considerably, treatment with anti-JAG-1 or anti-Notch2 antibodies leads towards a loss of activated Notch 2, while anti-Notch1 treatment heads to an increase in activation of Notch2[62]. These results have unveiled the therapeutic potentials of or anti-JAG-1 or anti-Notch2 antibodies for pathogenesis of CCA. It should also not be ignored that some toxicity effect has been noticed in terms of weight loss in higher dose mice[62]. Another worth considering study has showed that selective blocking of Notch 1 exhibits its inhibitory effect by two mechanisms. Out of these two, one follows the inhibition of the growth of cancer cells and other follows the deregulation of process of angiogenesis. This study has also revealed the intestinal toxicity on the combined inhibition of Notch and Notch 2, while the inhibition of either of the receptors alone exhibits lesser side effects. Thus, this study clearly demonstrates the benefits of using anti Notch receptor monoclonal antibodies for cancer treatment[90]. RNA interfering antisense oligonucleotide: RNA interfering antisense oligonucleotide, an advanced molecular therapeutic, has the potential to alter the activity of particular gene. A study conducted by Kaneez and her co-workers has showed that administration of the antisense oligonucleotides against specific Notch 2 nucleotide sequence might serve as future therapeutic agent with its ability of down regulating of Notch 2 activity in pathogenicity of B-cell chronc lymphocytic leukemia[91]. Anti-microRNA: Micro-RNA (miR)-21 has been reported to overexpress in both IH-CCA and EH-CCA[92]. Anti-miR-21 is a high affinity oligonucleotide. It is complementary to the active site of miR-21 with a phosphorothioate backbone containing modifications[65]. Tumour treatments with anti-miR-21 show an outstanding decrease in occurrence of cholangiocellular lesions[65]. Also, the average tumour weight in anti-miR-21 treated mouse has been considerably reduced at molecular level and has been associated with lower Notch 1 and Notch 3 expression in the liver and a low expression of Notch 2 in cholagiocellular tumours. Hence, these studies suggest the role of anti-miR-21 in suppressing the growth of CCA via inhibition of Notch 2 signalling[65,93]. As miRNAs and epigenetic molecules signify key therapeutic targets to be assessed for future treatment of CCA. The biological function and epigenetic regulation of miR-34a has been reported in human CCA[94]. It has been reported that miR-34a is an important tumour-suppressive miRNA that is silenced epigenetically by EZH2-mediated H3K27me3 and deoxyribonucleic acid methylation in CCA. It has been proved experimentally that miR-34a plays a significant role in suppressing the CCA cell growth in in vivo and in vitro via Notch signalling pathway related genes Notch 1, Notch 2 and JAG-1. Further to be noted that the expression of EZH2 is markedly increased in human CCA cell lines and tissues. Inhibition of EZH2 restores the tumour suppressive function of miR-34a and inhibits the cell growth of CCA in vitro and intrahepatic metastasis in vivo, thereby, suggesting the targeting of miR-34a and EZH2 in addition to Notch for effective therapy of CCA[94]. miR-200 family, comprising of miR-200a, miR-200b, miR-200c, miR-141 and miR-429, performs a significant function in suppressing metastasis of cancer by inhibiting the EMT. Reduced expression levels of miR-200c and miR-141 have been found associated with the overexpression of the transcription factor ZEB-1, which in turn induces the activation of the Notch signalling cascade by targeting Notch ligand JAG-1 and mastermind-like co-activators MAML-2 and MAML-3. Niclosamide, an anthelminthic drug, has been found to have the potential role of inhibiting the progression of colon cancer by up-regulating the miR-200 family members and by down-regulating the Notch signaling[95]. Stapled peptides: Stapled peptides have also been generated that include a cell permeable hydrocarbon-stapled α-helical peptide (SAHM1). It actually prevents the assembly of Notch1 trans-activation complex[96]. This compound is reported to inhibit the Notch pathway and have the suppressive effect on the development of in vivo and in vitro T-Cell acute leukemia models[96]. Small-molecule inhibitor (SMI), inhibitor (Inhibitor of Mastermind Recruitment-1 (IMR-1): In another study, a small-molecule inhibitor, named IMR-1, has been identified that disrupt the recruitment of Mastermind-like 1 to the Notch transcriptional activation complex on chromatin[97]. Specifically, IMR-1 has been reported to impair the development of patient-derived tumour xenografts and is also involved in inhibiting the growth of Notch-dependent cell lines[97]. Though these stapled peptides represents a promising therapeutic entities against Notch derived cancers, so far, none of them have been studied in preclinical trial of CCA. Other Biological/Natural Inhibitors: Natural compounds have been proposed for their inhibitory effect on the molecular components of this pathway. Currently, aspartate β-hydroxylase, a type II transmembrane protein, is under noteworthy consideration. This aspartate β-hydroxylase plays an important role in catalyzing the hydroxylation of asparaginyl and aspartyl residues, which are present in the epidermal growth factor-like domain of various proteins, including Notch[98]. A study employing a second-generation SMI of β-hydroxylase activity has demonstrated a significant suppression in the tumour growth and progression of CCA without having an effect on the body weight of mice. The treatment with SMI of β-hydroxylase also inhibits the overexpression of activated Notch 1 and Hey 1 in the subcutaneous CCA tumours[99]. Some compounds, like Corilagin[100] and Curcumin[101], also represents the inhibitory effects on the growth of CCA via Notch signalling cascade. Corilagin showed the inhibitory effect on cell proliferation, migration and invasion of CCA and promoted cell apoptosis. It inhibits Notch 1 and also showed the reduced Hes1 messenger ribose nucleic acid expression through impeding Hes1 promoter activity. Corilagin also inhibits the growth of CCA and reduces the expression of Notch 1 and mTOR in nude mice[100]. Another natural compound, Curcumin, effectively reduces the development of CCA cell growth. It also induces apoptosis at relatively low concentrations. A subsequent reduction in the expression of Notch1, HES-1, and Survivin in cell lines of CCA has also been observed on treatment with curcumin[101]. Chansu is a traditional Chinese compound extracted from the parotid glands of Chinese toad (Bufo gargarizan)[102]. It is extensively used for the treatment of variety of aliments including central nervous system respiratory disease, cardiac diseases and canner. An important component of Chansu is Cinobufagin that has an anti cancer effects including inhibition of prostate cancer cells by inducing apoptosis[103]. Antitumour effects of cinobufagin on CCA has illustrated that Cinobufagin exhibits significant suppression on CCA cell proliferation as well as induce the cellular apoptosis, both in vivo and in vitro. As Notch pathway plays a significant role in the basic processes of cancer cell proliferation and apoptosis, it has been found that Cinobufagin may inhibit the growth of tumour by inducing apoptosis via the activation of Notch pathways in CCA[104]. Xanthohumol, a prenylated chalcone, induces the apoptosis and cell cycle arrest via an increase in pro-apoptotic markers and reduction of cell cycle regulatory proteins. Xanthohumol has been found to reduce Notch 1 and AKT expression in a time-dependent fashion. In addition, Xanthohumol reduces growth of CCA in both SG-231 and CCLP-1 derived mouse xenografts. So, it significantly reduces CCA growth via the Notch1/AKT signalling axis[105]. (Table 2 summarizes the main therapeutic effects of these compounds). Anti-oxidants: Reactive oxygen species (ROS) and paracrine tumor necrosis factor (TNF) from Kupffer cells (macrophages) causes JNK-mediated CCA. Hence, treatment with antioxidants, depletion of Kupffer cells, depletion of TNFR1 and/or inhibition of JNK pathway reduces cholangiocellular lesions. Deletion of hepatic-specific JNK1/2 leads to reduction in tumor growth and enhances the survival in Akt/Notch induced IH-CCA models. As Kupffer cell-derived TNF induces the proliferation, differentiation and carcinogenesis, targeting of the ROS/TNF/JNK axis provide the potential strategy for IH-CCA therapy[106]. Cancer stem cells (CSCs) play a significant role in EH-CCA, so they are considered as a promising potential therapeutic target. They have been analysed in a tumour samples of EH-CCA via FACS for a cell surface markers of the CSCs and components of the Notch signalling cascade. The quantity of CSCs has been significantly correlated with higher nodal invasion. Henceforward, in vivo presence of CSCs in EH-CCA represents them as possible therapeutic objects[107]. In addition to various approaches to manipulate the Notch signalling pathway in CCA, a strategy to target the Notch signalling pathway in other disease models has also been of worth considering and employing in treating the CCA. Osteoclasts own a central role in bone physiology and interruption of these osteoclasts maturation process offers a potential therapeutic strategy for curing bone diseases. To study this phenomenon, Goel and his colleagues have employed an approach of genetically inhibiting the Notch signalling pathway in the myeloid lineage, which includes the osteoclast precursors. This genetically truncated Notch signalling actually involves the dominant negative form of MAML, called dnMAML, which play a significant role of inhibiting the transcriptional complex needed for the downstream Notch signalling pathway. Osteoclasts derived from dnMAML mice have shown no significant changes in early osteoclastic gene expression comparing to those of wild type. Further, these findings have demonstrated significantly reduced resorption activity using bone surfaces, whilst retaining their osteoblast stimulating ability using ex-vivo procedures. However, in vivo approaches show significantly higher rates of bone formation and osteoblast gene expression in dnMAML cohorts. These findings propose that therapeutic suppression of osteoclast Notch pathway could lower the osteoclastic resorption and shows a balanced model for increasing bone regeneration[108]. Hence, genetic manipulation of MAML could serve as a significant target to address the Notch signalling in CCA. Table 2 Anti-Notch therapeutic targets examined in vivo and/or in vitro for the treatment of CCA.
Table 2.
Anti-Notch therapeutic targets examined in vivo and/or in vitro for the treatment of cholangiocarcinoma
| Molecules/Targets and Functions | Experiments | Results and findings |
| GSI IX (Gamma Secretase Inhibitor) | In resected specimens of EH-CCA | In all of CC cell lines, treatment of GSI IX considerably reduces the subpopulation of CD24+ +CD44++ cells (Cancer Stem Cells, CScs)[60] |
| RO4929097 (Gamma Secretase Inhibitor) | Pancreatic adenocarcinoma patients | 25% treated patients attained the stable disease, while the 6-month survival rate was found to be 27.8%. Additionally, decrease in the expression of HeyL was observed following the drug administration[82] |
| DAPT (Gamma Secretase Inhibitor) | Cell lines (SZ1, MzChA1, EgI1 and TFK1) | Cause the decrease of the expression of the cleaved form of the Notch receptor and Hes1, with the subsequent overexpression of the cyclin kinase inhibitors (p21, p27 and p53)[30] |
| FLI-06 (Gamma Secretase Inhibitor) | IH-CCA cells | Found to be completely diminished the MFAP5- dependent Notch activation in CCA[84] |
| Brontictuzumab, OMP-52M51 (Anti-Notch1 antibody) | Phase 1 Clinical Trial | The treatment study was done on 07 CCA patients. It showed single-agent efficacy. While patients had a partial response, specifically in the NICD high population[87] |
| Tarextumab, OMP-59R5 (Anti-Notch2/3 antibody) | Phase 2 Clinical Trial | Phase1b/2 study conducted on SCLC (Small Cell Lung Cancer) and pancreatic cancer. Phase1b results showed a greater tumor reduction at high dose of TRXT[87] |
| Demcizumab, OMP- 21M18 [Anti-Delta-like ligand 4 (Dll4)] | Phase1/2 clinical trial | In NSCLC (Non-Squamous Non-small Cell Lung Cancer) patients treated with Demcizumab, 3% of patients had a valuable response, 48% had a partial response and 38% showed a stable disease. In pancreatic cancer patients, 50% of valuable patients who received Demcizumab showed a partial response[88,89] |
| RNA interfering antisense oligonucleotide (AS-ODN, a specific Notch2 Inhibitor) | B-CLL cells | AS-ODNs results in down-regulation of Notch 2 activity in pathogenicity of B-CLL[91] |
| miR-34a (Anti-tumorgenic effect) | Human CCA cells (CCLP1, TFK1, SG231 and HUCCT1) | miR-34a expression is silenced epigenetically by EZH2 that tends to result in progression of CCA cell growth via Notch cascade activation, so it’s activation via inhibition of EZH2 inhibitor results in the significant decrease of tumor burden[94] |
| Niclosamide (An antheliminthic drug) | Colon cancer cell lines (HCT116, LoVo and SW620 cells) | Niclosamide results in inhibition of the progression of colon cancer by up-regulating the miR-200 family members and by down-regulating the Notch signaling pathway[95] |
| Hydrocarbon-stapled α-helical peptide (SAHM1) | T-cell acute lymphoblastic leukaemia | It prevents the formation of Notch trans-activation complex and subsequently suppresses the development of in vivo and in vitro T- Cell acute leukemia[96] |
| Inhibitor of mastermind Recruitment-1 (IMR-1) | Notch-dependent cell lines and patient-derived tumor xenografts | It disrupts the recruitment of Mastermind-like to the Notch transcriptional activation complex and, thus attenuates the Notch target gene transcription[97] |
| MO-I-1100 (SMI, Small Molecule inhibitor of β-hydroxylase) | FOCUS, HCC cell line, BNLT3, Huh7, Hep3B and HepG2 | It reduces the ASPH enzymatic activity and inhibits the proliferation, invasion and growth of HCC cell lines and animal models. The mechanism of effect is based on the antitumor effect with the reduced activation of Notch Signalling cascade, both in vivo and in vitro[98] |
| MO-I-1151 (Second Generation Small Molecule Inhibitor, SMI of β-hydroxylase) | Human CCA cell lines (ETK1, RBE, SSP25 and BDE-Neu CL24 cells) | It exhibits the inhibition on CCA cell migration and proliferation and also significantly suppresses tumor growth and progression of CCA. The treatment also results in suppression of overexpression of Notch1 and Hey1 in the tumors[99] |
| Corilagin (A natural plant derivative, Polyphenol Tannic Acid) | Human CCA cell lines (MZ-Cha-1 and QBC9939) | It inhibits the CCA cell proliferation and invasion and increases the CCA cell apoptosis. It inhibits the AKT phosphorylation and counteracts the AKT phosphorylation that is induced by NICD1 and consequently inhibits the Notch1 and Hes1 mRNA expression[100] |
| Curcumin (A phytochemical derived from turmeric, Curcuma longa) | Human IH-CCA cell lines (SG-231 and CCLP-1) | It reduces the viability and colony-forming ability of CCA cells. Even at low concentration, it effectively reduces the growth of the cells and stimulates the apoptosis with subsequent suppression of Notch1 and Hes1 mRNA expression[101] |
| Cinobufagin (Chansu, Toad Venom) | Human prostate carcinoma cell lines (DU145, LNCaP and PC3 cells), Normal breast epithelial cell lines, Human CCA cell lines RBE (IH-CCA) and QBC939 (EX-CCA) | Cinobufagin causes and cell apoptosis and anti-proliferative effects in cancer cells[103]. Cinobufagin-derived inactivation of Notch signalling pathway results in the stimulation of apoptosis in CCA cells[104] |
| Xanthohumol (A Prenylated Chalcone) | SG-231and CCLP-1 derived mouse xenografts | Xanthohumol causes the cell cycle arrest via an increase in pro-apoptotic markers and induces the apoptosis. It also results in reduction of AKT and Notch1 expression level in a time dependent fashion[105] |
CCA: Cholangiocarcinoma; IH-CCA: Intrahepatic cholangiocarcinoma; NICD: Notch intracellular domain; IMR-1: Inhibitor of Mastermind Recruitment-1; SMI: Small-molecule inhibitor; GSI: Gamma-secretase inhibitors.
CONCLUSION
Notch signalling is juxtracrine mechanism that allows neighbouring cells to communicate and regulate various processes and in conjunction with other signalling pathways is critical in development, regeneration and repair of mammalian liver. Humans possess four Notch receptors and five ligands, each component plays unique role, some are widely reported and some are being studied. The pathway is critical in development and mutation of biliary system. In the embryonic liver Notch signalling induces differentiation of hepatoblasts into biliary epithelia. Similar mechanism has been noted in cellular response to liver injury where cells co-express hepatocyte and biliary markers and adopt changes leading to biliary morphology. These observations lead to research its role in both IH-CCA and EH-CCA. Notch signalling pathway inhibitors exhibit anti-proliferative tumour effects and represent a novel treatment for cancer; they can be used as single agent or in combination with other chemotherapeutic agents. GSIs and Notch inhibiting antibodies (mAbs), currently in clinical trials for various other tumours but few has been investigated for CCA. Although exploitation of this pathway has huge potential in improving survival in all types of CCA, as with any novel approach there are challenges that need to be overcome through research studies before its prospect is fully realized. There is gap in our understanding of the mechanism such as its relationship with other pathways. Many GSIs experienced several roadblocks in their development such as unacceptable intestinal toxicities and further research is needed to overcome them. Development of Notch isoform selective small molecules along with more targeted drug delivery and further advances in nano-medicine will pave the way for more effective therapeutic approach.
Footnotes
Conflict-of-interest statement: The authors declare no potential financial interests.
Manuscript source: Unsolicited manuscript
Peer-review started: June 3, 2020
First decision: July 21, 2020
Article in press: August 25, 2020
Specialty type: Oncology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Baiocchi L, Ju SQ, Messina C S-Editor: Zhang L L-Editor: A P-Editor: Li JH
Contributor Information
Bisma Rauff, Institute of Molecular Biology and Biotechnology, University of Lahore, Lahore 54000, Pakistan. bisma.rauff@imbb.uol.edu.pk.
Arif Malik, Institute of Molecular Biology and Biotechnology, University of Lahore, Lahore 54000, Pakistan.
Yasir Ali Bhatti, Institute of Molecular Biology and Biotechnology, University of Lahore, Lahore 54000, Pakistan.
Shafiq Ahmad Chudhary, Institute of Biomedical and Allied Health Sciences, University of Health Sciences, Lahore 54000, Pakistan.
Ishtiaq Qadri, Department of Biology, Faculty of Science, King Abdulaziz University Jeddah Kingdom of Saudi Arabia.
Shafquat Rafiq, Department of Gastrointestinal medicine, Croydon University Hospital, Croydon CR7 7YE, United Kingdom.
References
- 1.Kirstein MM, Vogel A. Epidemiology and Risk Factors of Cholangiocarcinoma. Visc Med. 2016;32:395–400. doi: 10.1159/000453013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghouri YA, Mian I, Blechacz B. Cancer review: cholangiocarcinoma. Journal of carcinogenesis. 2015 doi: 10.4103/1477-3163.151940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Intelligence Network. National Cancer Intelligence Network Rare and less common Cancers, Incidence and Mortality in England, 2010 to 2013. UK: Public Health England. [Google Scholar]
- 6.WHO. World Cholangiocarcinoma Day 2019 CCA: The statistics. http://worldcholangiocarcinomaday.org/the-statistics/ [Google Scholar]
- 7.Messina C, Merz V, Frisinghelli M, Trentin C, Grego E, Veccia A, Salati M, Messina M, Carnaghi C, Caffo O. Adjuvant chemotherapy in resected bile duct cancer: A systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol. 2019;143:124–129. doi: 10.1016/j.critrevonc.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 9.Cannito S, Milani C, Cappon A, Parola M, Strazzabosco M, Cadamuro M. Fibroinflammatory Liver Injuries as Preneoplastic Condition in Cholangiopathies. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19123875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17:722–735. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 12.Gil-García B, Baladrón V. The complex role of NOTCH receptors and their ligands in the development of hepatoblastoma, cholangiocarcinoma and hepatocellular carcinoma. Biol Cell. 2016;108:29–40. doi: 10.1111/boc.201500029. [DOI] [PubMed] [Google Scholar]
- 13.Cigliano A, Wang J, Chen X, Calvisi DF. Role of the Notch signaling in cholangiocarcinoma. Expert Opin Ther Targets. 2017;21:471–483. doi: 10.1080/14728222.2017.1310842. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Dong M, Xu Z, Song X, Zhang S, Qiao Y, Che L, Gordan J, Hu K, Liu Y, Calvisi DF, Chen X. Notch2 controls hepatocyte-derived cholangiocarcinoma formation in mice. Oncogene. 2018;37:3229–3242. doi: 10.1038/s41388-018-0188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Q, Li J, Zheng J, Wei A. The Carcinogenic Role of the Notch Signaling Pathway in the Development of Hepatocellular Carcinoma. J Cancer. 2019;10:1570–1579. doi: 10.7150/jca.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Che L, Fan B, Pilo MG, Xu Z, Liu Y, Cigliano A, Cossu A, Palmieri G, Pascale RM, Porcu A, Vidili G, Serra M, Dombrowski F, Ribback S, Calvisi DF, Chen X. Jagged 1 is a major Notch ligand along cholangiocarcinoma development in mice and humans. Oncogenesis. 2016;5:e274. doi: 10.1038/oncsis.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Z, Shang X, Zhang H, Wang G, Massey PA, Barton SR, Kevil CG, Dong Y. Notch Signaling in Osteogenesis, Osteoclastogenesis, and Angiogenesis. Am J Pathol. 2019;189:1495–1500. doi: 10.1016/j.ajpath.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- 20.D'Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ntziachristos P, Lim JS, Sage J, Aifantis I. From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell. 2014;25:318–334. doi: 10.1016/j.ccr.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi I, Minter LM, Telfer J, Demarest RM, Capobianco AJ, Aster JC, Sicinski P, Fauq A, Golde TE, Osborne BA. Notch signaling mediates G1/S cell-cycle progression in T cells via cyclin D3 and its dependent kinases. Blood. 2009;113:1689–1698. doi: 10.1182/blood-2008-03-147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis HD, Leveridge M, Strack PR, Haldon CD, O'neil J, Kim H, Madin A, Hannam JC, Look AT, Kohl N, Draetta G, Harrison T, Kerby JA, Shearman MS, Beher D. Apoptosis in T cell acute lymphoblastic leukemia cells after cell cycle arrest induced by pharmacological inhibition of notch signaling. Chem Biol. 2007;14:209–219. doi: 10.1016/j.chembiol.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Palomero T, Lim WK, Odom DT, Sulis ML, Real PJ, Margolin A, Barnes KC, O'Neil J, Neuberg D, Weng AP, Aster JC, Sigaux F, Soulier J, Look AT, Young RA, Califano A, Ferrando AA. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci. 2006;103:18261–18266. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S, Wu GS, Wu K. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 2015;369:20–27. doi: 10.1016/j.canlet.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 26.Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology. 2015;61:382–392. doi: 10.1002/hep.27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zong Y, Stanger BZ. Molecular mechanisms of liver and bile duct development. Wiley Interdiscip Rev Dev Biol. 2012;1:643–655. doi: 10.1002/wdev.47. [DOI] [PubMed] [Google Scholar]
- 28.Raynaud P, Carpentier R, Antoniou A, Lemaigre FP. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 2011;43:245–256. doi: 10.1016/j.biocel.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2012;20:251–257. doi: 10.1038/ejhg.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zender S, Nickeleit I, Wuestefeld T, Sörensen I, Dauch D, Bozko P, El-Khatib M, Geffers R, Bektas H, Manns MP, Gossler A, Wilkens L, Plentz R, Zender L, Malek NP. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013;23:784–795. doi: 10.1016/j.ccr.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Mazur PK, Riener MO, Jochum W, Kristiansen G, Weber A, Schmid RM, Siveke JT. Expression and clinicopathological significance of notch signaling and cell-fate genes in biliary tract cancer. Am J Gastroenterol. 2012;107:126–135. doi: 10.1038/ajg.2011.305. [DOI] [PubMed] [Google Scholar]
- 32.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekiya S, Suzuki A. Hepatocytes, rather than cholangiocytes, can be the major source of primitive ductules in the chronically injured mouse liver. Am J Pathol. 2014;184:1468–1478. doi: 10.1016/j.ajpath.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F, Arai Y, Takahashi H, Shirakihara T, Nagasaki M, Shibuya T, Nakano K, Watanabe-Makino K, Tanaka H, Nakamura H, Kusuda J, Ojima H, Shimada K, Okusaka T, Ueno M, Shigekawa Y, Kawakami Y, Arihiro K, Ohdan H, Gotoh K, Ishikawa O, Ariizumi S, Yamamoto M, Yamada T, Chayama K, Kosuge T, Yamaue H, Kamatani N, Miyano S, Nakagama H, Nakamura Y, Tsunoda T, Shibata T, Nakagawa H. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44:760–764. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Xue L, Cao Q, Lin Y, Ding Y, Yang P, Che L. Expression of Notch1, Jagged1 and beta-catenin and their clinicopathological significance in hepatocellular carcinoma. Neoplasma. 2009;56:533–541. doi: 10.4149/neo_2009_06_533. [DOI] [PubMed] [Google Scholar]
- 36.Qi R, An H, Yu Y, Zhang M, Liu S, Xu H, Guo Z, Cheng T, Cao X. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res. 2003;63:8323–8329. [PubMed] [Google Scholar]
- 37.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 38.Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin C, Zmoos AF, Mazur PK, Schaffer BE, Ostermeier A, Vogel H, Sylvester KG, Thorgeirsson SS, Grompe M, Sage J. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963–1976. doi: 10.1084/jem.20110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dill MT, Tornillo L, Fritzius T, Terracciano L, Semela D, Bettler B, Heim MH, Tchorz JS. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology. 2013;57:1607–1619. doi: 10.1002/hep.26165. [DOI] [PubMed] [Google Scholar]
- 40.Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, Sticht C, Tomasi ML, Delogu S, Evert M, Fan B, Ribback S, Jiang L, Brozzetti S, Bergmann F, Dombrowski F, Schirmacher P, Calvisi DF, Breuhahn K. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542.e12. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeliazkova P, Jörs S, Lee M, Zimber-Strobl U, Ferrer J, Schmid RM, Siveke JT, Geisler F. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology. 2013;57:2469–2479. doi: 10.1002/hep.26254. [DOI] [PubMed] [Google Scholar]
- 42.Akhoondi S, Sun D, von der Lehr N, Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D, Marth C, Mueller-Holzner E, Corcoran M, Dagnell M, Nejad SZ, Nayer BN, Zali MR, Hansson J, Egyhazi S, Petersson F, Sangfelt P, Nordgren H, Grander D, Reed SI, Widschwendter M, Sangfelt O, Spruck C. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–9012. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 43.Fryer CJ, Lamar E, Turbachova I, Kintner C, Jones KA. Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex. Genes Dev. 2002;16:1397–1411. doi: 10.1101/gad.991602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coffinier C, Gresh L, Fiette L, Tronche F, Schütz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- 45.Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Foltz DR, Santiago MC, Berechid BE, Nye JS. Glycogen synthase kinase-3beta modulates notch signaling and stability. Curr Biol. 2002;12:1006–1011. doi: 10.1016/s0960-9822(02)00888-6. [DOI] [PubMed] [Google Scholar]
- 47.Fryer CJ, White JB, Jones KA. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol Cell. 2004;16:509–520. doi: 10.1016/j.molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Pakkiriswami S, Couto A, Nagarajan U, Georgiou M. Glycosylated Notch and Cancer. Front Oncol. 2016;6:37. doi: 10.3389/fonc.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayward P, Brennan K, Sanders P, Balayo T, DasGupta R, Perrimon N, Martinez Arias A. Notch modulates Wnt signalling by associating with Armadillo/beta-catenin and regulating its transcriptional activity. Development. 2005;132:1819–1830. doi: 10.1242/dev.01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamizu K, Matsunaga T, Uosaki H, Fukushima H, Katayama S, Hiraoka-Kanie M, Mitani K, Yamashita JK. Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. J Cell Biol. 2010;189:325–338. doi: 10.1083/jcb.200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U, Ibáñez CF. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J Cell Biol. 2003;163:723–728. doi: 10.1083/jcb.200305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, Stanger BZ. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lemaigre FP. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009;137:62–79. doi: 10.1053/j.gastro.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 55.Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med. 2013;19:1054–1060. doi: 10.1038/nm.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xue TC, Zhang BH, Ye SL, Ren ZG. Differentially expressed gene profiles of intrahepatic cholangiocarcinoma, hepatocellular carcinoma, and combined hepatocellular-cholangiocarcinoma by integrated microarray analysis. Tumour Biol. 2015;36:5891–5899. doi: 10.1007/s13277-015-3261-1. [DOI] [PubMed] [Google Scholar]
- 58.Le Bras S, Loyer N, Le Borgne R. The multiple facets of ubiquitination in the regulation of notch signaling pathway. Traffic. 2011;12:149–161. doi: 10.1111/j.1600-0854.2010.01126.x. [DOI] [PubMed] [Google Scholar]
- 59.Wu WR, Shi XD, Zhang R, Zhu MS, Xu LB, Yu XH, Zeng H, Wang J, Liu C. Clinicopathological significance of aberrant Notch receptors in intrahepatic cholangiocarcinoma. Int J Clin Exp Pathol. 2014;7:3272–3279. [PMC free article] [PubMed] [Google Scholar]
- 60.Aoki S, Mizuma M, Takahashi Y, Haji Y, Okada R, Abe T, Karasawa H, Tamai K, Okada T, Morikawa T, Hayashi H, Nakagawa K, Motoi F, Naitoh T, Katayose Y, Unno M. Aberrant activation of Notch signaling in extrahepatic cholangiocarcinoma: clinicopathological features and therapeutic potential for cancer stem cell-like properties. BMC Cancer. 2016;16:854. doi: 10.1186/s12885-016-2919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Khatib M, Bozko P, Palagani V, Malek NP, Wilkens L, Plentz RR. Activation of Notch signaling is required for cholangiocarcinoma progression and is enhanced by inactivation of p53 in vivo. PLoS One. 2013;8:e77433. doi: 10.1371/journal.pone.0077433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huntzicker EG, Hötzel K, Choy L, Che L, Ross J, Pau G, Sharma N, Siebel CW, Chen X, French DM. Differential effects of targeting Notch receptors in a mouse model of liver cancer. Hepatology. 2015;61:942–952. doi: 10.1002/hep.27566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Q, Wang Y, Peng B, Liang L, Li J. The roles of Notch1 expression in the migration of intrahepatic cholangiocarcinoma. BMC Cancer. 2013;13:244. doi: 10.1186/1471-2407-13-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tchorz JS, Kinter J, Müller M, Tornillo L, Heim MH, Bettler B. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology. 2009;50:871–879. doi: 10.1002/hep.23048. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Jiao J, Cermelli S, Muir K, Jung KH, Zou R, Rashid A, Gagea M, Zabludoff S, Kalluri R, Beretta L. miR-21 Inhibition Reduces Liver Fibrosis and Prevents Tumor Development by Inducing Apoptosis of CD24+ Progenitor Cells. Cancer Res. 2015;75:1859–1867. doi: 10.1158/0008-5472.CAN-14-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guest RV, Boulter L, Dwyer BJ, Kendall TJ, Man TY, Minnis-Lyons SE, Lu WY, Robson AJ, Gonzalez SF, Raven A, Wojtacha D, Morton JP, Komuta M, Roskams T, Wigmore SJ, Sansom OJ, Forbes SJ. Notch3 drives development and progression of cholangiocarcinoma. Proc Natl Acad Sci. 2016;113:12250–12255. doi: 10.1073/pnas.1600067113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terada M, Horisawa K, Miura S, Takashima Y, Ohkawa Y, Sekiya S, Matsuda-Ito K, Suzuki A. Kupffer cells induce Notch-mediated hepatocyte conversion in a common mouse model of intrahepatic cholangiocarcinoma. Sci Rep. 2016;6:34691. doi: 10.1038/srep34691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morell CM, Fiorotto R, Fabris L, Strazzabosco M. Notch signalling beyond liver development: emerging concepts in liver repair and oncogenesis. Clin Res Hepatol Gastroenterol. 2013;37:447–454. doi: 10.1016/j.clinre.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 70.Singrang N, Kittisenachai S, Roytrakul S, Svasti J, Kangsamaksin T. NOTCH1 regulates the viability of cholangiocarcinoma cells via 14-3-3 theta. J Cell Commun Signal. 2019;13:245–254. doi: 10.1007/s12079-018-0488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brivio S, Cadamuro M, Fabris L, Strazzabosco M. Molecular Mechanisms Driving Cholangiocarcinoma Invasiveness: An Overview. Gene Expr. 2018;18:31–50. doi: 10.3727/105221617X15088670121925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsushima H, Kuroki T, Kitasato A, Adachi T, Tanaka T, Hirabaru M, Hirayama T, Kuroshima N, Hidaka M, Soyama A, Takatsuki M, Kinoshita N, Sano K, Nishida N, Eguchi S. Sox9 expression in carcinogenesis and its clinical significance in intrahepatic cholangiocarcinoma. Dig Liver Dis. 2015;47:1067–1075. doi: 10.1016/j.dld.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Imayoshi I, Shimogori T, Ohtsuka T, Kageyama R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008;135:2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- 74.Croquelois A, Blindenbacher A, Terracciano L, Wang X, Langer I, Radtke F, Heim MH. Inducible inactivation of Notch1 causes nodular regenerative hyperplasia in mice. Hepatology. 2005;41:487–496. doi: 10.1002/hep.20571. [DOI] [PubMed] [Google Scholar]
- 75.Razumilava N, Gradilone SA, Smoot RL, Mertens JC, Bronk SF, Sirica AE, Gores GJ. Non-canonical Hedgehog signaling contributes to chemotaxis in cholangiocarcinoma. J Hepatol. 2014;60:599–605. doi: 10.1016/j.jhep.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi ME, Cazanave SC, Mertens JC, Sirica AE, Gores GJ. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076–2088. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeng KS, Chang CF, Lin SS. Sonic Hedgehog Signaling in Organogenesis, Tumors, and Tumor Microenvironments. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21030758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie G, Karaca G, Swiderska-Syn M, Michelotti GA, Krüger L, Chen Y, Premont RT, Choi SS, Diehl AM. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology. 2013;58:1801–1813. doi: 10.1002/hep.26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang S, Wang J, Wang H, Fan L, Fan B, Zeng B, Tao J, Li X, Che L, Cigliano A, Ribback S, Dombrowski F, Chen B, Cong W, Wei L, Calvisi DF, Chen X. Hippo Cascade Controls Lineage Commitment of Liver Tumors in Mice and Humans. Am J Pathol. 2018;188:995–1006. doi: 10.1016/j.ajpath.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kitchen P, Lee KY, Clark D, Lau N, Lertsuwan J, Sawasdichai A, Satayavivad J, Oltean S, Afford S, Gaston K, Jayaraman PS. A Runaway PRH/HHEX-Notch3-Positive Feedback Loop Drives Cholangiocarcinoma and Determines Response to CDK4/6 Inhibition. Cancer Res. 2020;80:757–770. doi: 10.1158/0008-5472.CAN-19-0942. [DOI] [PubMed] [Google Scholar]
- 81.Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141:140–149. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Jesus-Acosta A, Laheru D, Maitra A, Arcaroli J, Rudek MA, Dasari A, Blatchford PJ, Quackenbush K, Messersmith W. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs. 2014;32:739–745. doi: 10.1007/s10637-014-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li JH, Zhu XX, Li FX, Huang CS, Huang XT, Wang JQ, Gao ZX, Li SJ, Xu QC, Zhao W, Yin XY. MFAP5 facilitates the aggressiveness of intrahepatic Cholangiocarcinoma by activating the Notch1 signaling pathway. J Exp Clin Cancer Res. 2019;38:476. doi: 10.1186/s13046-019-1477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mancarella S, Serino G, Dituri F, Cigliano A, Ribback S, Wang J, Chen X, Calvisi DF, Giannelli G. Crenigacestat, a selective NOTCH1 inhibitor, reduces intrahepatic cholangiocarcinoma progression by blocking VEGFA/DLL4/MMP13 axis. Cell Death Differ. 2020;27:2330–2343. doi: 10.1038/s41418-020-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Rourke CJ, Matter MS, Nepal C, Caetano-Oliveira R, Ton PT, Factor VM, Andersen JB. Identification of a Pan-Gamma-Secretase Inhibitor Response Signature for Notch-Driven Cholangiocarcinoma. Hepatology. 2020;71:196–213. doi: 10.1002/hep.30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pamela Munster SGE, Amita Patnaik, Anthony F. Shields, Anthony W. Tolcher, S. Lindsey Davis, John V. Heymach, Lu Xu, Ann M. Kapoun, Leonardo Faoro, Jakob Dupont and Renata Ferrarotto. Abstract C42: Safety and preliminary efficacy results of a first-in-human phase I study of the novel CSC targeting antibody brontictuzumab (OMP-52M51, anti-Notch1) administered intravenously to patients with certain advanced solid tumors. Molecular Cancer therapeutics 2015. [Google Scholar]
- 88.Ambrogio C, Gómez-López G, Falcone M, Vidal A, Nadal E, Crosetto N, Blasco RB, Fernández-Marcos PJ, Sánchez-Céspedes M, Ren X, Wang Z, Ding K, Hidalgo M, Serrano M, Villanueva A, Santamaría D, Barbacid M. Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med. 2016;22:270–277. doi: 10.1038/nm.4041. [DOI] [PubMed] [Google Scholar]
- 89.Previs RA, Coleman RL, Harris AL, Sood AK. Molecular pathways: translational and therapeutic implications of the Notch signaling pathway in cancer. Clin Cancer Res. 2015;21:955–961. doi: 10.1158/1078-0432.CCR-14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 91.Fatima K, Paracha RZ, Qadri I. Post-transcriptional silencing of Notch2 mRNA in chronic lymphocytic [corrected] leukemic cells of B-CLL patients. Mol Biol Rep. 2012;39:5059–5067. doi: 10.1007/s11033-011-1301-5. [DOI] [PubMed] [Google Scholar]
- 92.Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52:297–303. doi: 10.1002/mc.21864. [DOI] [PubMed] [Google Scholar]
- 93.Zakaria MK, Ali A, Fatima K, Suhail M, Mathew S, Alkarim S, Azhar E, Qadri I. Hepatic Cancer Stem Cells and Signaling Pathways. Int J Cancer Clin Res. 2015 [Google Scholar]
- 94.Kwon H, Song K, Han C, Zhang J, Lu L, Chen W, Wu T. Epigenetic Silencing of miRNA-34a in Human Cholangiocarcinoma via EZH2 and DNA Methylation: Impact on Regulation of Notch Pathway. Am J Pathol. 2017;187:2288–2299. doi: 10.1016/j.ajpath.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suliman MA, Zhang Z, Na H, Ribeiro AL, Zhang Y, Niang B, Hamid AS, Zhang H, Xu L, Zuo Y. Niclosamide inhibits colon cancer progression through downregulation of the Notch pathway and upregulation of the tumor suppressor miR-200 family. Int J Mol Med. 2016;38:776–784. doi: 10.3892/ijmm.2016.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Astudillo L, Da Silva TG, Wang Z, Han X, Jin K, VanWye J, Zhu X, Weaver K, Oashi T, Lopes PE, Orton D, Neitzel LR, Lee E, Landgraf R, Robbins DJ, MacKerell AD, Jr, Capobianco AJ. The Small Molecule IMR-1 Inhibits the Notch Transcriptional Activation Complex to Suppress Tumorigenesis. Cancer Res. 2016;76:3593–3603. doi: 10.1158/0008-5472.CAN-16-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aihara A, Huang CK, Olsen MJ, Lin Q, Chung W, Tang Q, Dong X, Wands JR. A cell-surface β-hydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60:1302–1313. doi: 10.1002/hep.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang CK, Iwagami Y, Aihara A, Chung W, de la Monte S, Thomas JM, Olsen M, Carlson R, Yu T, Dong X, Wands J. Anti-Tumor Effects of Second Generation β-Hydroxylase Inhibitors on Cholangiocarcinoma Development and Progression. PLoS One. 2016;11:e0150336. doi: 10.1371/journal.pone.0150336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gu Y, Xiao L, Ming Y, Zheng Z, Li W. Corilagin suppresses cholangiocarcinoma progression through Notch signaling pathway in vitro and in vivo. Int J Oncol. 2016;48:1868–1876. doi: 10.3892/ijo.2016.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koprowski S, Sokolowski K, Kunnimalaiyaan S, Gamblin TC, Kunnimalaiyaan M. Curcumin-mediated regulation of Notch1/hairy and enhancer of split-1/survivin: molecular targeting in cholangiocarcinoma. J Surg Res. 2015;198:434–440. doi: 10.1016/j.jss.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 102.Gao H, Zehl M, Leitner A, Wu X, Wang Z, Kopp B. Comparison of toad venoms from different Bufo species by HPLC and LC-DAD-MS/MS. J Ethnopharmacol. 2010;131:368–376. doi: 10.1016/j.jep.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 103.Yu CH, Kan SF, Pu HF, Jea Chien E, Wang PS. Apoptotic signaling in bufalin- and cinobufagin-treated androgen-dependent and -independent human prostate cancer cells. Cancer Sci. 2008;99:2467–2476. doi: 10.1111/j.1349-7006.2008.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ren J, Wang S, Jin L, Ma F, Zhou D, Cai Q. Cinobufagin inhibits tumor growth by inducing apoptosis through Notch signaling pathways in human cholangiocarcinoma. Transl Cancer Res. 2019;8:2461–2469. doi: 10.21037/tcr.2019.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Walden D, Kunnimalaiyaan S, Sokolowski K, Clark TG, Kunnimalaiyaan M. Antiproliferative and apoptotic effects of xanthohumol in cholangiocarcinoma. Oncotarget. 2017;8:88069–88078. doi: 10.18632/oncotarget.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan D, Huang S, Berger E, Liu L, Gross N, Heinzmann F, Ringelhan M, Connor TO, Stadler M, Meister M, Weber J, Öllinger R, Simonavicius N, Reisinger F, Hartmann D, Meyer R, Reich M, Seehawer M, Leone V, Höchst B, Wohlleber D, Jörs S, Prinz M, Spalding D, Protzer U, Luedde T, Terracciano L, Matter M, Longerich T, Knolle P, Ried T, Keitel V, Geisler F, Unger K, Cinnamon E, Pikarsky E, Hüser N, Davis RJ, Tschaharganeh DF, Rad R, Weber A, Zender L, Haller D, Heikenwalder M. Kupffer Cell-Derived Tnf Triggers Cholangiocellular Tumorigenesis through JNK due to Chronic Mitochondrial Dysfunction and ROS. Cancer Cell. 2017;31:771–789.e6. doi: 10.1016/j.ccell.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gogolok J, Seidel E, Strönisch A, Reutzel-Selke A, Sauer IM, Pratschke J, Bahra M, Schmuck RB. Characterization of Pancreatic and Biliary Cancer Stem Cells in Patient-derived Tissue. Anticancer Res. 2020;40:1267–1275. doi: 10.21873/anticanres.14068. [DOI] [PubMed] [Google Scholar]
- 108.Goel PN, Moharrer Y, Hebb JH, Egol AJ, Kaur G, Hankenson KD, Ahn J, Ashley JW. Suppression of Notch Signaling in Osteoclasts Improves Bone Regeneration and Healing. J Orthop Res. 2019;37:2089–2103. doi: 10.1002/jor.24384. [DOI] [PMC free article] [PubMed] [Google Scholar]