Abstract
BACKGROUND
Gallbladder carcinoma (GBC) carries a poor prognosis and requires a prediction method. Gamma-glutamyl transferase–to–platelet ratio (GPR) is a recently reported cancer prognostic factor. Although the mechanism for the relationship between GPR and poor cancer prognosis remains unclear, studies have demonstrated the clinical effect of both gamma-glutamyl transferase and platelet count on GBC and related gallbladder diseases.
AIM
To assess the prognostic value of GPR and to design a prognostic nomogram for GBC.
METHODS
The analysis involved 130 GBC patients who underwent surgery at Peking Union Medical College Hospital from December 2003 to April 2017. The patients were stratified into a high- or low-GPR group. The predictive ability of GPR was evaluated by Kaplan–Meier analysis and a Cox regression model. We developed a nomogram based on GPR, which we verified using calibration curves. The nomogram and other prognosis prediction models were compared using time-dependent receiver operating characteristic curves and the concordance index.
RESULTS
Patients in the high-GPR group had a higher risk of jaundice, were older, and had higher carbohydrate antigen 19-9 levels and worse postoperative outcomes. Univariate analysis revealed that GPR, age, body mass index, tumor–node–metastasis (TNM) stage, jaundice, cancer cell differentiation degree, and carcinoembryonic antigen and carbohydrate antigen 19-9 levels were related to overall survival (OS). Multivariate analysis confirmed that GPR, body mass index, age, and TNM stage were independent predictors of poor OS. Calibration curves were highly consistent with actual observations. Comparisons of time-dependent receiver operating characteristic curves and the concordance index showed advantages for the nomogram over TNM staging.
CONCLUSION
GPR is an independent predictor of GBC prognosis, and nomogram-integrated GPR is a promising predictive model for OS in GBC.
Keywords: Gamma-glutamyl transferase–to–platelet ratio, Gallbladder carcinoma, Prognosis, Nomogram, Tumor–node–metastasis, Patient management
Core Tip: We assessed the prognostic value of gamma-glutamyl transferase-to-platelet ratio (GPR) and designed a prognostic nomogram for gallbladder carcinoma (GBC). We retrospectively evaluated a group of 130 patients with GBC who underwent resection with either a high or low level of GPR. We proposed that GPR is an independent predictor of GBC prognosis, and nomogram-integrated GPR is a promising predictive model for overall survival in GBC patients.
INTRODUCTION
Gallbladder carcinoma (GBC) is the most common malignancy of the biliary tract[1,2], and accounts for 1% of the cancer incidence in China. The early symptoms of GBC are easily confused with those of chronic cholecystitis and cholelithiasis[3], so patients are likely to have reached the advanced stage of GBC upon diagnosis. Being insensitive to chemotherapy and radiotherapy and with no effective drugs[1], the prognostic outcomes of GBC remain poor, and the 5-year survival rate is less than 5%[4]. Therefore, there remains an unmet need for a more accurate patient stratification system to inform clinical decision-making and provide the rationale for designing trials, and this stratification strategy requires a prognosis prediction model as an important reference.
Previously, the most commonly used prognostic factor was tumor–node–metastasis (TNM) staging defined by the American Joint Committee on Cancer (AJCC) (8th edition)[5]. TNM staging ranks the degree of cancer by scoring the tumor, involved lymph nodes, and the presence or absence of metastasis. This method was developed for general cancer diagnosis and lacks personalized prediction for individual patients. Other inflammatory markers such as neutrophil–to–lymphocyte ratio (NLR) and monocyte–to–lymphocyte ratio (MLR) have been tested for their predictive value, but these ratios are limited to certain cancers[6]. There is an urgent need for a cost-effective prognostic prediction method for GBC patients.
Recently, Wang et al[8] developed a clinical prognostic index for hepatocellular carcinoma (HCC), the gamma-glutamyl transferase–to–platelet ratio (GPR). GPR was first proposed in 2014 as an inflammatory factor influencing liver fibrosis and cirrhosis[7], and further studies on GPR indicated ideal predictive ability for HCC. In 2016, Wang et al[8] proposed the predictive value of GPR in patients with hepatitis B-related HCC after curative hepatic resection[8]. Another study performed by Chiu, who developed a quality of life predictive model after surgical resection of HCC, also considered GPR an independent prognostic factor[9]. According to evidence that both gamma-glutamyl transferase (GGT)[10] and platelet count (PLT)[11] are proposed prognostic predictors of various cancers, GPR is also a potential clinical predictor of GBC; however, the relationship between GPR and prognosis and outcomes in patients with GBC remains unclear.
The current study aimed to investigate the prognostic role of GPR in patients with GBC, and to integrate GPR with other clinical variables to develop a nomogram for prognosis prediction in GBC patients.
MATERIALS AND METHODS
Population
A total of 130 patients with gallbladder adenocarcinoma who underwent resection at Peking Union Medical College Hospital from December 2003 to April 2017 were included in this study. The inclusion criteria were: (1) Histologically confirmed gallbladder adenocarcinoma; (2) Resectable gallbladder cancer; (3) No history of other malignancies; and (4) Available clinical data at the time of the first diagnosis. Patients with missing follow-up data or with other cancers such as adenosquamous cell carcinoma or papilla carcinoma were excluded from the study.
The study was approved by the Medical Ethics Committee of Peking Union Medical College Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College, and was performed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki[12]. The requirement for informed consent was waived because of the retrospective nature of this study.
Data collection
Clinical data including age, sex, jaundice, gallbladder stone, body mass index (BMI), maximum tumor diameter, TNM stage, postoperative complications, hospitalization days (HOD), and survival time were collected from the medical records. TNM stage was measured based on the 8th AJCC criteria for GBC.
Laboratory data including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), GGT, and different blood counts including platelet, monocyte, neutrophil, and lymphocyte counts were also obtained from the examination for cancer diagnosis.
GPR was defined as GGT divided by PLT. MLR was defined as an absolute monocyte count divided by lymphocyte count. NLR was defined as the ratio of absolute neutrophil count to lymphocyte count.
Differentiation degree of cancer cells was obtained from histological analysis result.
Statistical analysis
Statistical analyses were conducted with R 3.6.2 software (Institute for Statistics and Mathematics, Vienna, Austria) and Statistical Package for Social Sciences version 25.0 (SPSS, Chicago, IL, United States). Continuous variables which conformed to a normal distribution are summarized as the mean ± SD, while others are presented as the median and interquartile range. Comparisons of baseline characteristics between groups were performed using Chi-square tests, t test, and rank-sum test as appropriate.
GPR, BMI, CEA, CA19-9, tumor diameter, MLR, and NLR were divided into high and low groups. The optimal cutoff values for these factors were defined by receiver operating characteristic (ROC) analysis. Clinicopathological factors that potentially correlated with patients' prognosis were defined by the GPR level and were estimated.
The Kaplan-Meier method was used for calculating the long-term overall survival (OS) rates. Chi-square test and rank-sum test were used to estimate the effect of GPR on short-term clinical outcome as postoperative complications and HOD. Univariate and multivariate Cox regression analyses of potential factors affecting patients' outcomes were performed.
Based on multivariate analysis, a nomogram was developed by using the rms package in R version 3.6.2. The performance of the nomogram was assessed using calibration curve, concordance index (C-index), and decision curve. The prognostic abilities of the nomogram were compared with the TNM stage model, cancer marker CA 19-9, and prediction model from similar research by comparing the areas under the ROC curves (AUC) and C-index. All significance levels were set at 0.05, and all P values were two-sided.
RESULTS
Patients’ characteristics
The baseline features of the enrolled patients are provided in Table 1. Patients’ average age was 63.23 ± 1.20 years; 76 (58%) patients were men, and 54 (42%) were women. The mean BMI was 23.97 ± 0.38 kg/m2; 19 patients had jaundice (15%), and 62 patients had gallbladder stones (48%). Twelve (9%) patients had liver diseases including fatty liver disease (n = 7), hepatic cyst (n = 3), hemangiomas of the liver (n = 1), and cirrhosis (n = 1). There were 21, 26, 32, 19, and 18 patients with low, low-medium, medium, medium-high, and high degrees of cancer cell differentiation, respectively; 12% of patients were classified with TNM stage I disease, while 8% were classified with stage II, 65% with stage III, and 15% with stage IV. The median CEA value was 2.59 ng/mL (range, 1.62–5.50 ng/mL), median CA 19-9 level was 47.50 U/mL (13.03–220.85 U/mL), median tumor diameter was 2.70 cm (1.50–4.55 cm), and median GPR was 0.17 (0.09–0.44). Twenty-nine (22%) patients had postoperative complications, and the median HOD was 15 d (10–20 d), with a median survival time of 18 mo (6–34 mo). All patients were treated by radical cholecystectomy.
Table 1.
Clinicopathological characteristics of the 130 patients with gallbladder adenocarcinoma in this study
| Characteristic | mean ± SD or median (IQR) or n (%) |
| Age, yr | 63.23 ± 1.20 |
| Gender | |
| Male | 76 (58) |
| Female | 54 (42) |
| BMI, kg/m2 | 23.97 ± 0.38 |
| Jaundice | |
| No | 111 (85) |
| Yes | 19 (15) |
| Gallbladder stone | |
| No | 68 (52) |
| Yes | 62 (48) |
| Liver disease | |
| No | 118 (91) |
| Yes | 12 (9) |
| Differentiation stage of cancer cell | |
| Low | 21 (16) |
| Low-medium | 26 (20) |
| Medium | 32 (25) |
| Medium-high | 19 (15) |
| High | 18 (14) |
| TNM stage | |
| I | 16 (12) |
| II | 11 (8) |
| III | 83 (65) |
| IV | 20 (15) |
| CEA, ng/mL | 2.59 (1.62-5.50) |
| CA 19-9, U/mL | 47.50 (13.03-220.85) |
| Maximal tumor diameter, cm | 2.70 (1.50-4.55) |
| GPR | 0.17 (0.09-0.44) |
| Postoperative complications | |
| No | 101 (78) |
| Yes | 29 (22) |
| HOD, D | 15 (10-20) |
| OS, Mo | 18 (6-34) |
BMI: Body mass index; TNM: Tumor–node–metastasis; CEA: Carcinoembryonic antigen; CA 19-9: Carbohydrate antigen 19-9; GPR: Gamma-glutamyl transferase–to–platelet ratio; HOD: Hospitalization days; OS: Overall survival; SD: Standard deviation; IQR: Interquartile range.
Relationship between gamma-glutamyl transferase–to–platelet ratio and patients’ clinical characteristics
The optimal cutoff value for GPR obtained using the ROC analysis was 0.365. The cutoff values for other associated factors were obtained by the same method. We divided patients into a high and low group according to the cutoff values, and patients’ characteristics in each group are summarized in Table 2. Ninety-one patients had a GPR < 0.365 (low-GPR group), and 39 had a GPR ≥ 0.365 (high-GPR group). The frequency of jaundice was higher in the high-GPR group vs the low-GPR group (4% vs 15%, respectively; P < 0.001), and the proportion of patients with higher BMI was larger in the low-GPR group vs the high-GPR group (73% vs 34%, respectively; P < 0.001). The CA 19-9 level was also higher in the high-GPR group vs the low-GPR group (42% vs 62%, respectively; P = 0.049).
Table 2.
Clinical characteristics of the patients according to gamma-glutamyl transferase–to–platelet ratio
| Patients | GPR ≤ 0.365, n (%) | GPR > 0.365, n (%) | P value |
| Age, yr | 0.661 | ||
| ≤ 60 | 34 (37) | 13 (33) | |
| > 60 | 57 (63) | 26 (67) | |
| Gender | 0.484 | ||
| Male | 55 (60) | 21 (54) | |
| Female | 36 (40) | 18 (46) | |
| Jaundice | < 0.001 | ||
| No | 87 (96) | 24 (62) | |
| Yes | 4 (4) | 15 (38) | |
| Gallbladder stone | 0.319 | ||
| No | 45 (49) | 23 (59) | |
| Yes | 46 (51) | 16 (41) | |
| Liver disease | 0.509 | ||
| No | 81 (89) | 37 (95) | |
| Yes | 10 (11) | 2 (5) | |
| Differentiation stage of cancer cells | 0.640 | ||
| Low | 14 (18) | 7 (23) | |
| Low-medium | 18 (23) | 7 (23) | |
| Medium | 18 (23) | 8 (26) | |
| Medium-high | 16 (21) | 3 (10) | |
| High | 12 (15) | 6 (19) | |
| TNM stage | 0.053 | ||
| I + II | 23 (25) | 4 (10) | |
| III + IV | 68 (75) | 35 (90) | |
| BMI, kg/m2 | < 0.001 | ||
| ≥ 22.5 | 60 (73) | 11 (34) | |
| < 22.5 | 22 (27) | 21 (66) | |
| CEA, ng/mL | 0.732 | ||
| ≤ 5.30 | 56 (76) | 21 (72) | |
| > 5.30 | 18 (24) | 8 (28) | |
| CA19-9, U/mL | 0.049 | ||
| ≤ 47.8 | 47 (58) | 14 (38) | |
| > 47.8 | 35 (42) | 23 (62) | |
| Maximal tumor diameter, cm | 0.940 | ||
| ≤ 2.90 | 44 (50) | 20 (53) | |
| > 2.90 | 44 (50) | 18 (47) | |
BMI: Body mass index; TNM: Tumor–node–metastasis; CEA: Carcinoembryonic antigen; CA 19-9: Carbohydrate antigen 19-9; GPR: Gamma-glutamyl transferase–to–platelet ratio.
The short-term clinical outcomes are presented in Table 3. Patients in the high-GPR group had more postoperative complications vs the low-GPR group (16% vs 36%, respectively; P = 0.015), and the median HOD was also higher in the high-GPR group vs the low-GPR group (13 vs 19, respectively; P < 0.001).
Table 3.
Short-term clinical outcomes according to gamma-glutamyl transferase–to–platelet ratio
| Patients | GPR ≤ 0.365, medium (IQR) or n (%) | GPR > 0.365, medium (IQR) or n (%) | P value |
| Postoperative complications | 0.015 | ||
| No | 76 (84) | 25 (64) | |
| Yes | 15 (16) | 14 (36) | |
| HOD | 13 (10-17) | 19 (14-23) | < 0.001 |
GPR: Gamma-glutamyl transferase to platelet ratio; HOD: Hospitalization days; IQR: Interquartile range.
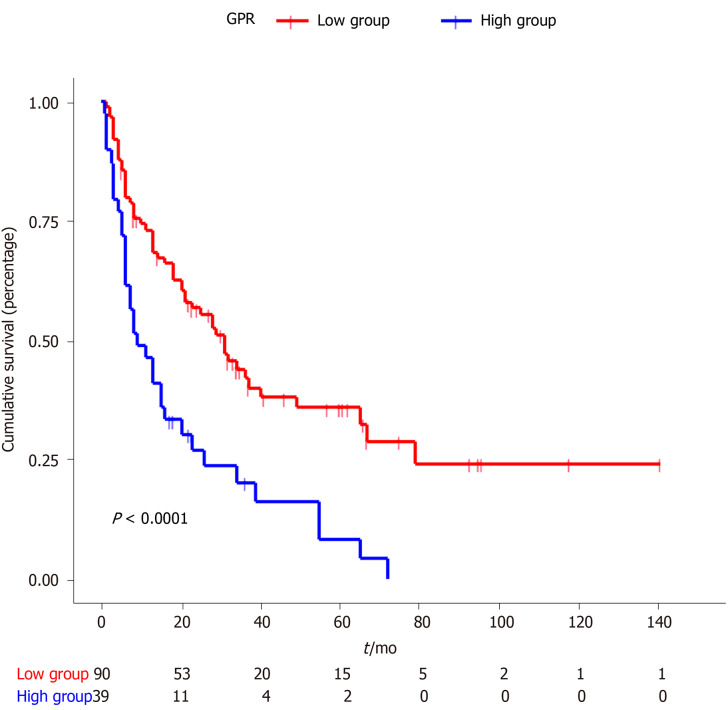
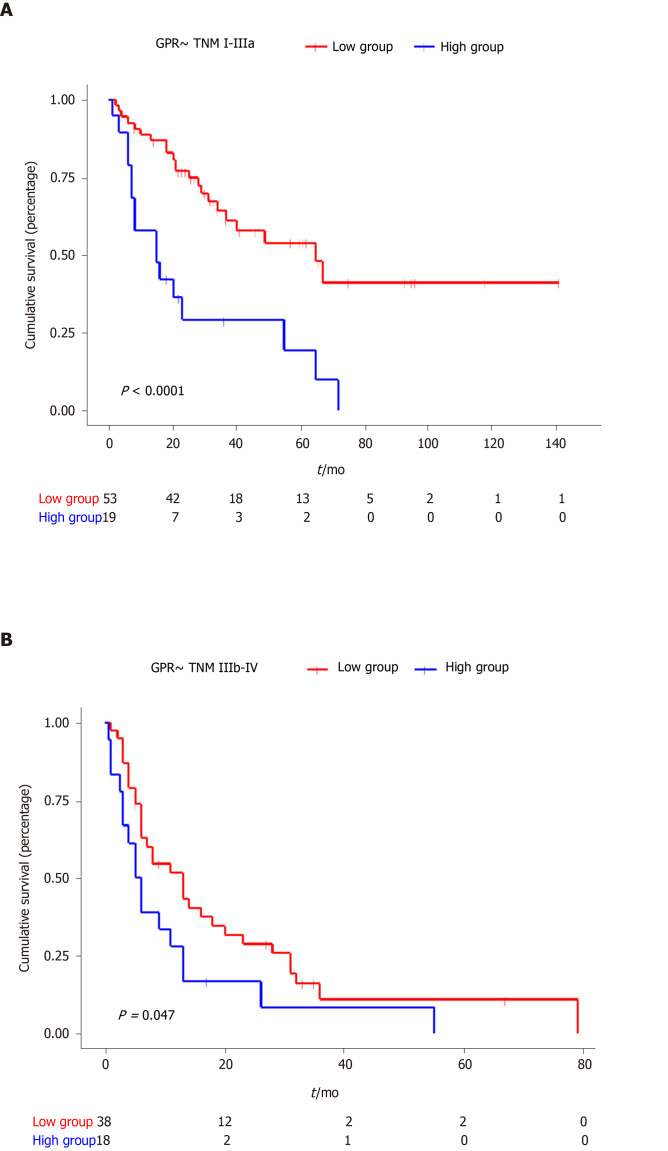
The Kaplan–Meier curves for GPR are shown in Figure 1. The median OS for the low-GPR group vs the high-GPR group was 31 mo and 9 mo, respectively (P < 0.0001). Subgroup Kaplan-Meier analysis for TNM stages I-IIIa (P < 0.0001) and IIIb-IV (P = 0.047) both showed a significant difference between the low GPR group and high GPR group (Figure 2).
Figure 1.
Kaplan-Meier curves for overall survival stratified according to Gamma-glutamyl transferase–to–platelet ratio.
Figure 2.
Kaplan-Meier curves for overall survival of different tumor–node–metastasis stages stratified according to gamma-glutamyl transferase–to–platelet ratio.
Univariate analysis showed that OS was significantly associated with age > 60 years, jaundice, cancer cell differentiation stage, BMI < 22.5 kg/m2, CEA > 5.30 ng/mL, CA 19-9 > 47.8 U/mL, TNM stage, and GPR > 0.365 (high-GPR group). Multivariate analysis identified four independent factors for poor OS: Age > 60 years [hazard ratio (HR) = 1.976, 95% confidence interval (CI): 1.063–3.675; P = 0.031], BMI ≤ 22.5 kg/m2 (HR = 2.776, 95%CI: 1.394–5.529; P = 0.004), TNM stage (HR = 9.093, 95%CI: 0.998–82.830; P = 0.050), and GPR > 0.365 (high-GPR group) (HR = 1.974, 95%CI: 1.008–3.867; P = 0.047) (Table 4).
Table 4.
Univariate and multivariate Cox proportional hazard analyses of factors associated with overall survival
|
Univariate test |
Multivariate test |
|||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | ||||||
| ≤ 60 | 1.000 | 1.000 | ||||
| > 60 | 1.722 | 1.089-2.723 | 0.020 | 1.976 | 1.063-3.675 | 0.031 |
| Gender | ||||||
| Male | 1.000 | |||||
| Female | 1.033 | 0.834-1.278 | 0.769 | |||
| Jaundice | ||||||
| No | 1.000 | 1.000 | ||||
| Yes | 2.378 | 1.423-3.977 | 0.001 | 1.064 | 0.462-2.450 | 0.883 |
| Gallbladder stone | ||||||
| No | 1.000 | |||||
| Yes | 0.983 | 0.798-1.211 | 0.873 | |||
| Differentiation degree of cancer cells | 0.018 | 0.513 | ||||
| Low | 5.583 | 1.870-16.666 | 0.002 | 0.860 | 0.089-8.327 | 0.897 |
| Low-medium | 3.403 | 1.162-9.969 | 0.026 | 0.414 | 0.043-4.015 | 0.447 |
| Medium | 3.264 | 1.134-9.394 | 0.028 | 0.366 | 0.037-3.576 | 0.388 |
| Medium-high | 1.980 | 0.630-6.226 | 0.243 | 0.549 | 0.057-5.277 | 0.604 |
| High | 2.510 | 0.807-7.803 | 0.112 | 0.421 | 0.042-4.192 | 0.461 |
| BMI, kg/m2 | ||||||
| ≥ 22.5 | 1.000 | 1.000 | ||||
| < 22.5 | 3.128 | 1.956-5.004 | < 0.001 | 2.776 | 1.394-5.529 | 0.004 |
| CEA, ng/mL | ||||||
| ≤ 5.30 | 1.000 | 1.000 | ||||
| > 5.30 | 2.485 | 1.478-4.178 | 0.001 | 1.477 | 0.800-2.726 | 0.212 |
| CA19-9, U/mL | ||||||
| ≤ 47.8 | 1.000 | 1.000 | ||||
| > 47.8 | 3.305 | 2.079-5.251 | < 0.001 | 1.665 | 0.840-3.297 | 0.144 |
| Maximal tumor diameter, cm | ||||||
| ≤ 2.90 | 1.000 | |||||
| > 2.90 | 0.794-1.833 | 0.499 | ||||
| TNM stage | ||||||
| I + II | 1.000 | 1.000 | ||||
| III + IV | 6.810 | 2.952-15.711 | < 0.001 | 9.093 | 0.998-82.830 | 0.050 |
| GPR | ||||||
| ≤ 0.365 | 1.000 | 1.000 | ||||
| > 0.365 | 2.298 | 1.493-3.537 | < 0.001 | 1.974 | 1.008-3.867 | 0.047 |
BMI: Body mass index; TNM: Tumor–node–metastasis; CEA: Carcinoembryonic antigen; CA 19-9: Carbohydrate antigen 19-9; GPR: Gamma-glutamyl transferase–to–platelet ratio.
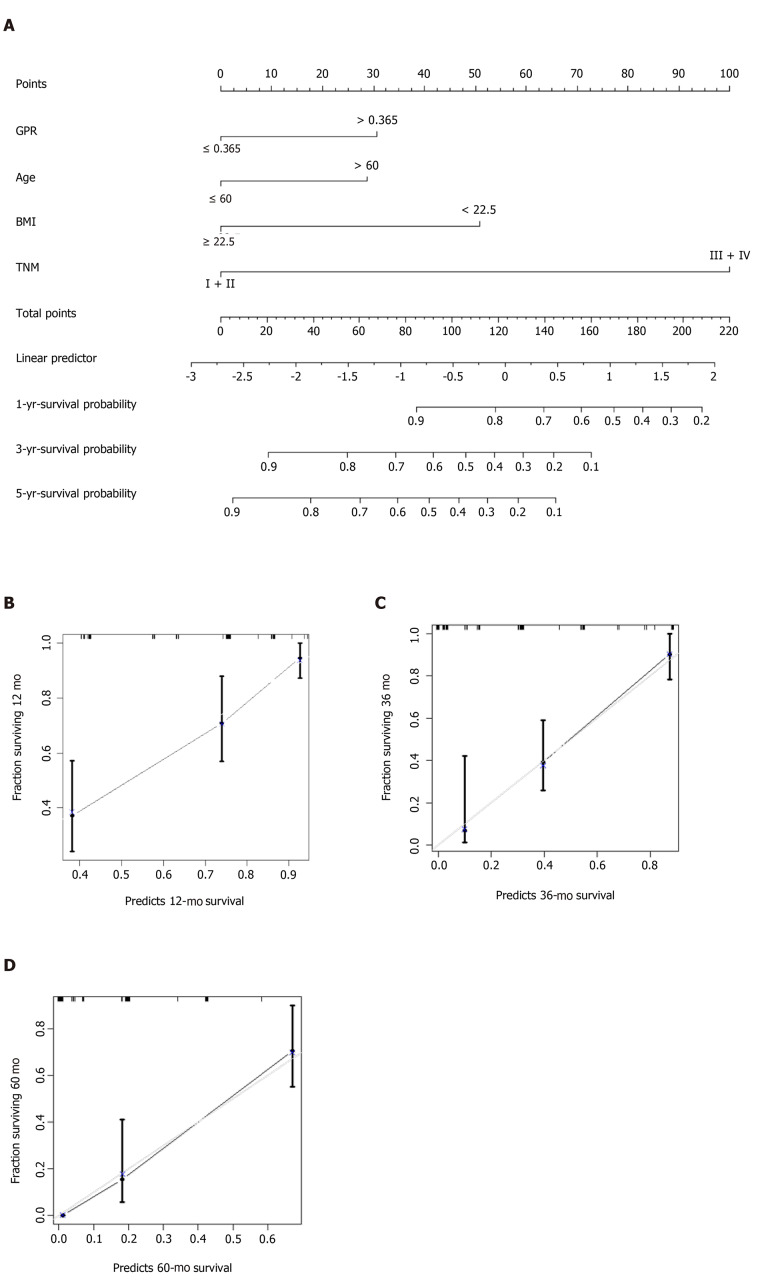
Development and verification of a nomogram
Multivariate Cox regression analysis identified age, BMI, TNM stage, and GPR as independent predictors for prognosis prediction of GBC (Table 4). The model incorporating the independent parameters is shown as a nomogram in Figure 3A. The 1-, 3-, and 5-year calibration curves for OS prediction of the nomogram demonstrated good agreement between nomogram prediction and actual observation (Figure 3B-D). The C-index for the prediction nomogram was 0.770 (95%Cl: 0.717–0.823) by internal bootstrapping validation.
Figure 3.
Prediction nomogram for survival probability. A: Nomogram for overall survival; B: Calibration curve for the nomogram for predicting 1-year survival probability; C: Calibration curve for the nomogram for predicting 3-year survival probability; and D: Calibration curve for the nomogram for predicting 5-year survival probability.
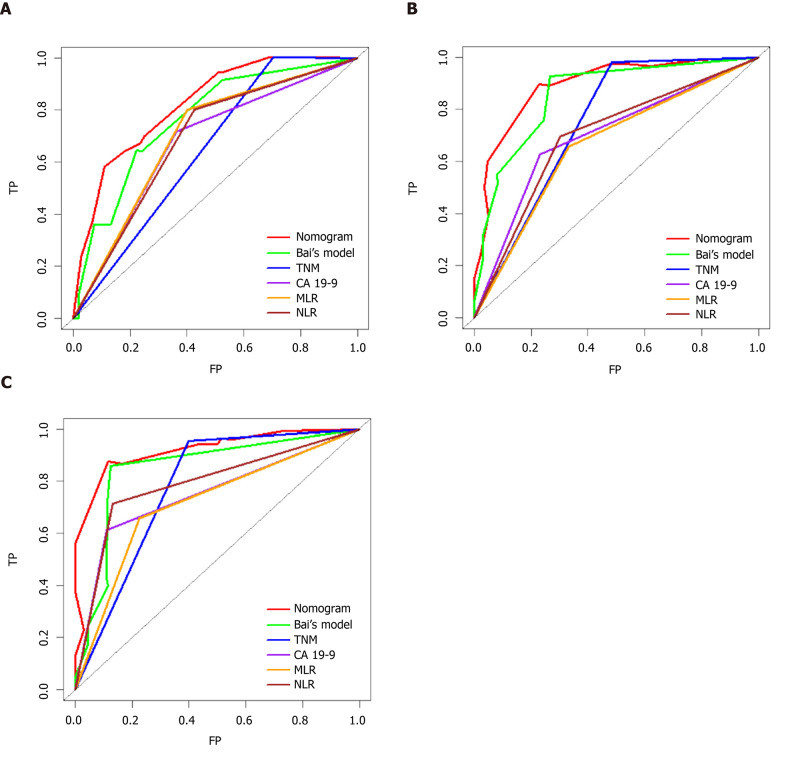
Comparing different prediction models or factors
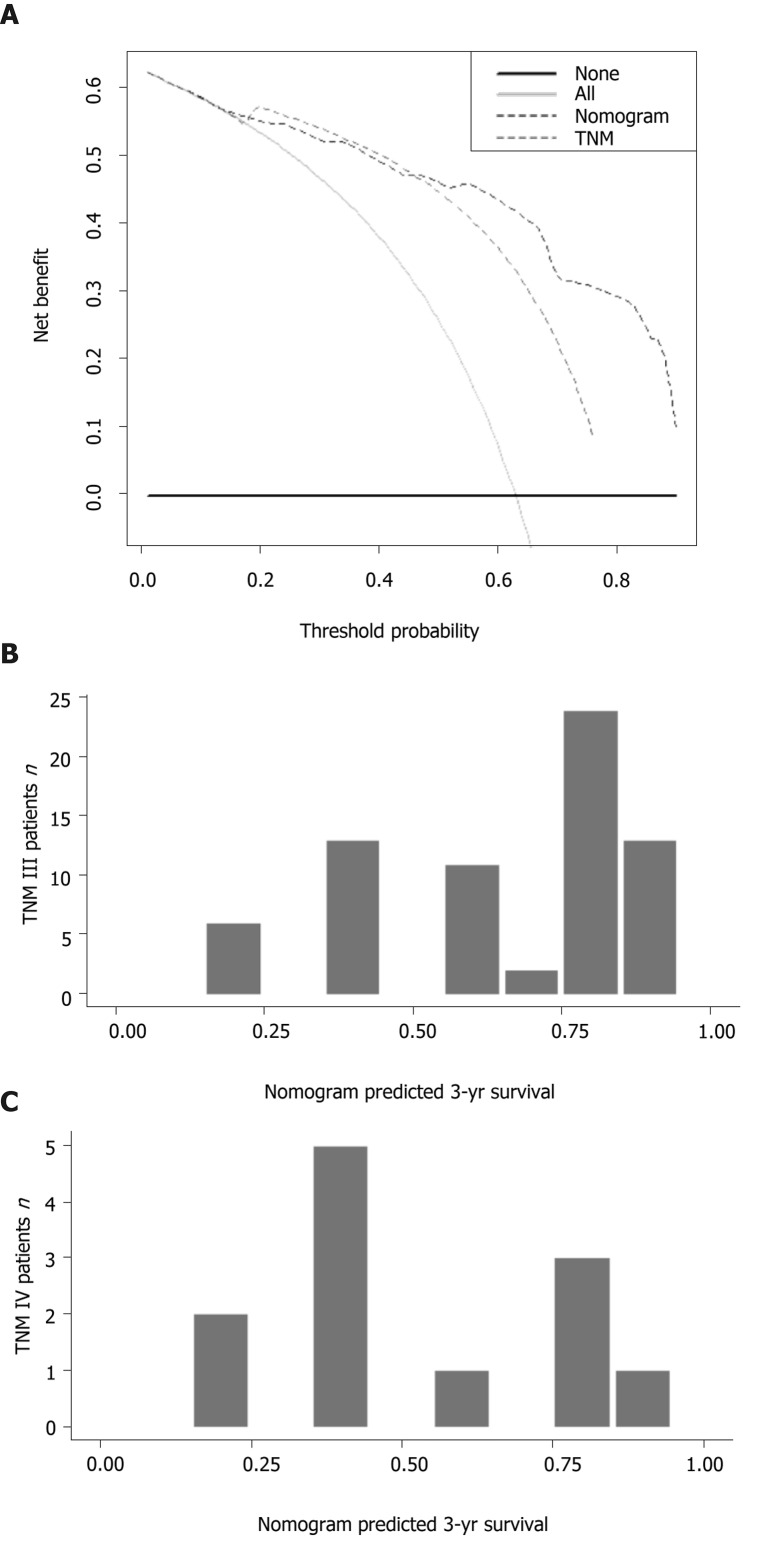
Time-dependent ROC curves for the 1-, 3-, and 5-year OS rates were generated to compare the performance of several prediction models or factors, and the results showed that the nomogram model was superior to the other models (Figure 4). Next, we calculated the AUC values at the same time points to further compare the prediction models. The details of the AUC and C-index values are listed in Table 5. The C-index of the nomogram model was 0.770, which was greater than those for TNM stage (0.631), jaundice + CA 19-9 + TNM stage + R stage (0.715), CA19-9 (0.658), MLR (0.632), and NLR (0.644). Specifically, the 3-year decision curve showed that if the threshold of probability was > 50%, the nomogram model showed better net benefit for predicting OS compared with the TNM stage-based model (Figure 5A). We also developed a histogram for the nomogram-predicted probability of 3-year survival for stages III and IV GBC. Notably, even for the same TNM stage, there was considerable heterogeneity in the nomogram-predicted probabilities (Figure 5B).
Figure 4.
Time-dependent receiver operating characteristic curves for the nomogram. Bai et al[34]’s model1, tumor–node–metastasis staging, CA 19-9, monocyte–to–lymphocyte ratio, and neutrophil–to–lymphocyte ratio. A: Time-dependent receiver operating characteristic (ROC) curves at 1 year; B: Time-dependent ROC curves at 3 years; and C: Time-dependent ROC curves at 5 years. 1Bai’s model: Nomogram based on jaundice, CA19-9, tumor–node–metastasis stage, and R status[34].
Table 5.
Comparison of the performance and discriminative ability between different prognosis prediction models
| 1-yr AUROC | 3-yr AUROC | 5-yr AUROC | C-index | |
| Nomogram | 0.823 | 0.893 | 0.920 | 0.770 |
| TNM stage | 0.649 | 0.748 | 0.778 | 0.631 |
| Bai’s model1 | 0.766 | 0.857 | 0.848 | 0.773 |
| CA19-9 | 0.677 | 0.698 | 0.750 | 0.658 |
| MLR | 0.700 | 0.662 | 0.716 | 0.632 |
| NLR | 0.688 | 0.697 | 0.790 | 0.644 |
Bai’s model: Nomogram based on jaundice, CA19-9, tumor–node–metastasis stage, and R status[34]. BMI: Body mass index; TNM: Tumor–node–metastasis; MLR: Monocyte–to–lymphocyte ratio; NLR: Neutrophil–to–lymphocyte ratio; AUROC: Area under the ROC curve; ROC: Receiver operating characteristic.
Figure 5.
Comparisons of the nomogram with the American Joint Committee on Cancer tumor–node–metastasis stage model. A: Decision curve analysis of the nomogram and tumor–node–metastasis stage model for 3-year survival probability; B: Comparison of the nomogram prediction with tumor–node–metastasis staging.
DISCUSSION
GBC is the most common biliary duct cancer[1] and carries a poor prognosis. Accurate prediction of GBC prognosis could benefit clinical decision-making for personalized treatment after surgery. Therefore, in this study, we aimed to assess the prognostic value of GPR and to develop a prognosis prediction model as a nomogram for GBC patients. Our results showed that higher GPR, older age, lower BMI, and late TNM stage were independent predictors of GBC prognosis. In addition, GPR of patients with either early or terminal stage of GBC show a similar correlativity to OS. According to the score given to each clinical variable, our nomogram model predicted the 1-, 3-, and 5-year survival probability of GBC patients. This nomogram could serve as a reference for patient stratification and clinical decision-making.
Jaundice, BMI, and CA19-9 level had significant correlations with GPR. Preoperative jaundice indicates a higher risk of postoperative complications and adverse events, which indicates a poor prognosis[13]. According to the study by Rai et al[14], low BMI is related to malnutrition in GBC patients, and nutritional deterioration leads to adverse outcomes[14]. CA19-9 is a tumor-associated antigen, synthesized by normal human pancreatic and biliary ductular epithelial cells under physiological conditions, and increased CA19-9 levels imply biliary and pancreatic malignancy[15]. These three factors are clinicopathological factors related to poor GBC outcomes. Thus, the relationship between poor prognosis in GBC and GPR could also indicate correlations between GPR and the three described characteristics.
Even though previous studies simply showed GPR to be a confounding prognostic predictor for HCC, only limited patients involved in current study had liver complications such as fatty liver, cirrhosis, and HCC. The irrelevance of overall clinical characteristics of involved patients with either cirrhosis or HCC proved GPR’s prediction value for GBC to be independent of liver disease burden. In addition, GPR serves as an independent predictor of GBC prognosis for both long-term survival and short-term clinical outcomes. Patients with higher GPR levels tend to have higher risks of developing postoperative complications and require longer hospital stays because of poor outcomes.
The mechanism of GPR’s relationship with poor cancer prognosis remains unclear, but studies have demonstrated the clinical effect of both GGT and PLT on GBC and related gallbladder diseases. Study on surgical resection for GBC has revealed GGT’s diagnostic value[16]. Clinically, GGT has been administered in the evaluation of gallbladder diseases such as cholangiocarcinoma[17], biliary atresia[18], and cholecystitis[19]. Emerging evidence also indicates that higher GGT levels may be linked to a high cancer risk. In 2015, Kunutsor et al[20] indicated a positive association between GGT levels and overall cancer risk[20]. Several potential mechanisms of GGT’s effect on tumor growth have also been proposed. Reactive oxygen species, a result of the tumor microenvironment, could up-regulate GGT expression[21]. GGT, in turn, plays an essential role in maintaining the production of intracellular glutathione, which acts as a key antioxidant[22], and GGT also induces the production of an additional source of endogenous reactive oxygen species[21]; therefore, abnormal GGT levels could contribute to the formation of the tumor microenvironment and promote tumor growth. However, the exact mechanisms of elevated GGT in cancer are poorly described and require further research.
PLT has been proposed as a preoperative prognostic factor for GBC, two studies on PLT’s diagnostic value both show a correlation between high PLT level and poor post-surgery outcomes[11,23]. Mechanisms of PLT’s contribution to cancer development are involved in tumor growth factor synthesis[24], promotion of tumor adhesion of epithelial cells[25], and the morbidity of tumor cells[26]. A study by Andrade et al[27] showed that PLT is related to angiogenesis, microenvironment maintenance, and tumor masses[27]. PLT could promote tumor recurrence and serve as a resource for cytokines such as vascular endothelial growth factor or tumor growth factor-β. Additionally, tumor cells release inflammatory cytokines, and transference of cytokines such as platelet-derived growth factor and tumor necrosis factor by platelets could enhance tumor growth[28].
In this study, GPR appeared to be more significant than GGT to predict GBC, when we compared AUCs and the C-index. PLT was not a predictor of GBC in this study, but the combination of PLT and GGT as GPR showed good results regarding prognosis.
In 2008, Wang et al[29] published a predictive model related to RT based on patients’ records from the SEER database developed by the National Cancer Institute[29]. In 2016, Zhou et al[30] improved the predictive model by adding more clinical factors and using a nomogram scoring method[30]. However, these models were based on analyses of the SEER database, and patients’ characteristics may differ from patients in other areas. More studies have been proposed regarding patients’ gene expression levels, but these methods are not convenient to use clinically[31-33]; thus, an appropriate model to evaluate the prognosis of GBC patients in China is still an urgent need.
TNM stage defined by the AJCC is now the most widely used prognostic model for GBC[5]. However, the TNM staging system is designed for a broad cancer diagnosis and lacks a personal examination reference for individual patients. Compared with the TNM stage model defined by the AJCC (8th edition), adding more clinical factors significantly improves the accuracy and discriminability of prediction. GPR, age, and BMI all contribute to a better prognostic model by adding specific patients’ characteristics. The AUC of the time-dependent ROC and C-index both have advantages over the TNM stage system. Furthermore, nomogram models discriminate between patients with the same TNM stage, and better correspond with clinical observations.
Studies also show advantages of nomograms over other previously studied clinical predictive models or factors. In 2018, Bai et al[34] published a nomogram model aimed at predicting OS after GBC resection in China[34]. The authors’ study involved a similar patient population as in our study, and evaluated jaundice, CA19-9, TNM stage, and R stage as predictors. A comparison between these two models demonstrated an advantage regarding accuracy for our nomogram over Bai et al[34]’s nomogram.
MLR, NLR, and CA19-9 are clinical factors that have been evaluated in previous studies for evaluating GBC prognosis[6]. Comparisons of the related AUCs and the C-index showed a significant advantage of nomogram over these three factors.
In conclusion, GPR is an independent prognostic factor when predictnig OS in patients with GBC. Our nomogram model based on GPR successfully predicts the survival probability, and has advantages compared with the 8th edition of the AJCC system and other prognostic models.
Limits of the study
Our study has several limitations. First, because of the small sample size, we evaluated only a training cohort; our study had no validation cohort. Second, our study was a retrospective analysis; multicenter research based on our nomogram model is required to confirm the prediction outcomes of our model. Third, also because of the small number of patients included, the heterogeneity of involved patients could lead to statistical bias, and further research should expand the study population and confirm the prediction value of GPR. Finally, we analyzed only laboratory results and patients’ medical records. Previous studies evaluated multiple methods of examination such as computed tomography and magnetic resonance imaging[35]; therefore, further research should broaden the database and combine more clinical data[36].
ARTICLE HIGHLIGHTS
Research background
Gallbladder carcinoma (GBC) carries a poor prognosis and requires a prediction method. Gamma-glutamyl transferase–to–platelet ratio (GPR) is a recently-reported cancer prognostic factor. Although the mechanism of GPR’s relationship with poor cancer prognosis remains unclear, studies have demonstrated the clinical effect of both GGT and platelet count on GBC and related gallbladder diseases.
Research motivation
We aimed to elucidate the prognostic value of GPR and to improve the current prognostic system for GBC patients
Research objectives
We aimed to assess the prognostic value of GPR and to design a prognostic nomogram for GBC.
Research methods
The analysis involved 130 GBC patients who underwent surgery at Peking Union Medical College Hospital from December 2003 to April 2017. Patients were stratified into a high- or low-GPR group. The predictive ability of GPR was evaluated by Kaplan–Meier analysis and a Cox regression model. We developed a nomogram based on GPR, which we verified using calibration curves. The nomogram and other prognosis prediction models were compared using time-dependent receiver operating characteristic curves and the C-index.
Research results
Patients in the high-GPR group had a higher risk of jaundice, were older, and had higher carbohydrate antigen 19-9 levels and worse postoperative outcomes. Univariate analysis revealed that GPR, age, body mass index, tumor–node–metastasis (TNM) stage, jaundice, cancer cell differentiation degree, and carcinoembryonic antigen and carbohydrate antigen 19-9 levels were related to overall survival (OS). Multivariate analysis confirmed that GPR, body mass index, age, and TNM stage were independent predictors of poor OS. Calibration curves were highly consistent with actual observations. Comparisons of time-dependent receiver operating characteristic curves and the C-index showed advantages for the nomogram over TNM staging.
Research conclusions
GPR is an independent predictor of GBC prognosis, and nomogram-integrated GPR is a promising predictive model for OS in GBC.
Research perspectives
First, multicenter research based on our nomogram model is required to confirm the prediction outcomes of our model. Second, further research should expand the study population and confirm the prediction value of GPR. Finally, further research should also broaden the database and combine more clinical data.
Footnotes
Institutional review board statement: All procedures were approved by the Medical Ethics Committee of Peking Union Medical College Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College and were conducted in accordance with the Helsinki Declaration of 1965 and later versions.
Informed consent statement: The requirement for informed consent was waived because of the retrospective nature of this study.
Conflict-of-interest statement: The authors declare no potential financial interests.
Manuscript source: Unsolicited manuscript
Peer-review started: May 2, 2020
First decision: May 26, 2020
Article in press: August 4, 2020
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casella C S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
Contributor Information
Le-Jia Sun, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Ai Guan, Department of Clinical Medicine, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Wei-Yu Xu, Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, Beijing 100730, China.
Mei-Xi Liu, Department of Clinical Medicine, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Huan-Huan Yin, Department of Clinical Medicine, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Bao Jin, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Gang Xu, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Fei-Hu Xie, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Hai-Feng Xu, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Shun-Da Du, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Yi-Yao Xu, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Hai-Tao Zhao, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Xin Lu, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Xin-Ting Sang, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Hua-Yu Yang, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.
Yi-Lei Mao, Department of Liver Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China. pumch-liver@hotmail.com.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakić M, Patrlj L, Kopljar M, Kliček R, Kolovrat M, Loncar B, Busic Z. Gallbladder cancer. Hepatobiliary Surg Nutr. 2014;3:221–226. doi: 10.3978/j.issn.2304-3881.2014.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haq N, Khan BA, Imran M, Akram A, Jamal AB, Bangash F. Frequency of gall bladder carcinoma in patients with acute and chronic cholecystitis. J Ayub Med Coll Abbottabad. 2014;26:191–193. [PubMed] [Google Scholar]
- 4.Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder Cancer in the 21st Century. J Oncol. 2015;2015:967472. doi: 10.1155/2015/967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 6.Choi YH, Lee JW, Lee SH, Choi JH, Kang J, Lee BS, Paik WH, Ryu JK, Kim YT. A High Monocyte-to-Lymphocyte Ratio Predicts Poor Prognosis in Patients with Advanced Gallbladder Cancer Receiving Chemotherapy. Cancer Epidemiol Biomarkers Prev. 2019;28:1045–1051. doi: 10.1158/1055-9965.EPI-18-1066. [DOI] [PubMed] [Google Scholar]
- 7.Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, Goldin R, Njai HF, Ndow G, Taal M, Cooke G, D'Alessandro U, Vray M, Mbaye PS, Njie R, Mallet V, Thursz M. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369–1376. doi: 10.1136/gutjnl-2015-309260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang WL, Zheng XL, Zhang ZY, Zhou Y, Hao J, Tang G, Li O, Xiang JX, Wu Z, Wang B. Preoperative γ-glutamyl transpeptidase to platelet ratio (GPR) is an independent prognostic factor for HBV-related hepatocellular carcinoma after curative hepatic resection. Medicine (Baltimore) 2016;95:e4087. doi: 10.1097/MD.0000000000004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu CC, Lee KT, Lee HH, Wang JJ, Sun DP, Huang CC, Shi HY. Comparison of Models for Predicting Quality of Life After Surgical Resection of Hepatocellular Carcinoma: a Prospective Study. J Gastrointest Surg. 2018;22:1724–1731. doi: 10.1007/s11605-018-3833-7. [DOI] [PubMed] [Google Scholar]
- 10.Xu XS, Miao RC, Zhang LQ, Wang RT, Qu K, Pang Q, Liu C. Model Based on Alkaline Phosphatase and Gamma-Glutamyltransferase for Gallbladder Cancer Prognosis. Asian Pac J Cancer Prev. 2015;16:6255–6259. doi: 10.7314/apjcp.2015.16.15.6255. [DOI] [PubMed] [Google Scholar]
- 11.Wang RT, Zhang LQ, Mu YP, Li JB, Xu XS, Pang Q, Sun LK, Zhang X, Dong SB, Wang L, Liu C. Prognostic significance of preoperative platelet count in patients with gallbladder cancer. World J Gastroenterol. 2015;21:5303–5310. doi: 10.3748/wjg.v21.i17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Association WM. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 13.Yang XW, Yuan JM, Chen JY, Yang J, Gao QG, Yan XZ, Zhang BH, Feng S, Wu MC. The prognostic importance of jaundice in surgical resection with curative intent for gallbladder cancer. BMC Cancer. 2014;14:652. doi: 10.1186/1471-2407-14-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai A, Tewari M, Mohapatra SC, Shukla HS. Correlation of nutritional parameters of gallbladder cancer patients. J Surg Oncol. 2006;93:705–708. doi: 10.1002/jso.20539. [DOI] [PubMed] [Google Scholar]
- 15.Wen Z, Si A, Yang J, Yang P, Yang X, Liu H, Yan X, Li W, Zhang B. Elevation of CA19-9 and CEA is associated with a poor prognosis in patients with resectable gallbladder carcinoma. HPB (Oxford) 2017;19:951–956. doi: 10.1016/j.hpb.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Lin HM, Cai ZX, Du SJ, Zeng H, Xu LB, Wang J, Liu C. Clinical strategies for differentiating IgG4-related cholecystitis from gallbladder carcinoma to avoid unnecessary surgical resection. Sci China Life Sci. 2020;63:764–770. doi: 10.1007/s11427-019-9539-6. [DOI] [PubMed] [Google Scholar]
- 17.Boyd S, Mustonen H, Tenca A, Jokelainen K, Arola J, Färkkilä MA. Surveillance of primary sclerosing cholangitis with ERC and brush cytology: risk factors for cholangiocarcinoma. Scand J Gastroenterol. 2017;52:242–249. doi: 10.1080/00365521.2016.1250281. [DOI] [PubMed] [Google Scholar]
- 18.Dong C, Zhu HY, Chen YC, Luo XP, Huang ZH. Clinical Assessment of Differential Diagnostic Methods in Infants with Cholestasis due to Biliary Atresia or Non-Biliary Atresia. Curr Med Sci. 2018;38:137–143. doi: 10.1007/s11596-018-1857-6. [DOI] [PubMed] [Google Scholar]
- 19.Barut B, Gönültaş F, Gök AFK, Şahin TT. Management of Acute Cholecystitis during Pregnancy: A Single Center Experience. Ulus Travma Acil Cerrahi Derg. 2019;25:154–158. doi: 10.5505/tjtes.2018.82357. [DOI] [PubMed] [Google Scholar]
- 20.Kunutsor SK, Apekey TA, Van Hemelrijck M, Calori G, Perseghin G. Gamma glutamyltransferase, alanine aminotransferase and risk of cancer: systematic review and meta-analysis. Int J Cancer. 2015;136:1162–1170. doi: 10.1002/ijc.29084. [DOI] [PubMed] [Google Scholar]
- 21.Hanigan MH. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Adv Cancer Res. 2014;122:103–141. doi: 10.1016/B978-0-12-420117-0.00003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo C, Xu B, Fan Y, Yu W, Zhang Q, Jin J. Preoperative Gamma-Glutamyltransferase Is Associated with Cancer-Specific Survival and Recurrence-Free Survival of Nonmetastatic Renal Cell Carcinoma with Venous Tumor Thrombus. Biomed Res Int. 2017;2017:3142926. doi: 10.1155/2017/3142926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Miao R, Zhang X, Chen W, Zhou Y, Wang R, Zhang R, Pang Q, Xu X, Liu C. Exploring the diagnosis markers for gallbladder cancer based on clinical data. Front Med. 2015;9:350–355. doi: 10.1007/s11684-015-0402-2. [DOI] [PubMed] [Google Scholar]
- 24.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagi S, Sato S, Oh-hara T, Takami M, Koike S, Mishima Y, Hatake K, Fujita N. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS One. 2013;8:e73609. doi: 10.1371/journal.pone.0073609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho MS, Bottsford-Miller J, Vasquez HG, Stone R, Zand B, Kroll MH, Sood AK, Afshar-Kharghan V. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120:4869–4872. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade SS, Sumikawa JT, Castro ED, Batista FP, Paredes-Gamero E, Oliveira LC, Guerra IM, Peres GB, Cavalheiro RP, Juliano L, Nazário AP, Facina G, Tsai SM, Oliva ML, Girão MJ. Interface between breast cancer cells and the tumor microenvironment using platelet-rich plasma to promote tumor angiogenesis - influence of platelets and fibrin bundles on the behavior of breast tumor cells. Oncotarget. 2017;8:16851–16874. doi: 10.18632/oncotarget.15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li AJ, Karlan BY. Androgen mediation of thrombocytosis in epithelial ovarian cancer biology. Clin Cancer Res. 2005;11:8015–8018. doi: 10.1158/1078-0432.CCR-05-1058. [DOI] [PubMed] [Google Scholar]
- 29.Wang SJ, Fuller CD, Kim JS, Sittig DF, Thomas CR, Jr, Ravdin PM. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26:2112–2117. doi: 10.1200/JCO.2007.14.7934. [DOI] [PubMed] [Google Scholar]
- 30.Zhou D, Wang JD, Yang Y, Yu WL, Zhang YJ, Quan ZW. Individualized nomogram improves diagnostic accuracy of stage I-II gallbladder cancer in chronic cholecystitis patients with gallbladder wall thickening. Hepatobiliary Pancreat Dis Int. 2016;15:180–188. doi: 10.1016/s1499-3872(16)60073-5. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Yang ZL, Miao X, Zou Q, Li J, Liang L, Zeng G, Chen S. ATP5b and β2-microglobulin are predictive markers for the prognosis of patients with gallbladder cancer. J Mol Histol. 2015;46:57–65. doi: 10.1007/s10735-014-9597-9. [DOI] [PubMed] [Google Scholar]
- 32.Yadav S, Chandra A, Kumar A, Mittal B. Association of TERT-CLPTM1L and 8q24 Common Genetic Variants with Gallbladder Cancer Susceptibility and Prognosis in North Indian Population. Biochem Genet. 2018;56:267–282. doi: 10.1007/s10528-018-9843-z. [DOI] [PubMed] [Google Scholar]
- 33.Yang P, Javle M, Pang F, Zhao W, Abdel-Wahab R, Chen X, Meric-Bernstam F, Chen H, Borad MJ, Liu Y, Zou C, Mu S, Xing Y, Wang K, Peng C, Che X. Somatic genetic aberrations in gallbladder cancer: comparison between Chinese and US patients. Hepatobiliary Surg Nutr. 2019;8:604–614. doi: 10.21037/hbsn.2019.04.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y, Liu ZS, Xiong JP, Xu WY, Lin JZ, Long JY, Miao F, Huang HC, Wan XS, Zhao HT. Nomogram to predict overall survival after gallbladder cancer resection in China. World J Gastroenterol. 2018;24:5167–5178. doi: 10.3748/wjg.v24.i45.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi SY, Kim JH, Park HJ, Han JK. Preoperative CT findings for prediction of resectability in patients with gallbladder cancer. Eur Radiol. 2019;29:6458–6468. doi: 10.1007/s00330-019-06323-4. [DOI] [PubMed] [Google Scholar]
- 36.Chen M, Lin J, Cao J, Zhu H, Zhang B, Wu A, Cai X. Development and validation of a nomogram for survival benefit of lymphadenectomy in resected gallbladder cancer. Hepatobiliary Surg Nutr. 2019;8:480–489. doi: 10.21037/hbsn.2019.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.