Abstract
OBJECTIVE
To examine whether HbA1c, outpatient diabetes treatment regimen, demographics, and clinical characteristics are associated with mortality in hospitalized patients with diabetes and coronavirus disease 2019 (COVID-19).
RESEARCH DESIGN AND METHODS
This was a retrospective cohort analysis of patients with diabetes hospitalized with confirmed COVID-19 infection from 11 March to 7 May 2020 at a large academic medical center in New York City. Multivariate modeling was used to assess the independent association of HbA1c levels and outpatient diabetes treatment regimen with mortality, in addition to independent effects of demographic and clinical characteristics.
RESULTS
We included 1,126 hospitalized patients with diabetes and COVID-19 for analysis, among whom mean age was 68 years, 50% were male, 75% were Black, mean BMI was 30 kg/m2, 98% had type 2 diabetes, mean HbA1c was 7.5%, and 33.1% died. HbA1c levels were not associated with mortality in unadjusted or adjusted analyses, but an outpatient regimen with any insulin treatment was strongly predictive. Additionally, age, sex, and BMI interacted such that in all age categories, mortality was higher with increasing BMI in males compared with females.
CONCLUSIONS
In this large U.S. cohort of hospitalized patients with diabetes and COVID-19, insulin treatment, as a possible proxy for diabetes duration, and obesity rather than long-term glycemic control were predictive of mortality. Further investigation of underlying mechanisms of mortality and inpatient glycemic control is needed.
Introduction
Since the first cases of coronavirus disease 2019 (COVID-19) emerged in China in December 2019 (1), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused high rates of transmission and mortality worldwide, especially among people with diabetes (2). Diabetes affects approximately one-half billion people in the world (3), with incidence rising steadily. People with diabetes are known to be more prone to complications and death from infectious pulmonary diseases, including COVID-19 (2,4,5). Given how vulnerable patients with diabetes are and the high prevalence of diabetes, a deeper understanding of diabetes-specific risk factors for COVID-19–related morbidity and mortality is critical to optimizing management and to improve outcomes.
Numerous studies from Asia, Europe, and the U.S. have demonstrated higher rates of mechanical ventilation and death from COVID-19 among people with diabetes compared with people without diabetes (2,4,6,7). These reports showed that people with diabetes of older age, higher BMI, and more comorbidities had increased mortality, ranging from 10% to 45% (2,4). Prior viral pneumonia pandemics including H1N1 influenza and Middle East respiratory syndrome demonstrated similar increased risk in people with diabetes (8–10). However, the roles of diabetes treatment regimen and glycemic control prior to COVID-19 infection and their contribution to the higher mortality risk in diabetes remain unclear.
Few studies have specifically examined the effect of preadmission glycemic control in patients with diabetes and risk of in-hospital mortality from COVID-19, despite having great implications for outpatient glycemic management during the pandemic. Zhu et al. (6) used a large Chinese cohort to match patients based on preadmission glycemic control and found that higher preadmission HbA1c levels were associated with higher in-hospital mortality. However, this study’s limitations included a higher prevalence of cardiovascular disease and older age in the higher HbA1c group that could have confounded the relationship between preadmission HbA1c levels and mortality. The Coronavirus SARS-CoV-2 and Diabetes Outcomes (CORONADO) study was a nationwide French study of people with diabetes and COVID-19 examining preadmission and admission characteristics with a primary composite outcome of mechanical ventilation or mortality and a secondary outcome of mortality alone within 7 days of admission and demonstrated that neither HbA1c nor BMI was associated with mortality alone (7). This study examined independent effects after accounting for multiple variables, which strengthens its results, but has not been confirmed yet by others.
While prior studies provide early insight into relationships between preadmission glycemic control and mortality among people with diabetes and COVID-19, characterization of U.S. cohorts is still needed for several reasons: 1) the reported death rate in the U.S. is significantly higher than in China or Europe (4); 2) differences in virulence of COVID-19 strains and transmission in the U.S. compared with Europe and China may result in different outcomes among U.S. diabetes cohorts (11); and 3) treatment regimens for both COVID-19 and diabetes may differ significantly between the U.S. and other countries (12), which limits generalizability of the previously published studies.
The goal of this study was to use a U.S. cohort to examine the association of outpatient glycemic control, diabetes treatment, and other characteristics with mortality among hospitalized patients with diabetes and COVID-19. We used a diverse cohort from New York City, at the height of the U.S. COVID-19 pandemic, with high hospitalization and mortality rates from COVID-19. We hypothesized that HbA1c, outpatient insulin treatment, and other comorbidities would be associated with mortality after multiple adjustment.
Research Design and Methods
Setting and Participants
Montefiore Medical Center (MMC) comprises three hospitals with a total of 1,536 beds and with a high proportion of patients from racial/ethnic minority populations and low socioeconomic status (13). From 11 March to 7 May 2020, there were 3,992 COVID-19–positive admissions at MMC.
The study population included all patients with documented diabetes defined by Clinical Modification code (ICD-10-CM) or HbA1c ≥6.5% (11) prior to or during the first week of hospitalization who were admitted to MMC from 11 March to 7 May 2020 and were confirmed to be COVID-19 positive by PCR testing. Participants were excluded from this analysis if they were still hospitalized at the end of data collection because the end points of hospital discharge or death were not known.
Study Design, Data Collection, and Measures
This is a retrospective electronic health record analysis of all patients meeting inclusion criteria admitted during the study time frame. A priori decisions were made to extract data on measures that were associated with diabetes and mortality. A data analyst used automated chart extraction (Epic Clarity) to collect all variables, and the data were cross-checked for integrity before analysis with other institutional projects using different methods of data retrieval (Clinical Looking Glass).
Primary Outcome
The primary outcome was mortality at any point during hospitalization for COVID-19 in the study time frame or live discharge. If the person was admitted and discharged alive and subsequently readmitted after which they expired during readmission, their data from both admission time frames were used.
Demographic and Clinical Characteristics
Age, sex, race, ethnicity, and insurance status were collected for every participant when available. For race/ethnicity information, there was a high amount of missing data (56%), so this information was not included in further analyses.
Glycemic Control
HbA1c levels were used to define glycemic control. The most recent HbA1c level within 3 years prior to or during the first week of admission was used for analysis.
Treatment Regimen
Outpatient diabetes treatment regimen prior to admission was collected from medication reconciliation files and categorized into insulin only, insulin plus noninsulin, noninsulin medications (oral or injectable), and no diabetes medications.
Comorbidities and Long-term Diabetes Complications
Hypertension, chronic kidney disease, and chronic obstructive pulmonary disease were directly extracted using ICD-10-CM codes from outpatient visits for 2 years prior to admission or admission diagnoses. Cardiovascular disease was a composite measure using ICD-10-CM codes for cardiovascular disease, myocardial infarction, congestive heart failure, prior history of coronary artery bypass graft surgery or percutaneous coronary stent placement, cerebrovascular disease, and peripheral arterial disease.
Data Analysis
Descriptive statistics were calculated for all variables, with mean ± SD or median and interquartile range reported for continuous variables, and number and percentage of total for categorical variables.
To assess the associations of preadmission attributes with in-hospital mortality, we performed logistic regression analyses with death as the outcome variable. In bivariate analyses, each of the attributes was the sole predictor used. In multivariate logistic regression analyses, we used all of these variables as predictors simultaneously. The odds ratios from both the bivariate (unadjusted) and multivariate (adjusted) analyses are reported along with their 95% CIs.
We also tested for interactions between age, sex, and BMI in their association with mortality using a logistic regression model with death as the outcome and a three-way interaction of these parameters as predictors. As the three-way interaction term was found to make no discernible contribution to the model, it was dropped and results are presented from a model containing these three predictors and all of their pairwise interactions.
Results
Patient Characteristics
Table 1 displays the clinical and demographic characteristics of our cohort. Out of 3,992 COVID-19 admissions between 11 March and 7 May 2020, 1,399 patients had HbA1c levels within the past 3 years. Of 1,399 patients, 1,279 had a prior history of diabetes or HbA1c levels ≥6.5% (48 mmol/mol). Of 1,279 eligible patients, 1,126 had complete data on all of the variables tested for inclusion in the study.
Table 1.
Characteristics of hospitalized patients with diabetes and COVID-19
| Outcome | |
| Vital status (at discharge) | |
| Alive, n = 798 | 66.9 |
| Deceased, n = 394 | 33.1 |
| Median survival ± IQR (days), n = 1,279 | 18 ± 20 |
| Demographic characteristics | |
| Age (years), n = 1,279 | 67.9 ± 13.7 |
| Sex, n = 1,279 | |
| Male, n = 630 | 49.3 |
| Female, n = 649 | 50.7 |
| Race, n = 690 | |
| White, n = 112 | 15.5 |
| Black or African American, n = 538 | 74.5 |
| Other, n = 40 | 5.8 |
| Ethnicity, n = 1,170 | |
| Spanish/Hispanic/Latino, n = 473 | 40.4 |
| Not Spanish/Hispanic/Latino, n = 697 | 59.6 |
| Insurance | |
| Medicaid, n = 556 | 43.0 |
| Medicare, n = 602 | 47.0 |
| Commercial, n = 120 | 9.0 |
| Clinical characteristics | |
| HbA1c (%), n = 1,279 | 7.5 ± 2.0 |
| HbA1c (mmol/mol), n = 1,279 | 58 ± 21.9 |
| BMI (kg/m2), n = 1,271 | 30.1 ± 7.5 |
| Hypertension, n = 1,163 | 90.9 |
| Cardiovascular disease, n = 754 | 59.0 |
| Chronic kidney disease, n = 544 | 42.5 |
| Chronic obstructive pulmonary disease, n = 163 | 13.5 |
| Type of diabetes, n = 1,279 | |
| Type 1 diabetes, n = 21 | 1.6 |
| Type 2 diabetes, n = 1,258 | 98.4 |
| Outpatient diabetes treatment regimen, n = 1,258 | |
| None, n = 259 | 20.3 |
| Insulin only, n = 119 | 9.3 |
| Insulin plus noninsulin, n = 412 | 32.2 |
| Noninsulin only, n = 489 | 38.2 |
| Any noninsulin treatment | |
| Metformin, n = 585 | 45.7 |
| Sulfonylurea, n = 280 | 21.9 |
| DPP-4 inhibitor, n = 329 | 25.7 |
| GLP-1 agonist, n = 136 | 10.6 |
| SGLT2 inhibitor, n = 57 | 4.5 |
Data are mean ± SD or % unless otherwise indicated. n data show the number of patients for each variable. DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; IQR, interquartile range; SGLT2, sodium–glucose cotransporter 2.
The mortality rate for the cohort of hospitalized patients with diabetes and COVID-19 was 33.1%. The majority of patients had type 2 diabetes, and mean HbA1c level was 7.5% (58 mmol/mol). Most recent HbA1c level was within 1 year of admission for 75% of participants and within 1 week of hospitalization for 18% of participants. Participants were treated in roughly equal amounts with insulin and noninsulin medications prior to admission. Proportions of Medicaid and Medicare insurance were equally high.
Association of Age, Sex, and BMI With Mortality
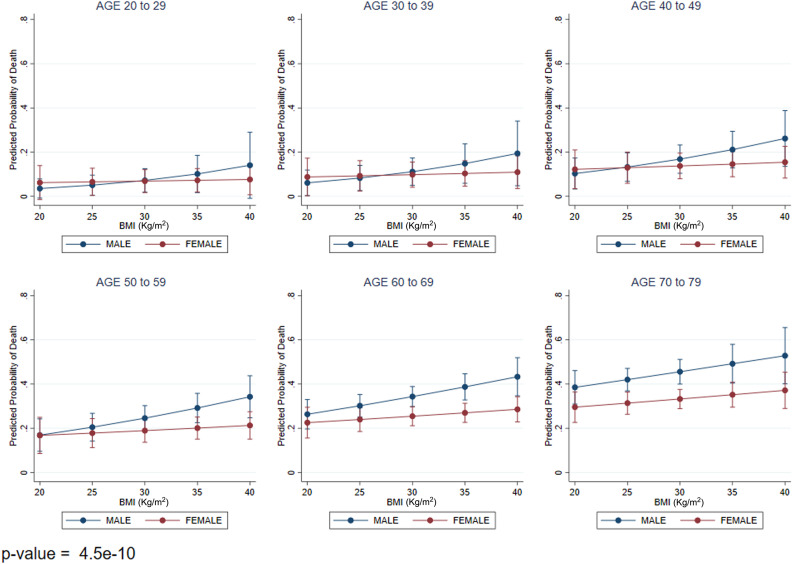
The combination of age, sex, and BMI was strongly predictive of mortality (Fig. 1). As age increased, mortality increased as BMI increased and was higher for males compared with females (Fig. 1).
Figure 1.
Mortality by age (years), sex, and BMI in hospitalized patients with diabetes and COVID-19. P value compares all age-groups.
Association of Preadmission Glycemic Control and Outpatient Diabetes Treatment Regimen With Mortality
There was no significant association of HbA1c levels with mortality in unadjusted analyses or when adjusted for demographic characteristics, BMI, treatment regimen, and comorbidities or complications (Table 2). Several a priori subanalyses were performed to examine interactions of HbA1c with other variables, including age, sex, BMI, and treatment regimen, which revealed no significant interactions (results not shown). In addition, we performed a priori sensitivity analyses for poorly controlled diabetes (HbA1c ≥9%, 75 mmol/mol [14]), which showed no association between HbA1c and mortality (results not shown). Lastly, we examined whether more or less recent testing for HbA1c level would change results. Seventy-five percent of the cohort had most recent HbA1c levels within 1 year of admission, and 18% had in-hospital HbA1c levels within 1 week of admission. Analyses of patients with HbA1c within 1 year or 1 week of hospitalization revealed no differences in results compared with the cohort that included patients with HbA1c levels for up to 3 years prior to admission.
Table 2.
Mortality odds ratios of preadmission clinical characteristics in hospitalized patients with diabetes and COVID-19 (N = 1,126)
| Unadjusted OR | Unadjusted 95% CI | Adjusted OR* | Adjusted 95% CI* | |
|---|---|---|---|---|
| Glycemic control: HbA1cƗ | 1.02 | 0.96, 1.08 | 1.01 | 0.94, 1.09 |
| Treatment regimen (Ref: no treatment) | ||||
| Noninsulin only | 1.45 | 1.00, 2.10 | 1.30 | 0.89, 1.91 |
| Insulin + noninsulin | 1.68 | 1.15, 2.45 | 1.74 | 1.13, 2.68 |
| Insulin only | 1.98 | 1.20, 3.26 | 2.30 | 1.32, 4.01 |
| Comorbidity or long-term diabetes complication | ||||
| Hypertension | 0.84 | 0.44, 1.58 | 0.54 | 0.28, 1.05 |
| Cardiovascular disease | 1.57 | 1.21, 2.04 | 1.18 | 0.88, 1.57 |
| Chronic kidney disease | 1.34 | 1.05, 1.72 | 1.11 | 0.84, 1.45 |
| Chronic obstructive pulmonary disease | 1.63 | 1.16, 2.29 | 1.46 | 1.02, 2.08 |
Ref, reference.
Adjustment: each variable was adjusted for age, sex, BMI, insurance, and the other variables in the table.
Most recent HbA1c within 3 years prior to or 1 week after hospitalization was used for analyses.
For treatment regimen, there was increased risk for mortality for the outpatient treatment regimens containing insulin (insulin only and insulin plus noninsulin), compared with no treatment (Table 2). In addition, there was a dose-response relationship of insulin use with mortality such that patients on insulin only prior to admission had the highest risk of mortality, followed by patients on insulin plus noninsulin treatments (Table 2). These relationships were unchanged when insurance was included in the multivariate model, indicating that the relationship between treatment regimen and mortality was not explained by disadvantages in treatment regimen due to insurance.
Association of Comorbidities and Long-term Diabetes Complications With Mortality
Cardiovascular disease, chronic kidney disease, and chronic obstructive pulmonary disease were all strongly associated with mortality in univariate analyses, while hypertension was protective (Table 2). In adjusted analyses, the associations of cardiovascular disease, chronic kidney disease, and hypertension were attenuated, while chronic obstructive pulmonary disease remained strong (Table 2).
Conclusions
Recent data suggest that diabetes is a risk factor for mortality in patients with COVID-19 (2,4,15,16). We investigated whether HbA1c levels, outpatient diabetes treatment, and other characteristics were associated with in-hospital mortality among patients with COVID-19 and preexisting diabetes. Intriguingly, our results showed that HbA1c was not associated with mortality during hospitalization, while any outpatient insulin treatment was strongly predictive. In addition, BMI was independently predictive of mortality, especially among older men compared with women.
Explanations for the lack of association of HbA1c levels with in-hospital mortality remain unclear. Given how closely tied HbA1c is to overall disease progression and complications (17), which were associated with mortality in our study, one would expect higher HbA1c levels to be associated with increased risk of mortality during hospitalizations for serious infections, such as COVID-19. Moreover, mechanistic studies have demonstrated that chronic hyperglycemia is associated with immune dysregulation and susceptibility to severe prolonged lung disease, suggesting that high HbA1c levels should be associated with poor outcomes from infectious diseases like COVID-19 (18). It is possible that in the setting of such high mortality rates in our cohort (33%), the impact of glycemic control on mortality was comparably too small to be detected. However, results similar to ours were reported in the CORONADO study from France, which used multivariable modeling to evaluate the relationship of HbA1c with mortality among patients with diabetes hospitalized for COVID-19 and found no relationship of HbA1c with either mortality or invasive mechanical ventilation (7). Interestingly, a study from China found that hospitalized patients with diabetes with higher preadmission HbA1c levels had higher mortality from COVID-19 (6). However, this study had unmatched variables between groups, which limited conclusions, with disproportionate representation of older age, male sex, higher BMI, and more comorbidities in the higher HbA1c group.
Other large studies prior to the COVID-19 pandemic have demonstrated a complex relationship of HbA1c with in-hospital mortality. A large meta-analysis of mortality from acute coronary syndrome in hospitalized patients with and without diabetes found that HbA1c was not associated with mortality in patients with diabetes, even though patients with diabetes had higher mortality than those without diabetes (19). In two separate studies examining thousands of patients postoperatively, HbA1c was not associated with 30-day or 6-month mortality (20), while HbA1c was strongly associated with in-hospital glucose levels, surgical complications, intensive care unit admissions, and length of stay (21). These studies suggest that HbA1c levels have effects on in-hospital glucose levels but are not independently predictive of mortality. Thus, in-hospital mortality may be affected by in-hospital factors such as severity of illness, inpatient insulin treatment, and other hyperglycemia-inducing hospital interventions and need to be further investigated.
Our findings suggest that outpatient insulin treatment may be a better marker for hospital mortality risk from COVID-19 than HbA1c level. In our analysis, insulin treatment was strongly predictive of mortality and acted in a dose-dependent fashion, with treatment regimens of insulin only conferring higher risk than that for regimens of insulin plus noninsulin. In this cohort of predominantly type 2 diabetes, insulin treatment is likely a surrogate marker of diabetes duration and progression of disease with accompanying β-cell loss. Recent evidence suggests that COVID-19 itself might induce β-cell loss/dysfunction (5). Thus, there may be an additive effect of COVID-19 to the mortality risk of diabetes disease progression, but more studies are needed to confirm this hypothesis.
We also found that age, sex, and BMI interacted and strongly predicted mortality in this diabetes cohort; specifically, as age increased, mortality was higher with increasing BMI in men compared with women. Results from other studies examining the relationship of age, sex, and BMI individually with mortality are consistent with our results (2,4), but none of the previous studies have tested the intersection of these three important variables or controlled for common comorbidities in older age such as hypertension, cardiovascular disease, chronic kidney disease, and chronic obstructive pulmonary disease. Obesity has been a subject of much study in COVID-19 and has been shown to be an independent risk factor for mortality compared with other diseases (4,22,23). However, few studies have looked at the effect of BMI as a risk factor of COVID-19 mortality independent of diabetes. For diabetes-specific risk factors, obesity could worsen insulin resistance, thus complicating inpatient glycemic management. For the sex-based differences in mortality we found, it has been hypothesized that there may be a protective effect of estrogens in COVID-19, which needs to be further elucidated (24). Our results of a three-way interaction provide a basis for risk stratification for proactive intensification of therapy in the hospital and should be considered in the design of trials testing COVID-19 treatments for high-risk subgroups (older obese men with diabetes).
Lastly, we found that cardiovascular disease, chronic kidney disease, and chronic obstructive pulmonary disease were strongly associated with mortality and hypertension was protective in unadjusted analyses but that these relationships were attenuated after adjustment, except for chronic obstructive pulmonary disease. Other studies have found significant associations of comorbidities and complications of diabetes with mortality in COVID-19 (4,7). For cardiovascular disease, COVID-19 is associated with cardiac involvement and increased thromboembolic risk, both of which may contribute to the association between cardiovascular disease and mortality among COVID-19 patients with dysglycemia (25,26). Chronic kidney disease may lead to electrolyte and fluid shifts that increase risk for COVID-19 cardiovascular mortality (27). Chronic obstructive pulmonary disease may be associated with alterations in lung microbiome imbalance and structural damage, which confer increased risk for mortality (28). Lastly, hypertension may have appeared protective if antihypertensive medications commonly used in diabetes, such as ACE inhibitors and angiotensin receptor blockade agents, decreased the cardiovascular risk of mortality from COVID-19 (29,30). Nevertheless, these factors did not appear to be as strongly predictive of mortality after full adjustment of all variables.
This study has several limitations. First, this study is cross-sectional. Thus, we can only make conclusions about associations and not causality. Second, only the most recent HbA1c value was used in analyses, which may not fully reflect the relationship of glycemic control with mortality. Nevertheless, the majority of participants had HbA1c levels within 1 year of admission and there was no difference in results in comparison of HbA1c values for up to 3 years prior to admission with HbA1c values within 1 year or 1 week of admission. We could not include a measure of glycemic variability, which may have been important, given prior studies demonstrating that variability in HbA1c independently predicts mortality and thus should be further studied (31,32). Lastly, this study was performed in a single medical center located in New York City and therefore our results may not be applicable to other geographic regions in the U.S. Nevertheless, our study included a large cohort of patients at the epicenter of the COVID-19 pandemic, and our null HbA1c results are consistent with studies from other areas (7).
In conclusion, we analyzed the association of mortality with HbA1c levels, outpatient diabetes treatment, and other outpatient characteristics in a large cohort of hospitalized patients with diabetes and COVID-19 and, importantly, found that HbA1c was not predictive of in-hospital mortality. We also found that insulin treatment and obesity were strongly and independently predictive of mortality. Our results should help guide risk stratification for clinicians caring for patients with diabetes and COVID-19 in the hospital setting as well as guide outpatient management and mitigation of risk factors. More research is needed to examine the role of inpatient glycemic control on mortality in patients with diabetes and COVID-19.
Article Information
Acknowledgments. The authors greatly appreciate the efforts of their fellow health care workers and support staff at MMC for providing outstanding patient care at considerable personal risk on the front lines of this pandemic. The authors express their solidarity with those who are or have been ill with COVID-19 and their families.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.A. researched the data and wrote the manuscript. C.S. analyzed the data and reviewed and edited the manuscript. W.S. reviewed and edited the manuscript. J.P.C. and Y.T. researched the data, contributed to the discussion, and reviewed and edited the manuscript. S.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/diabetes-and-COVID19.
References
- 1.World Health Organization WHO Timeline-COVID-19. WHO Newsroom, 2020. Accessed 22 June 2020. Available from https://www.who.int/news-room/detail/29-06-2020-covidtimeline
- 2.Guan WJ, Ni ZY, Hu Y, et al.; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al.; Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 2020;8:546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31:1068–1077.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cariou B, Hadjadj S, Wargny M, et al.; CORONADO Investigators . Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 2020;63:1500–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allard R, Leclerc P, Tremblay C, Tannenbaum TN. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care 2010;33:1491–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013;13:752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alqahtani FY, Aleanizy FS, Ali El Hadi Mohamed R, et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect 2018;147:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Reiche AS, Hernandez MM, Sullivan MJ, et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science 2020;369:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020;323:2466–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The United States Census Bureau QuickFacts: Bronx County (Bronx Borough), New York, 2019. Accessed 13 June 2020. Available from https://www.census.gov/quickfacts/bronxcountybronxboroughnewyork.
- 14.Johnson EL, Feldman H, Butts A, et al.; American Diabetes Association . Standards of Medical Care in Diabetes-2020 abridged for primary care providers. Clin Diabetes 2020;38:10–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bello-Chavolla O, Bahena-López J, Antonio-Villa N, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinal Metab 2020:105:dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020;395:1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan DM; DCCT/EDIC Research Group . The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study at 30 years: overview. Diabetes Care 2014;37:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 2019;4:e131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan W, Lu H, Lian B, Liao P, Guo L, Zhang M. Prognostic value of HbA1c for in-hospital and short-term mortality in patients with acute coronary syndrome: a systematic review and meta-analysis. Cardiovasc Diabetol 2019;18:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care 2018;41:782–788 [DOI] [PubMed] [Google Scholar]
- 21.Yong PH, Weinberg L, Torkamani N, et al. The presence of diabetes and higher HbA1c are independently associated with adverse outcomes after surgery. Diabetes Care 2018;41:1172–1179 [DOI] [PubMed] [Google Scholar]
- 22.Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020;43:1392–1398 [DOI] [PubMed] [Google Scholar]
- 23.Simonnet A, Chetboun M, Poissy J, et al.; LICORN and the Lille COVID-19 and Obesity study group . High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandi G, Facchinetti F, Bitzer J. The gendered impact of coronavirus disease (COVID-19): do estrogens play a role? Eur J Contracept Reprod Health Care 2020;25:233–234 [DOI] [PubMed] [Google Scholar]
- 25.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. Eur J Contracept Reprod Health Care 2020;25:233–234 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020;97:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med 2020;167:105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;382:2431–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126:1671–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Critchley JA, Carey IM, Harris T, DeWilde S, Cook DG. Variability in glycated hemoglobin and risk of poor outcomes among people with type 2 diabetes in a large primary care cohort study. Diabetes Care 2019;42:2237–2246 [DOI] [PubMed] [Google Scholar]
- 32.Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol 2018;6:476–486 [DOI] [PubMed] [Google Scholar]