Abstract
OBJECTIVE
To investigate the impact of residual β-cell function on continuous glucose monitoring (CGM) outcomes following acute exercise in people with type 1 diabetes (T1D).
RESEARCH DESIGN AND METHODS
Thirty participants with T1D for ≥3 years were recruited. First, participants wore a blinded CGM unit for 7 days of free-living data capture. Second, a 3-h mixed-meal test assessed stimulated C-peptide and glucagon. Peak C-peptide was used to allocate participants into undetectable (Cpepund <3 pmol/L), low (Cpeplow 3–200 pmol/L), or high (Cpephigh >200 pmol/L) C-peptide groups. Finally, participants completed 45 min of incline treadmill walking at 60% VO2peak followed by a further 48-h CGM capture.
RESULTS
CGM parameters were comparable across groups during the free-living observation week. In the 12- and 24-h postexercise periods (12 h and 24 h), the Cpephigh group had a significantly greater amount of time spent with glucose 3.9–10 mmol/L (12 h, 73.5 ± 27.6%; 24 h, 76.3 ± 19.2%) compared with Cpeplow (12 h, 43.6 ± 26.1%, P = 0.027; 24 h, 52.3 ± 25.0%, P = 0.067) or Cpepund (12 h, 40.6 ± 17.0%, P = 0.010; 24 h, 51.3 ± 22.3%, P = 0.041). Time spent in hyperglycemia (12 h and 24 h glucose >10 and >13.9 mmol/L, P < 0.05) and glycemic variability (12 h and 24 h SD, P < 0.01) were significantly lower in the Cpephigh group compared with Cpepund and Cpeplow. Change in CGM outcomes from pre-exercise to 24-h postexercise was divergent: Cpepund and Cpeplow experienced worsening (glucose 3.9–10 mmol/L: −9.1% and −16.2%, respectively), with Cpephigh experiencing improvement (+12.1%) (P = 0.017).
CONCLUSIONS
Residual β-cell function may partially explain the interindividual variation in the acute glycemic benefits of exercise in individuals with T1D. Quantifying C-peptide could aid in providing personalized and targeted support for exercising patients.
Introduction
Individuals with type 1 diabetes (T1D) are encouraged to regularly engage in physical activity and exercise because of many health benefits, such as reduced cardiovascular risk factors and improvements in physical fitness (1). However, exercise can cause disruption to maintaining euglycemia, particularly when causing hypoglycemia, and can be complex to manage (2). This may explain the lower physical activity levels within the population with T1D compared with the general public (3).
One major obstacle to providing exercise support to people with T1D is a high interindividual variability in the blood glucose responses to exercise (2). Even within tightly controlled research studies that have adopted a strict inclusion criteria, recruited a homogenous cohort of participants, had standardized insulin and dietary intake, and used continuous glucose monitoring (CGM) to stabilize pretrial glucose, a large unexplained interindividual variability in the acute glycemic responses to exercise remains (4–7). This is despite a high intraindividual reproducibility under repeated conditions (4,5). Indeed, outside of formal research, both clinical observations and feedback from patient support groups report potential for both an improvement and detrimental impact of regular exercise on HbA1c. Wide-ranging challenges to successfully avoiding hypoglycemia persist, despite advancement and availability of supportive strategies including CGM and patient education.
Recent research has shown that even in long-duration T1D, β-cell function—as measured by C-peptide—can persist. There is some disparity within the evidence regarding the prevalence of residual β-cell function within the T1D population, but it is estimated that between 35% and 80% of participants have detectable β-cell function at >5 years postdiagnosis (8,9). Moreover, it is estimated that 8–16% of individuals diagnosed with T1D as an adult have a relatively high C-peptide level, above the threshold found in the Diabetes Control and Complications Trial (DCCT) (>200 pmol/L) to have some clinical benefits (10), compared with 5–6% of individuals with childhood onset of diabetes (8,9,11).
Evidence from recently diagnosed individuals and after islet transplantation, when consequently C-peptide levels are relatively high, demonstrates that as residual β-cell function declines, CGM parameters such as time in a state of euglycemia (time in range 3.9–10 mmol/L) and coefficient of variation (CV) worsen (12,13). A recent study by Rickels et al. (14) demonstrated that individuals with short-duration T1D and very high stimulated C-peptide (>400 pmol/L) had greater time in euglycemia at rest compared with negative, low (17–200 pmol/L), and intermediate (200–400 pmol/L) C-peptide groupings. How this translates to people with established, longer-duration T1D and during and following exercise is unclear. Potentially, diminished but functioning β-cells may convey some level of intrinsic glucose regulation that offers benefits under an intense metabolic stressor (including increased metabolic rate, carbohydrate oxidation, and insulin sensitivity) such as exercise. Moreover, it can be hypothesized that β-cell function is associated with CGM outcomes explaining (at least in part) interindividual variability in the exercise response. This information could be valuable for provision of targeted exercise support, based on C-peptide status.
This study examined the impact of residual β-cell function on CGM outcomes after a bout of aerobic exercise in people with T1D. We hypothesized that individuals with higher C-peptide will have increased amount of time with an interstitial glucose in the euglycemia range (3.9–10 mmol/L)—the primary outcome.
Research Design and Methods
Participants
Eligibility criteria comprised a clinical diagnosis of T1D (primary osmotic symptoms, weight loss, hyperglycemia, ketosis, insulin initiation at diagnosis), age 18–65 years with diabetes duration ≥3 years at enrollment, HbA1c <86 mmol/mol (10.0%), absence of diabetes-related complications apart from retinopathy, and stable multiple daily injections or continuous subcutaneous insulin infusion regimen without changes over the preceding 6 months. All participants provided written informed consent, and this study was approved by the local National Health Service Research Ethics Committee, Newcastle, U.K. (code: 16/NE/0192).
Sample Size
Sample size estimation was calculated using available C-peptide and CGM data from studies previously conducted by our group (13). Specifically, percentage time in range 3.9–10 mmol/L from 5 days of CGM capture from islet transplant recipients with a stimulated C-peptide >200 pmol/L (mean ± SD 71 ± 21%) or <150 pmol/L (45 ± 16%) was used. With an estimated difference of at least 10% in the primary outcome, a sample of 10 participants per group would be needed to test the null hypothesis that mean time within range (3.9–10.0 mmol/L) of all groups is equal with a probability of 0.8. Type 1 error associated with this calculation is 0.05.
Participant Identification and Recruitment
Potential participants with ≥3 years’ duration were first identified using a home urine C-peptide–to–creatinine ratio (UCPCR) kit (15). The time frame of 3 years was used to allow a clear gap from the approximate 2-year point, often referred to as the “honeymoon” (16). UCPCR results were used to preliminarily allocate participants in one of three UCPCR groupings: undetectable (<0.001), low (0.001–0.19), and high (≥0.2 nmol/mmol). Supplementary Fig. 1 has a schematic of the study recruitment numbers and protocol.
Visit 1: Free-living Observational CGM Week
Participants attended the National Institute for Health Research (NIHR) Newcastle Clinical Research Facility (CRF) for insertion of a blinded CGM unit (Enlite sensor with iPro2 Professional CGM, MiniMed; Medtronic Diabetes). During the observational free-living week, patients self-recorded insulin dosages and capillary blood glucose (CBG) concentrations. CBG was recorded four or more times per day for calibration purposes with sensor data retrospectively processed using CareLink software (Medtronic Diabetes). If a day’s CGM recording, from midnight to midnight, failed any of the CareLink optimal data thresholds (valid calibrations, mean absolute relative difference (%), correlations) (17) or had missing data of >15 min segments, data from throughout that day were deemed suboptimal and not used. If the iPro2 failed to collect four valid days of data, the testing process was repeated.
Visit 2: Mixed-Meal Tolerance Test
Participants attended the CRF at ∼8:30 a.m. after an overnight fast, and a cannula was inserted into an antecubital vein. Individuals were instructed to maintain their normal basal insulin regimen. A mixed-meal tolerance test (MMTT) protocol was used, with participants given 240 mL Fortisip (Nutricia, Trowbridge, U.K.) (360 kcal, 14.4 g protein, 13.92 g fat, and 44.16 g carbohydrate) to drink within 2 min (18). Blood samples were drawn at baseline and every 30 min up to and including 180 min. Samples were centrifuged with plasma, and serum was stored at −80°C in the Newcastle Biobank facility.
Visit 3: Health Screening and Maximum Exercise Test
Participant height, weight (seca 220 stadiometer/seca 889 scale; seca, Hamburg, Germany), and medical history were taken. Participants underwent a modified 12-lead resting and exercising electrocardiogram to screen for cardiac abnormalities.
A maximal graded walking treadmill (Valiant 2 cpet; Lode, Groningen, the Netherlands) test (Bruce protocol [19]) was performed to determine peak oxygen uptake (VO2peak) and peak heart rate. Glycemic strategy was managed as per the guidance of Riddell et al. (2).
Visit 4: Main Trial Exercise Bout
Prior to the submaximal exercise phase, participants attended the CRF 24–48 h before the final testing visit to have a CGM inserted. Individuals arrived at the exercise laboratory at ∼8:30 a.m. after an overnight fast, having been instructed to maintain their normal basal insulin regimen. If participants had a hypoglycemic event overnight prior to the study visit, the visit was rearranged, while if participants awoke with blood glucose >10 mmol/L they were instructed to have a small corrective bolus of insulin upon waking (≤2 units). A carbohydrate snack (belVita; Mondelēz International) providing 204 kcal (31 g carbohydrate) was consumed, and participants remained rested for 20 min. Target CBG was >7 mmol/L for the duration of the exercise, with participants given 10 g carbohydrate if CBG fell below this level. Participants walked at 60% VO2peak for 45 min at a comfortable stride length (7.15 ± 3.58% gradient at 5.09 ± 0.28 km/h). Individual treadmill speed and gradient were calculated using VO2, speed, and gradient data from the preliminary exercise test (20). Heart rate and expired air were captured and analyzed throughout (MetaLyzer 3B-R3 CPET; CORTEX Biophysik, Leipzig, Germany), with gradient adjusted at 10 and 30 min if VO2 was >10% different than target VO2. Upon completion of the exercise, participants rested for 60 min before discharge from the laboratory. For the 48 h following the exercise bout, free-living interstitial glucose responses were captured and participants recorded CBG.
Blood Sample Analysis
Samples from visit 2 were transported to Exeter Clinical Laboratories for analysis of serum C-peptide, glucagon, and autoantibodies. C-peptide was analyzed using a direct electrochemiluminescence immunoassay (E170 analyzer; Roche Diagnostics, Mannheim, Germany) as previously described (21). Lower limit of detection was 3.3 pmol/L with a reported intra- and interassay CV of 3.3% and 4.5%, respectively (22). Individuals’ peak serum C-peptide recorded during the MMTT was used to confirm which C-peptide group participants were sorted into: undetectable (Cpepund), <3 pmol/L; low (Cpeplow), 3–200 pmol/L; and high (Cpephigh), >200 pmol/L. The high C-peptide grouping was based upon the clinically significant threshold found in the DCCT (10), while the low C-peptide threshold was based on the lower limit of detection of the assay. Serum glucagon was measured using a Glucagon ELISA (Mercodia AB, Uppsala, Sweden) on the Dynex DS2 automated platform (Dynex Technologics, Worthing, U.K.) with a lower limit of detection of 1.5 pmol/L.
Autoantibody analysis was performed using ELISA assays (RSR Ltd., Cardiff, U.K.) on the DS2 automated platform (Dynex Technologics) as previously reported (23). The cutoffs for positivity were ≥7.5 units/mL (IA-2), ≥11 units/mL (GAD65), and ≥65 units/mL (ZnT8) for subjects aged <30 years or ≥9.1 units/mL for those aged >30 years. Positive result was defined as >97.5th centile of 1,559 control subjects without diabetes (23).
Statistical and Data Analysis
Data are presented as mean ± SD throughout unless otherwise stated, with statistical significance set at P < 0.05. The primary outcome was amount of time with an interstitial glucose in a euglycemia range (3.9–10 mmol/L) in the 24 h postexercise. Secondary outcomes were euglycemia at 12 h and glycemic variability (SD and CV), time spent in hypoglycemia, and time spent in hyperglycemia in the 12 and 24 h postexercise. CGM ranges were defined as 3.9–10 mmol/L (euglycemia), <3.9 mmol/L (hypoglycemia 1), <3.0 mmol/L (hypoglycemia 2), >10 mmol/L (hyperglycemia 1), and >13.9 mmol/L (hyperglycemia 2) as recommended by international consensus (24). CV was calculated as SD divided by mean glucose.
Statistically significant differences between the means of Cpepund, Cpeplow, and Cpephigh were determined by one-way ANOVA with Tukey post hoc analysis. Data were assessed for normality and outliers by Shapiro-Wilk test and box plots, with skewed data assessed by Kruskal-Wallis H test. Pearson product-moment or Spearman rank-order correlation was used to determine the strength and direction of a linear relationship between peak MMTT serum C-peptide and glucagon versus CGM data. GraphPad Prism 8.0.1 (GraphPad Software, San Diego, CA) and SPSS Statistics (version 24; IBM, Armonk, NY) software package were used to analyze the data.
Results
Three participants who were initially recruited with a low UCPCR subsequently demonstrated an undetectable peak serum C-peptide. Additionally, two participants with undetectable UCPCR subsequently showed low C-peptide positivity during the MMTT.
Participants were allocated into three groups according to MMTT peak serum C-peptide. Demographic and MMTT group data are shown in Table 1. Age, HbA1c, BMI, insulin, and VO2peak were comparable between groups. However, the Cpephigh group had significantly higher age of diagnosis and shorter duration of diabetes than the Cpepund. Although C-peptide metrics differed between groups (in keeping with the study design), MMTT glucagon values were comparable. Fasting glucose was comparable at baseline of the MMTT, with the Cpephigh group having significantly lower peak and change (Δ) compared with the Cpepund.
Table 1.
Demographic and MMTT results for each C-peptide grouping
| Cpepund | Cpeplow | Cpephigh | P | |
|---|---|---|---|---|
| N | 11 | 9 | 10 | |
| n male/n female | 5/6 | 6/3 | 5/5 | |
| Age (years) | 40.09 ± 11.18 (26–58) | 38.67 ± 14.73 (25–61) | 35.80 ± 10.98 (18–52) | 0.738 |
| Age at diagnosis (years) | 13.27 ± 4.50 (8–24) | 16.56 ± 8.57 (8–32) | 25.10 ± 8.20* (13–35) | 0.003 |
| Duration of diabetes (years) | 26.82 ± 13.24 (13–47) | 21.89 ± 13.34 (9–44) | 10.70 ± 6.15* (3–20) | 0.015 |
| HbA1c (mmol/mol) | 61.64 ± 10.64 (42–78) | 58.11 ± 7.11 (51–74) | 55.40 ± 8.47 (41–69) | 0.297 |
| HbA1c (%) | 7.8 ± 3.1 (6.0–9.3) | 7.5 ± 2.8 (6.8–8.9) | 7.2 ± 2.9 (5.9–8.5) | |
| BMI (kg/m2) | 25.65 ± 3.27 | 24.20 ± 4.13 | 25.67 ± 4.04 | 0.259 |
| Daily insulin (units) | 39.93 ± 15.15 | 47.88 ± 23.21 | 38.30 ± 31.23 | 0.242 |
| Insulin units/kg/day | 0.54 ± 0.19 | 0.63 ± 0.25 | 0.49 ± 0.29 | 0.332 |
| Method of control (n MDI/n CSII) | 5/6 | 4/5 | 6/4 | |
| VO2peak (mL/kg/min) | 35.61 ± 7.69 (21.05–49.00) | 43.93 ± 9.03 (31.80–58.25) | 35.67 ± 10.77 (21.25–51.00) | 0.194 |
| MMTT | ||||
| Peak C-peptide (pmol/L) | 0.00 ± 0.00 (0–0) | 42.00 ± 32.58* (4–83) | 671.70 ± 435.15*† (221–1,640) | <0.001 |
| Median C-peptide | 0.00 | 53.00 | 568.50 | |
| AUC0–180 min C-peptide (pmol/L) | 0.00 ± 0.00 | 6,026 ± 4,452* | 89,459 ± 48,095*† | <0.001 |
| Peak glucagon (pmol/L) | 14.04 ± 6.74 | 18.60 ± 13.49 | 12.45 ± 4.34 | 0.802 |
| AUC0–180 min glucagon (pmol/L) | 1,557 ± 905.8 | 2,072 ± 1,370 | 1,259 ± 674.5 | 0.252 |
| Pre-MMTT glucose (mmol/L) | 10.12 ± 3.38 | 9.55 ± 1.62 | 8.47 ± 3.15 | 0.428 |
| Peak glucose (mmol/L) | 21.91 ± 2.75 | 20.03 ± 2.34 | 17.74 ± 3.59* | 0.016 |
| ΔPre-MMTT to peak glucose (mmol/L) | 11.76 ± 2.77 | 10.48 ± 2.12 | 9.27 ± 3.02* | 0.045 |
| Autoantibody positivity | 6 of 11 | 7 of 9 | 8 of 10 |
Data are means ± SD unless otherwise indicated (data in parentheses are ranges). Boldface type indicates statistically significant P values. CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections.
Significantly different from Cpepund.
Significantly different from Cpeplow.
Observational Week
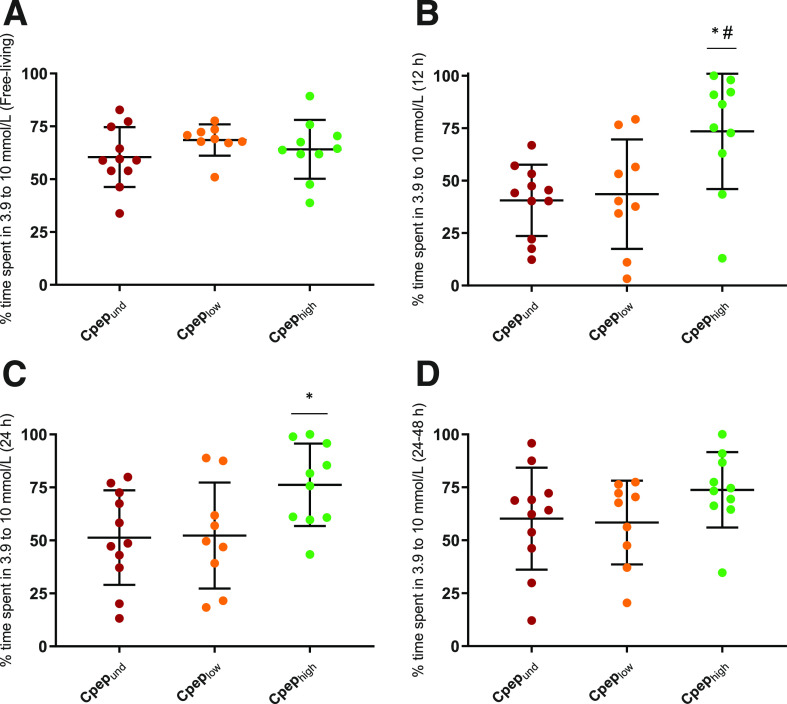
Data were collected for mean ± SD 5.1 ± 0.96 days, with no differences between groups (P = 0.730). During the observational week, there were no differences between the C-peptide groups in time spent in euglycemia (Fig. 1A), hypoglycemia, or hyperglycemia; mean glucose; SD; or CV. MMTT C-peptide and glucagon values did not predict any CGM outcomes during the observational week (P > 0.05) (Table 2).
Figure 1.
Group mean ± SD and individual data points for time spent in a euglycemic range, 3.9–10 mmol/L, during the observational free-living week (A), 12 h post–submaximal exercise bout (B), 24 h post–submaximal exercise bout (C), and between 24 and 48 h post–submaximal exercise bout (D). Cpepund, n = 11; Cpeplow, n = 9; Cpephigh, n = 10. *Significantly different from Cpepund; #significantly different from Cpeplow.
Table 2.
One-way ANOVA results for the CGM outcomes of each C-peptide grouping at different time points
| Free-living observational week | 12 h postexercise | 24 h postexercise | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cpepund | Cpeplow | Cpephigh | P | Cpepund | Cpeplow | Cpephigh | P | Cpepund | Cpeplow | Cpephigh | P | |
| % time <3 mmol/L | 0.7 ± 1.4 | 1.3 ± 1.9 | 0.9 ± 1.2 | 0.710 | 0.7 ± 2.4 | 3.0 ± 8.4 | 0.0 ± 0.0 | 0.284 | 1.3 ± 3.7 | 5.3 ± 15.4 | 0.5 ± 1.5 | 0.773 |
| % time <3.9 mmol/L | 3.5 ± 3.2 | 8.7 ± 9.7 | 5.7 ± 5.4 | 0.540 | 3.6 ± 5.1 | 5.9 ± 9.1 | 1.9 ± 3.2 | 0.586 | 3.2 ± 5.1 | 9.3 ± 16.2 | 4.1 ± 9.8 | 0.471 |
| % time >10 mmol/L | 36.1 ± 14.7 | 22.8 ± 10.0 | 30.2 ± 16.3 | 0.129 | 55.8 ± 17.5 | 50.5 ± 30.3 | 24.6 ± 27.6* | 0.015 | 45.5 ± 23.5 | 38.4 ± 24.8 | 19.7 ± 19.6* | 0.043 |
| % time >13.9 mmol/L | 8.8 ± 5.9 | 4.3 ± 3.4 | 6.8 ± 8.6 | 0.206 | 20.2 ± 15.7 | 23.6 ± 18.1 | 2.3 ± 6.0*† | 0.001 | 12.0 ± 10.2 | 19.1 ± 20.9 | 1.3 ± 3.2*† | 0.001 |
| Mean glucose (mmol/L) | 9.1 ± 1.2 | 7.8 ± 1.3 | 8.5 ± 1.6 | 0.149 | 10.7 ± 1.6 | 10.7 ± 2.9 | 8.2 ± 1.6* | 0.006 | 9.8 ± 1.7 | 9.6 ± 3.0 | 7.7 ± 1.5 | 0.065 |
| SD (mmol/L) | 3.2 ± 0.6 | 3.0 ± 0.6 | 3.1 ± 0.6 | 0.604 | 3.4 ± 1.2 | 3.7 ± 1.0 | 2.0 ± 1.0*† | 0.003 | 3.0 ± 0.9 | 3.8 ± 1.0 | 2.0 ± 0.7*† | <0.001 |
| CV (%) | 36.7 ± 7.6 | 38.2 ± 7.3 | 36.5 ± 6.0 | 0.848 | 32.5 ± 11.5 | 36.8 ± 14.2 | 24.8 ± 9.9 | 0.098 | 31.9 ± 10.8 | 42.1 ± 15.4 | 26.2 ± 9.6† | 0.025 |
Data are means ± SD.
Boldface type indicates statistically significant P values.
Significantly different from Cpepund.
Significantly different from Cpeplow.
Laboratory Phase: Exercise Bout
On average, participants exercised at mean ± SD 59.4 ± 4.1% of their VO2peak, with no differences between the C-peptide groups (P = 0.542). The Cpepund group had higher CBG on arrival (Cpepund 9.83 ± 2.17, Cpeplow 7.96 ± 3.11, Cpephigh 7.25 ± 1.52 mmol/L, P = 0.045), pre-exercise (Cpepund 11.42 ± 2.76, Cpeplow 9.37 ± 1.61, Cpephigh 8.30 ± 1.14 mmol/L, P = 0.007), and postexercise (Cpepund 13.00 ± 4.38, Cpeplow 9.26 ± 4.37, Cpephigh 9.00 ± 2.83 mmol/L, P = 0.048), as well as on leaving the laboratory at 1 h postexercise (Cpepund 13.34 ± 3.21, Cpeplow 11.23 ± 3.86, Cpephigh 9.32 ± 2.58 mmol/L, P = 0.029), compared with the Cpephigh but not the Cpeplow group. There were no incidences of hypoglycemia within the laboratory phase of the study either during the exercise or throughout the 60-min postexercise recovery. Six participants (one Cpepund, two Cpeplow, and three Cpephigh) were given 10 g additional carbohydrates during the exercise bout, as their blood glucose had dropped to <7 mmol/L.
Postexercise
Twelve- and 24-h postexercise interstitial glucose responses are presented in Fig. 1B and C and Table 2. The Cpephigh group spent mean ± SD 73.51 ± 27.64% of the 12 h postexercise in a state of euglycemia compared with 43.58 ± 26.07% for Cpeplow (P = 0.027) and 40.61 ± 16.97% for Cpepund (P = 0.010) (Fig. 1B). The Cpephigh group also had significantly less time spent in a state of hyperglycemia (categories 1 and 2) and lower mean glucose and SD compared with Cpeplow and Cpepund (P < 0.05). No difference existed between groups for time spent with CGM glucose <3.9 mmol/L (P = 0.766) or <3.0 mmol/L (P = 0.370), although, notably, mean time with CGM <3.0 mmol/L was zero in the Cpephigh group.
Similar patterns were observed in the interstitial glucose response in the 24-h postexercise period, with the Cpephigh group having more time in a state of euglycemia (76.25 ± 19.16%) than Cpepund (51.33 ± 22.26%, P = 0.041), although not statistically more than Cpeplow (52.31 ± 24.98%, P = 0.067) (Fig. 1C). Cpephigh had significantly less time spent in a state of hyperglycemia and reduced measures of glycemic variability compared with both Cpeplow and Cpepund.
In the 24–48 h following the exercise bout, the effects were largely lost, with only time spent with glucose >13.9 mmol/L and SD significantly lower in the Cpephigh group compared with Cpepund and Cpeplow (Table 2 and Fig. 1D).
Peak stimulated glucagon was comparable across groups and did not predict time in hypoglycemia or any CGM measure postexercise (P > 0.05).
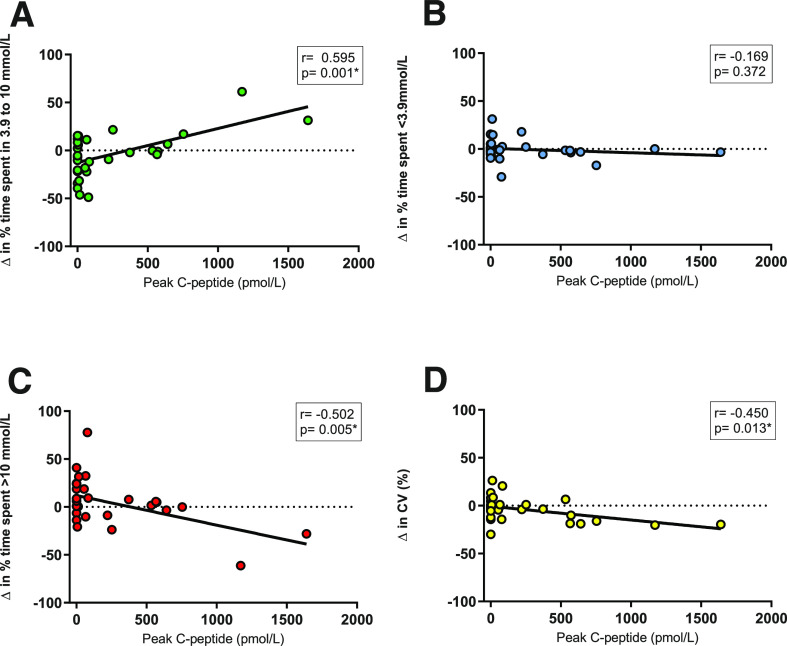
Change (Δ) in interstitial glucose parameters from the observational week to 24 h postexercise showed significant correlations between peak C-peptide and time in euglycemia (Fig. 2A), time spent with glucose >10 mmol/L (Fig. 2C), time spent with glucose >13.9 mmol/L, and measures of glucose variability (Fig. 2D).
Figure 2.
Scatterplots displaying linear relationships between peak serum C-peptide vs. the Δ in glycemic control measures from the free-living observational week to the 24 h postexercise (n = 30). Δ in the percentage of time spent in 3.9–10 mmol/L range (A), Δ in the percentage of time spent <3.9 mmol/L (B), Δ in the percentage of time spent >10 mmol/L (C), and Δ in the CV (%) (D). *Significant correlation.
The Cpephigh group had increased percentage of time in euglycemia in the 24 h following the exercise bout compared with their free-living observational week (Δ12.11 ± 21.54%), whereas individuals in the Cpeplow (Δ−16 ± 24%, P = 0.018) and Cpepund (Δ−9.1 ± 18%, P = 0.073) groups had reduced time in euglycemia compared with the observational week.
Autoantibody Status
Individual autoantibody positivity status is displayed in Supplementary Table 1. Nine of the 30 participants were autoantibody negative, including 2 participants within the Cpephigh group (duration of diabetes 17 and 20 years and peak C-peptide 532 and 1,170 pmol/L, respectively). To reduce the possibility of misdiagnoses of type 2 or monogenic diabetes influencing the results, we reassessed the data excluding these participants.
Between-group differences within the first 12 h postexercise mirrored those seen within the whole group analysis, with time spent in euglycemia significantly higher for Cpephigh than Cpeplow and Cpepund (P = 0.023). When extended out to 24 h, the trends persisted, with clinically relevant, but not statistically significant, mean ± SD differences (Cpepund 51.33 ± 22.26%, Cpeplow 52.31 ± 24.98%, and Cpephigh 73.35 ± 19.88%, P = 0.093). Furthermore, the same relationships exist between C-peptide and Δ from the observational week to 24 h postexercise for euglycemia (r = 0.473, P = 0.041), <3.9 mmol/L (r = −0.192, P = 0.328), >10 mmol/L (r = −0.355, P = 0.064), and CV (r = −0.432, P = 0.022).
Conclusions
We investigated how residual β-cell function impacts CGM outcomes following exercise in people with T1D. We show in the cohort studied that under free-living conditions, time in euglycemia is comparable despite wide-ranging residual β-cell function. Regardless, and for the first time, we demonstrate that individuals with T1D with higher residual β-cell function (stimulated C-peptide >200 pmol/L) displayed a substantially greater amount of time spent in euglycemia in the hours following a bout of moderate-intensity exercise. Furthermore, we show divergence in the impact of exercise on glycemic profiles, with high residual C-peptide associated with improved control compared with pre-exercise free-living conditions and low/absent C-peptide associated with worsened control following exercise.
Results from the baseline observational free-living CGM data are similar to those of Rickels et al. (14). While they demonstrated that individuals with C-peptide >400 pmol/L spent more time in a state of euglycemia under free-living conditions, there were no differences between their negative, low (17–200 pmol/L), and what they have defined as intermediate (200–400 pmol/L) groups. Participants in the current study were all attending a single diabetes center. They had mainly good to moderate HbA1c and similar insulin treatment, with access to the same clinical management and education. These factors likely contributed to the comparable time in euglycemia, despite different levels of C-peptide, under these stable free-living conditions.
Our primary findings that individuals with higher C-peptide had substantially increased time in euglycemia postexercise compared with those with lower C-peptide, in addition to the clear divergence in whether there is a positive or negative impact of exercise on CGM parameters depending on residual C-peptide status, have not previously been reported. These findings were despite the cohort having comparable free-living CGM outcomes and HbA1c. We hypothesize that the endogenous insulin secretion within the Cpephigh group combined with increased insulin sensitivity following the exercise bout attenuated high blood glucose excursions. Indeed, the results from the MMTT demonstrated an attenuated glucose response within the high C-peptide group. Exercise can independently increase glucose uptake into the skeletal muscles via the redistribution of GLUT4 to the cell membrane (25). A single bout of endurance exercise also increases insulin’s action (26), with sensitivity to insulin persisting up to 48 h postexercise (27). These mechanisms may contribute to the difficulties in maintaining time in euglycemia after exercise in those with low C-peptide, while enhancing the beneficial impact of endogenous insulin secretion within individuals with higher C-peptide.
Authors from previous secondary analysis of glycemic control during and after exercise have postulated that insulin resistance may play a role in the interindividual variability (28). As a longer duration of diabetes is associated with increased insulin resistance (29), and the Cpephigh group had a lower mean duration, this study cannot rule out the role that insulin resistance plays in postexercise glycemic control. However, it is important to note that the mean ± SD BMI (25.22 ± 3.73 kg/m2), total daily insulin dose (41.77 ± 23.40 units), and dose per kilogram (0.55 ± 0.24 units/kg/day) were comparable across groups and were not high enough to indicate insulin resistance.
Avoidance of hypoglycemia, in everyday life as well as during and after exercise, is of central importance for people with T1D. A wide range of methods, including nutritional and insulin adjustments, have been reported and discussed, yet difficulties in maintaining euglycemia during and following exercise are prevalent (2). Previous studies have reported that preserved β-cell function was associated with reduced self-reported hypoglycemia (30,31); however, neither this study nor previous studies have seen time spent in hypoglycemia as measured by CGM influenced by C-peptide (14). In the current study, time spent in hypoglycemia (<3.9 and 3 mmol/L) in the postexercise period was ≥2.0-fold less in the Cpephigh group, which may be clinically meaningful although it is not statistically different. Future studies should carefully consider how to most meaningfully measure hypoglycemia in free-living conditions, with a combination of CGM and diaries likely to be needed (32).
This study provides further evidence that the paradoxical glucagon secretion in response to oral ingestion is not influenced by C-peptide status and that peak glucagon measured by these methods does not associate with time spent in a state of hypoglycemia (14,33). However, recent research demonstrates that during a hyperinsulinemic-hypoglycemic clamp, those with persistent β-cell function have residual counterregulatory responses to hypoglycemia including increased glucagon (34). Additionally, there is a reduction in biochemical hypoglycemia and an increase in glucagon response to hypoglycemic clamp in C-peptide–positive islet transplant recipients (16). The α-cell’s ability to secrete glucagon in response to hypoglycemia is impaired around diagnosis of T1D (35), with further functional losses as duration of diabetes increases (36). It is hypothesized that functioning β-cells within the islets of Langerhans enable residual α-cell function, allowing some hypoglycemia protection, although underlying mechanisms remain unclear (37). Whether responses to a hyperinsulinemic clamp have a significant impact in real-world conditions requires studies such as the current one.
To further understand the participants’ responses in our study, autoantibody status was assessed to minimize the possibility of misdiagnosed diabetes impacting the results, despite a large proportion of individuals with T1D being autoantibody negative with this longer duration of the disease (38). Even in the Cpephigh group, the two autoantibody-negative participants met our inclusion criteria of classical presentation of T1D at diagnosis. When these participants were excluded, similar patterns were observed, with residual β-cell function associated with postexercise CGM outcomes. Moreover, the same positive relationship between C-peptide and the Δ in free-living to 24-h postexercise euglycemia exists. Limitations of this study include participants being a single cohort from the same diabetes center and predominantly being in moderate or good control. While the CGM capture was largely from free-living periods, the exercise bout was laboratory based with carefully managed blood glucose. It thus remains unclear whether results can be generalized to the wider exercising population with T1D.
Keeping in mind the potential for residual β-cell function to help stabilize time in euglycemia during and after exercise, future research should explore longer-term exercise and its associations with hypoglycemia. Previous studies have demonstrated that exercise can blunt counterregulatory responses to subsequent hypoglycemia (39) and, conversely, that antecedent hypoglycemia can blunt hormone responses to exercise (40). Potentially, residual β-cell function may limit the burden of hypoglycemia by preserving some of these counterregulatory responses to repeated bouts of physiological stress, helping facilitate effective and safe long-term exercise. Investigations into whether residual β-cell function influences the glycemic responses to differing modalities of exercise (i.e., resistance, high-intensity intermittent training), as well as under a range of different insulin and nutritional strategies used before, during, and after exercise (i.e., fasted morning exercise) are warranted. Finally, a large long-term trial is needed to explore whether C-peptide predicts HbA1c changes with exercise, as well as to explore further glycemic and cardiovascular outcomes, teasing apart whether reported improvements in diabetes complications are due to glycemic improvements or potentially a direct impact of C-peptide upon vasculature.
In conclusion, people with T1D who have higher residual β-cell function show improved time in euglycemia following exercise. C-peptide may be useful in identification of patients most at risk for exercise-associated dysglycemia. We show that future exercise research should consider level of C-peptide as a factor that may impact study outcomes.
Article Information
Acknowledgments. The authors thank the study participants for their time, effort, and commitment, as well as the research team at the NIHR Newcastle Clinical Research Facility, Newcastle-upon-Tyne, U.K., for their assistance.
Funding. This study was funded by the Diabetes Research and Wellness Foundation (SCA/OF/12/15) award to D.J.W. CGM equipment was provided by an equipment award to D.J.W. by Medtronic UK.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.S.T. recruited participants, designed the study, researched data, and wrote the manuscript. D.J.W. designed the study, researched data, and wrote the manuscript. J.A.S. recruited participants, designed the study, provided clinical cover, and reviewed and edited the manuscript. A.B. and A.F. recruited participants, provided clinical cover, and reviewed and edited the manuscript. T.J.M. and R.A.O. analyzed samples and reviewed and edited the manuscript. E.J.S. reviewed and edited the manuscript. K.S., T.E.C., and J.H.S. contributed to data collection and reviewed and edited the manuscript. D.J.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
Clinical trial reg. no. ISRCTN50072340, www.isrctn.org
This article contains supplementary material online at https://doi.org/10.2337/figshare.12616118.
References
- 1.Colberg SR, Sigal RJ, Yardley JE, et al. . Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riddell MC, Gallen IW, Smart CE, et al. . Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol 2017;5:377–390 [DOI] [PubMed] [Google Scholar]
- 3.Bohn B, Herbst A, Pfeifer M, et al.; DPV Initiative . Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross-sectional multicenter study of 18,028 patients. Diabetes Care 2015;38:1536–1543 [DOI] [PubMed] [Google Scholar]
- 4.Temple MYM, Bar-Or O, Riddell MC. The reliability and repeatability of the blood glucose response to prolonged exercise in adolescent boys with IDDM. Diabetes Care 1995;18:326–332 [DOI] [PubMed] [Google Scholar]
- 5.Abraham MB, Davey RJ, Cooper MN, et al. . Reproducibility of the plasma glucose response to moderate-intensity exercise in adolescents with type 1 diabetes. Diabet Med 2017;34:1291–1295 [DOI] [PubMed] [Google Scholar]
- 6.Tansey MJ, Tsalikian E, Beck RW, et al.; Diabetes Research in Children Network (DirecNet) Study Group . The effects of aerobic exercise on glucose and counterregulatory hormone concentrations in children with type 1 diabetes. Diabetes Care 2006;29:20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilbride L, Charlton J, Aitken G, Hill GW, Davison RC, McKnight JA. Managing blood glucose during and after exercise in type 1 diabetes: reproducibility of glucose response and a trial of a structured algorithm adjusting insulin and carbohydrate intake. J Clin Nurs 2011;20:3423–3429 [DOI] [PubMed] [Google Scholar]
- 8.Williams GM, Long AE, Wilson IV, et al. . Beta cell function and ongoing autoimmunity in long-standing, childhood onset type 1 diabetes. Diabetologia 2016;59:2722–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oram RA, McDonald TJ, Shields BM, et al.; UNITED Team . Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care 2015;38:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lachin JM, McGee P, Palmer JP; DCCT/EDIC Research Group . Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 2014;63:739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis AK, DuBose SN, Haller MJ, et al.; T1D Exchange Clinic Network . Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 2015;38:476–481 [DOI] [PubMed] [Google Scholar]
- 12.Buckingham B, Cheng P, Beck RW, et al.; Diabetes Research in Children Network (DirecNet) and Type 1 Diabetes TrialNet Study Groups . CGM-measured glucose values have a strong correlation with C-peptide, HbA1c and IDAAC, but do poorly in predicting C-peptide levels in the two years following onset of diabetes. Diabetologia 2015;58:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks AM, Oram R, Home P, Steen N, Shaw JA. Demonstration of an intrinsic relationship between endogenous C-peptide concentration and determinants of glycemic control in type 1 diabetes following islet transplantation. Diabetes Care 2015;38:105–112 [DOI] [PubMed] [Google Scholar]
- 14.Rickels MR, Evans-Molina C, Bahnson HT, et al.; T1D Exchange β-Cell Function Study Group . High residual C-peptide likely contributes to glycemic control in type 1 diabetes. J Clin Invest 2020;130:1850–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besser RE, Ludvigsson J, Jones AG, et al. . Urine C-peptide creatinine ratio is a noninvasive alternative to the mixed-meal tolerance test in children and adults with type 1 diabetes. Diabetes Care 2011;34:607–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schölin A, Berne C, Schvarcz E, Karlsson FA, Björk E. Factors predicting clinical remission in adult patients with type 1 diabetes. J Intern Med 1999;245:155–162 [DOI] [PubMed] [Google Scholar]
- 17.Medtronic CareLink iPro User Guide - 8-Sep-2017 [Internet], 2017. Available from http://www.medtronicdiabetes.com/download-library/ipro-2. Accessed 4 February 2020
- 18.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al.; Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group . Mixed-meal tolerance test versus glucagon stimulation test for the assessment of β-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 1973;85:546–562 [DOI] [PubMed] [Google Scholar]
- 20.Glass S, Dwyer GB, American College of Sports Medicine. ACSM’S Metabolic Calculations Handbook, Baltimore, MD, Lippincott Williams & Wilkins, 2007 [Google Scholar]
- 21.Oram RA, Jones AG, Besser RE, et al. . The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014;57:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hope SV, Knight BA, Shields BM, Hattersley AT, McDonald TJ, Jones AG. Random non-fasting C-peptide: bringing robust assessment of endogenous insulin secretion to the clinic. Diabet Med 2016;33:1554–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald TJ, Colclough K, Brown R, et al. . Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from type 1 diabetes. Diabet Med 2011;28:1028–1033 [DOI] [PubMed] [Google Scholar]
- 24.Danne T, Nimri R, Battelino T, et al. . International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douen AG, Ramlal T, Rastogi S, et al. . Exercise induces recruitment of the “insulin-responsive glucose transporter”. Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem 1990;265:13427–13430 [PubMed] [Google Scholar]
- 26.Gulve EA, Cartee GD, Zierath JR, Corpus VM, Holloszy JO. Reversal of enhanced muscle glucose transport after exercise: roles of insulin and glucose. Am J Physiol 1990;259:E685–E691 [DOI] [PubMed] [Google Scholar]
- 27.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 1988;254:E248–E259 [DOI] [PubMed] [Google Scholar]
- 28.Tagougui S, Goulet-Gelinas L, Taleb N, Messier V, Suppere C, Rabasa-Lhoret R. Association between body composition and blood glucose during exercise and recovery in adolescent and adult patients with type 1 diabetes. Can J Diabetes 2020;44:192–195 [DOI] [PubMed] [Google Scholar]
- 29.Teixeira MM, Diniz MdeF, Reis JS, et al. . Insulin resistance and associated factors in patients with type 1 Diabetes. Diabetol Metab Syndr 2014;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marren SM, Hammersley S, McDonald TJ, et al.; TIGI Consortium . Persistent C-peptide is associated with reduced hypoglycaemia but not HbA1c in adults with longstanding type 1 diabetes: evidence for lack of intensive treatment in UK clinical practice? Diabet Med 2019;36:1092–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhtreiber WM, Washer SL, Hsu E, et al. . Low levels of C-peptide have clinical significance for established type 1 diabetes. Diabet Med 2015;32:1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriksen MM, Andersen HU, Thorsteinsson B, Pedersen-Bjergaard U. Asymptomatic hypoglycaemia in type 1 diabetes: incidence and risk factors. Diabet Med 2019;36:62–69 [DOI] [PubMed] [Google Scholar]
- 33.Thivolet C, Marchand L, Chikh K. Inappropriate glucagon and GLP-1 secretion in individuals with long-standing type 1 diabetes: effects of residual C-peptide. Diabetologia 2019;62:593–597 [DOI] [PubMed] [Google Scholar]
- 34.Zenz S, Mader JK, Regittnig W, et al. . Impact of C-peptide status on the response of glucagon and endogenous glucose production to induced hypoglycemia in T1DM. J Clin Endocrinol Metab 2018;103:1408–1417 [DOI] [PubMed] [Google Scholar]
- 35.Arbelaez AM, Xing D, Cryer PE, et al.; Diabetes Research in Children Network (DirecNet) Study Group . Blunted glucagon but not epinephrine responses to hypoglycemia occurs in youth with less than 1 yr duration of type 1 diabetes mellitus. Pediatr Diabetes 2014;15:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siafarikas A, Johnston RJ, Bulsara MK, O’Leary P, Jones TW, Davis EA. Early loss of the glucagon response to hypoglycemia in adolescents with type 1 diabetes. Diabetes Care 2012;35:1757–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes 2010;59:2333–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tridgell DM, Spiekerman C, Wang RS, Greenbaum CJ. Interaction of onset and duration of diabetes on the percent of GAD and IA-2 antibody-positive subjects in the Type 1 Diabetes Genetics Consortium database. Diabetes Care 2011;34:988–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandoval DA, Guy DL, Richardson MA, Ertl AC, Davis SN. Acute, same-day effects of antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes mellitus. Am J Physiol Endocrinol Metab 2006;290:E1331–E1338 [DOI] [PubMed] [Google Scholar]
- 40.Galassetti P, Tate D, Neill RA, Morrey S, Wasserman DH, Davis SN. Effect of antecedent hypoglycemia on counterregulatory responses to subsequent euglycemic exercise in type 1 diabetes. Diabetes 2003;52:1761–1769 [DOI] [PubMed] [Google Scholar]