In spite of being at target for glucose (1) or traditional cardiovascular (CV) risk factors (2), individuals with type 1 diabetes (T1D) still have an excess of CV mortality and morbidity implying a role for other mechanisms including insulin resistance (IR). Impaired insulin action in T1D was established by clamp technique long ago (3). Estimated glucose disposal rate (eGDR) correlates well with the clamp technique (4) and is a risk marker for microangiopathy (5,6), diabetic kidney disease (DKD) (6), CV risk, and mortality (5,7).
In this observational single-center study, we investigated to what extent eGDR is a predictor of CV events, coronary artery disease (CAD), and all-cause mortality irrespective of CV risk factors and DKD in 774 subjects with T1D over a 10-year follow-up, as previously described (8). eGDR (mg/kg/min) was calculated at baseline as follows (4): eGDR = 21.158 − (0.09 × WC) − (3.407 × HTN) − (0.551 × HbA1c), where WC is waist circumference (cm), HTN is hypertension (yes = 1, no = 0), and HbA1c is in %. Follow-up data were retrieved from the national and regional health care registers (ICD-9, Clinical Modification, codes) by searching for CV outcomes (primary outcome) up to 31 December 2017 and for all-cause death up to 31 October 2018. Incidence of CV outcomes was available for 736 participants (95.1%), and vital status was available for all individuals (8). We used univariate and multivariate Cox proportional hazards models to identify key covariates, with impact of eGDR evaluated for each SD. Results are expressed as hazard ratio (HR) and 95% CI. A two-sided P value ≤0.05 was considered significant.
At baseline, as previously reported (8), mean ± SD age was 40.2 ± 11.7 years, diabetes duration was 19.4 ± 12.2 years, and HbA1c was 7.8 ± 1.2% (62.1 ± 12.9 mmol/mol); 52.6% were male, and 10.6% had DKD. Mean eGDR was 7.52 ± 2.28 mg/kg/min (median 8.29 mg/kg/min [interquartile range 5.54–9.31]) with bimodal distribution. Overall, the lower the eGDR, the worse the CV risk profile. For proper assessment of the most reliable relationship between eGDR and outcomes, eGDR was included into Cox models as a linear or quadratic term, as both linear and quadratic terms, and as square root. Goodness of fit was evaluated by Akaike information criterion. The best fitting model, the one minimizing Akaike information criterion, was the linear model for all outcomes. The shape of these relationships is reported in Fig. 1.
Figure 1.
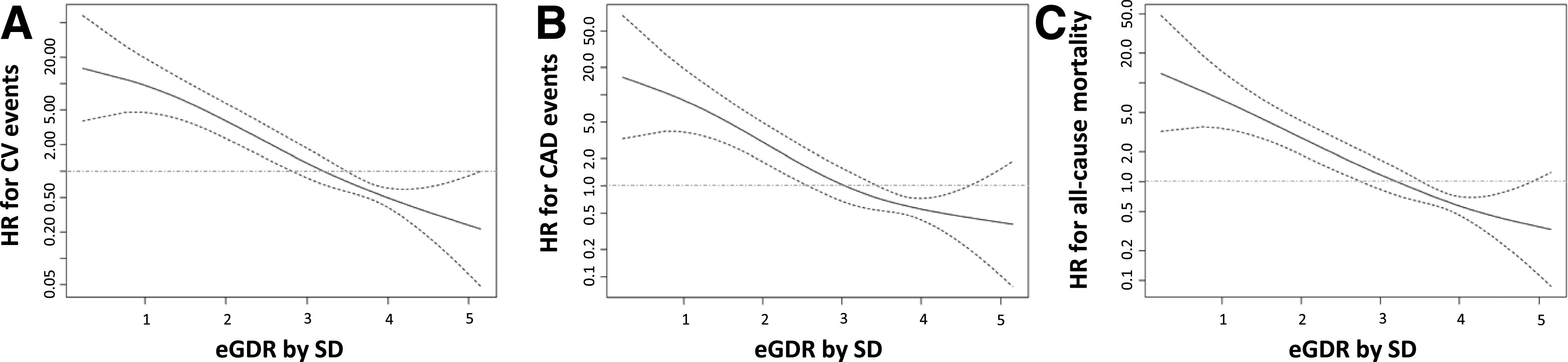
The spline plots display the HR (solid black lines) and 95% CIs (dashed lines) for the association between the baseline eGDR (expressed as SD) and major CV events (panel A: 49 events [6.7%], incidence density 6.35 × 1,000 person-years, mean ± SD 10.4 ± 2.9 years of follow-up), CAD (panel B: 35 events [4.8%], incidence density 4.50 × 1,000 person-years, 10.5 ± 2.6 years of follow-up), and all-cause mortality (panel C: 57 deaths [7.4%], incidence density 6.4 × 1,000 person-years, 11.6 ± 2.6 years of follow-up). The baseline eGDR was modeled using penalized spline in a Cox regression model. The eGDR by SD reference levels were set at their mean, i.e., 3.3 SD, for major CV events, CAD events, and all-cause mortality, respectively, for the estimation of HRs. The gray lines represent an HR of 1.0. y-axes were appropriately reported in a log scale.
Rates and incidence density of outcomes are given in the Fig. 1 legend. eGDR was an independent covariate of CV events in all regression models and remained so after adjustment for IR-related variables (models 4 or 5: HDL cholesterol, triacylglycerol, and urinary albumin-to-creatinine ratio [uACR] or DKD), yielding, in model 5, an HR 0.56 (95% CI 0.39–0.80; P = 0.002) with independent effects for age, prior CV events, and DKD (Table 1). An independent role of eGDR was confirmed for CAD (HR 0.63, 95% CI 0.42–0.96; P = 0.033), with independent effects for the same covariates (Table 1). Finally, eGDR remained independently associated with all-cause mortality after adjustment for several CV risk factors (model 3 HR 0.66, 95% CI 0.48–0.91; P = 0.011) but not after further correction for uACR or DKD, HDL cholesterol, and triacylglycerol.
Table 1.
Outcomes analyses by unadjusted and adjusted Cox regression models according to eGDR by SD
| HR (95% CI) | |||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Major CV events | |||||
| eGDR, 1 SD | 0.36 (0.27–0.48)* | 0.45 (0.32–0.62)* | 0.53 (0.37–0.75)* | 0.57 (0.39–0.82)† | 0.56 (0.39–0.80)† |
| Age, 1 year | 1.06 (1.04–1.09) | 1.04 (1.01–1.06) | 1.04 (1.01–1.06) | 1.04 (1.01–1.06) | |
| Sex, male | 0.96 (0.55–1.70) | — | — | — | |
| Prior CV disease | 3.74 (1.88–7.43) | 4.33 (2.16–8.69) | 4.64 (2.32–9.28) | ||
| Retinopathy | — | ||||
| No retinopathy | 1 | 1 | |||
| Nonadvanced | 2.11 (0.90–4.94) | 2.08 (0.89–4.87) | |||
| Advanced | 3.46 (1.50–7.99) | 2.84 (1.19–6.78) | |||
| eGFR (CKD-EPI), mL/min/1.73 m2 | 0.99 (0.98–1.01) | 1.00 (0.98–1.02) | |||
| uACR, mg/mmol | 1.02 (1.01–1.03) | ||||
| DKD | 3.03 (1.61–5.71) | ||||
| CAD events | |||||
| eGDR, 1 SD | 0.40 (0.28–0.56)* | 0.49 (0.34–0.72)* | 0.57 (0.38–0.86)‡ | 0.63 (0.41–0.96)§ | 0.63 (0.42–0.96)§ |
| Age, 1 year | 1.06 (1.02–1.09) | 1.03 (1.00–1.06) | 1.04 (1.01–1.07) | 1.03 (1.00–1.06) | |
| Sex, male | 1.37 (0.69–2.74) | — | — | — | |
| Prior CV disease | 4.26 (1.84–9.85) | 6.10 (2.61–14.24) | 5.12 (2.21–11.86) | ||
| Retinopathy | — | — | |||
| No retinopathy | 1 | ||||
| Nonadvanced | 1.26 (0.48–3.31) | ||||
| Advanced | 2.54 (1.01–6.39) | ||||
| eGFR (CKD-EPI), mL/min/1.73 m2 | 0.99 (0.97–1.01) | 1.00 (0.98–1.02) | |||
| uACR, mg/mmol | 1.03 (1.02–1.04) | ||||
| DKD | 3.25 (1.54–6.87) | ||||
| All-cause mortality | |||||
| eGDR, 1 SD | 0.44 (0.34–0.56)* | 0.61 (0.45–0.82)† | 0.66 (0.48–0.91)§ | — | — |
| Age, 1 year | 1.07 (1.05–1.10) | 1.05 (1.03–1.08) | 1.06 (1.04–1.09) | 1.07 (1.05–1.09) | |
| Sex, male | 1.56 (0.90–2.71) | 1.69 (0.96–2.96) | — | — | |
| Active smoking | 2.28 (1.22–4.26) | 2.07 (1.11–3.86) | 1.85 (0.99–3.45) | ||
| Retinopathy | |||||
| No retinopathy | 1 | 1 | 1 | ||
| Nonadvanced | 2.21 (1.04–4.66) | 2.74 (1.30–5.79) | 2.78 (1.32–5.87) | ||
| Advanced | 2.56 (1.13–5.78) | 2.46 (1.07–5.68) | 2.62 (1.16–5.93) | ||
| eGFR (CKD-EPI), mL/min/1.73 m2 | 0.98 (0.96–0.99) | 0.99 (0.97–1.00) | |||
| uACR, mg/mmol | 1.02 (1.01–1.03) | ||||
| Triacylglycerol, mmol/L | 1.68 (1.28–2.22) | 1.43 (1.08–1.90) | |||
| DKD | 3.46 (1.86–6.43) | ||||
Data are reported only for those variables selected as significant in each model. Major CV events have been defined as first event of myocardial infarction, coronary revascularization, stroke, carotid revascularization, and ulcer, gangrene, amputation, and peripheral revascularization. Coronary artery events have been defined as first event of myocardial infarction or coronary revascularization. DKD has been defined as uACR ≥3.4 mg/mmol or eGFR <60 mL/min/1.73 m2. Model 1, unadjusted Cox regression; model 2, adjustment for age and sex; model 3, adjustment for age and sex and further for diabetes duration, active smoking, LDL cholesterol, eGFR (Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]), lipid-lowering drugs, metformin use, total daily dose of insulin, peripheral neuropathy, retinopathy, and prior CV events; model 4, model 3 adjustments plus further adjustment for HDL cholesterol, triacylglycerol, and uACR; model 5, model 4 adjustments with exclusion of uACR and eGFR (Chronic Kidney Disease Epidemiology Collaboration) as continuous variables and inclusion of DKD as categorical covariate.
P < 0.0001;
P < 0.005;
P < 0.01;
P < 0.05.
The results of our single-center 10-year observation study show that insulin sensitivity is an independent predictor of major CV events, CAD, and all-cause mortality. Importantly, these associations were maintained after adjustment for multiple confounders including IR-related parameters. A similar association pertains to all-cause death, although it lost significance upon correction for uACR or DKD. Previous cross-sectional studies showed an association of eGDR with retinopathy, DKD, or CV disease (4). Moreover, in the Diabetes Control and Complications Trial (DCCT), eGDR was associated with risk of CV disease, although uACR and estimated glomerular filtration rate (eGFR) were not accounted for (5). Similar to our results, in the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study cohort, eGDR was a predictor of CAD independent of several confounders, including DKD (9). We now show this is independent of the combination of uACR and eGFR, or DKD. At variance, in the Pittsburgh EDC Study, eGDR was an independent predictor for mortality (10). Finally, in 17,050 Swedish individuals with T1D the steep increase in all-cause mortality associated with eGDR reduction persisted after adjustment for several covariates (7).
Our study relies on robust national and regional registries and on availability of survival information for the entire cohort and prospective CV data for virtually all subjects. Nonetheless, the number of events is relatively small, limiting the confidence for some estimates, and data on CV death could not be retrieved.
In conclusion, our study suggests that eGDR, estimated by handy clinical parameters, could improve risk stratification beyond traditional CV risk factors.
Article Information
Acknowledgments. The authors are indebted to the patients attending the Outpatients Diabetic Clinic and to the staff of the Renzo Navalesi Diabetes Centre in Pisa.
Funding. This work was supported by a grant from Regione Toscana, Italy, Resolution 1157 (19 December 2011), identification number D55E11002680005. M.G.S. is supported by Fondazione Umberto Veronesi (Grant 2020).
The funder had no role in the original study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit the manuscript for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.G., S.D.P., and G.P. designed the study, drafted the manuscript, approved the final version, and made the decision to submit and publish the manuscript. M.G., M.G.S., D.L., R.M., and G.P. contributed to analysis and interpretation of data, revised the article’s intellectual content, and approved the final version. M.G., E.G., C.B., M.A., F.C., G.D., and P.F. acquired data, revised the article’s intellectual content, and approved the final version. S.D.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
References
- 1.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014;371:1972–1982 [DOI] [PubMed] [Google Scholar]
- 2.Rawshani A, Rawshani A, Franzén S, et al. Range of risk factor levels: control, mortality, and cardiovascular outcomes in type 1 diabetes mellitus. Circulation 2017;135:1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Prato S, Nosadini R, Tiengo A, et al. Insulin-mediated glucose disposal in type I diabetes: evidence for insulin resistance. J Clin Endocrinol Metab 1983;57:904–910 [DOI] [PubMed] [Google Scholar]
- 4.Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care 2013;36:2280–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007;30:707–712 [DOI] [PubMed] [Google Scholar]
- 6.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int 2002;62:963–970 [DOI] [PubMed] [Google Scholar]
- 7.Nyström T, Holzmann MJ, Eliasson B, Svensson AM, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab 2018;20:556–563 [DOI] [PubMed] [Google Scholar]
- 8.Garofolo M, Gualdani E, Giannarelli R, et al. Microvascular complications burden (nephropathy, retinopathy and peripheral polyneuropathy) affects risk of major vascular events and all-cause mortality in type 1 diabetes: a 10-year follow-up study. Cardiovasc Diabetol 2019;18:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orchard TJ, Olson JC, Erbey JR, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003;26:1374–1379 [DOI] [PubMed] [Google Scholar]
- 10.Olson JC, Erbey JR, Williams KV, et al. Subclinical atherosclerosis and estimated glucose disposal rate as predictors of mortality in type 1 diabetes. Ann Epidemiol 2002;12:331–337 [DOI] [PubMed] [Google Scholar]


