Abstract
OBJECTIVE
The principle of replacing prandial insulin lispro with a once-weekly glucagon-like peptide 1 receptor agonist (GLP-1RA) for type 2 diabetes inadequately controlled on a multiple daily insulin injections regimen was tested with albiglutide.
RESEARCH DESIGN AND METHODS
In this treat-to-target study, basal plus prandial insulin was optimized over 4 weeks before participants were randomized (1:1) to albiglutide plus optimized basal insulin glargine and lispro (dose reduced by 50% at randomization; subsequently, lispro injections were fully discontinued 4 weeks later) (n = 402) or to continued optimized lispro plus optimized glargine (n = 412).
RESULTS
Mean ± SD HbA1c at baseline, 7.8 ± 0.6% (61 ± 7 mmol/mol) in the albiglutide + glargine group and 7.7 ± 0.6% (60 ± 7 mmol/mol) in the lispro + glargine group, was reduced at week 26 to 6.7 ± 0.8% (49 ± 8 mmol/mol) and 6.6 ± 0.8% (48 ± 8 mmol/mol), respectively (least squares [LS] difference 0.06% [95% CI −0.05 to 0.17]; noninferiority P < 0.0001). In the albiglutide + glargine group, 218 participants (54%) replaced all prandial insulin without reintroducing lispro up to week 26. Total daily prandial insulin dose was similar at baseline but was lower by 62 units/day (95% CI −65.9 to −57.8; P < 0.0001) at week 26 in the albiglutide + glargine group, and the total number of weekly injections was also reduced from 29 to 13 per week. Less severe/documented symptomatic hypoglycemia (57.2% vs. 75.0%) occurred in the albiglutide + glargine group with meaningful weight differences (LS mean ± SE −2.0 ± 0.2 vs. +2.4 ± 0.2 kg; P < 0.0001) vs. lispro + glargine. Gastrointestinal adverse events were higher with albiglutide + glargine (26% vs. 13%).
CONCLUSIONS
A once-weekly GLP-1RA was able to substitute for prandial insulin in 54% of people, substantially reducing the number of prandial insulin injections; glycemic control improved, with the added benefits of weight loss and less hypoglycemia in the GLP-1RA arm. Replacing prandial insulin with a weekly GLP-1RA can simplify basal plus prandial insulin treatments and achieve better outcomes in type 2 diabetes.
Introduction
Insulin remains the cornerstone therapy for longer-duration type 2 diabetes and β-cell failure (1). However, basal insulin regimens that include prandial insulin can be difficult for most people to manage and many fail to attain individualized glycated hemoglobin (HbA1c) targets, despite increasing dosage and intensification with basal insulin plus multiple prandial insulin injections (2–4). Critical limitations of prandial insulin include lack of adherence to such complex regimens, increased risk of hypoglycemia, weight gain, erratic pre- and postprandial glucose control, and fear among users and physicians about these unintended effects (2,3,5,6). Accordingly, less complex therapeutic approaches are needed to improve glycemic control in people using insulin while avoiding these shortcomings and enhancing adherence (3,4).
Albiglutide is a glucagon-like peptide 1 receptor agonist (GLP-1RA) that was indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes at the time this study was conducted (7,8). In people failing to achieve glycemic targets with basal insulin, GLP-1RAs (exenatide, liraglutide, lixisenatide, dulaglutide, semaglutide) (9–15) have been shown to be better than placebo and similar (albiglutide, exenatide) (16,17) or better (liraglutide) (18) than basal plus prandial insulin at reducing HbA1c, without weight gain and generally with less hypoglycemia but with higher frequency of gastrointestinal adverse events (AEs) that tend to subside over time.
No large randomized controlled studies have previously explored, as in the current study, the efficacy and safety of substituting prandial insulin with a weekly GLP-1RA in people with type 2 diabetes with inadequately controlled blood glucose levels despite intensive basal plus prandial insulin therapy (three or more injections/day).
Research Design and Methods
Trial Design
This 26-week, randomized, open-label, parallel-group, active-control, multicenter, treat-to-target study evaluated the efficacy and safety of a once-weekly single injection of albiglutide to replace prandial insulin in participants with type 2 diabetes experiencing inadequate glycemic control (HbA1c ≥7.0% to ≤9.5% [≥53 to ≤80 mmol/mol]) on a basal plus prandial insulin regimen (three or more injections/day and ≤140 units/day) with or without metformin. Optimized basal plus prandial insulin therapy (actively titrated insulin glargine [Lantus; Sanofi, Bridgewater, NJ] and insulin lispro [Humalog; Lilly, Indianapolis, IN]), based on predefined treat-to-target titration algorithms, served as the active control (18–22). (GlaxoSmithKline has made the decision to discontinue commercial sale of albiglutide effective July 2018. The decision was not related to any known safety concerns.)
The protocol was approved by the independent ethics committee or institutional review board for every study site. Written informed consent was obtained from all study participants. The trial was conducted in accordance with the Declaration of Helsinki/International Conference on Harmonization good clinical practice guidelines.
The study included four periods: screening (2 weeks), insulin standardization (4 weeks), treatment (26 weeks), and posttreatment follow-up (4 weeks) (Supplementary Fig. 1). During insulin standardization, participants transitioned from their current basal plus prandial insulin regimen to once-daily glargine and thrice-daily lispro to reduce any confounding variability associated with other insulin combinations. In this phase, glargine and lispro were actively adjusted to glycemic targets as close to normal as possible without untoward hypoglycemia in accordance with local product labeling and standards of care.
At randomization, study participants were stratified by screening HbA1c (<8.0% vs. ≥8.0% [<64 vs. ≥64 mmol/mol]), age (<65 vs. ≥65 years), and current background metformin (yes/no). Participants were randomized 1:1 to the albiglutide + glargine group (with lispro reduced by 50% at randomization followed by full discontinuation at week 4) or lispro + glargine group.
Trial Participants
The complete inclusion/exclusion criteria are listed in the Supplemental Material. Key eligibility criteria at screening were age 18−75 years, HbA1c ≥7.0% to ≤9.5% (≥53 to ≤80 mmol/mol), fasting C-peptide ≥0.8 ng/mL (≥0.26 nmol/L), BMI ≤40.0 kg/m2, and current use of a basal plus prandial insulin regimen (three or more injections/day and ≤140 units/day) for ≥3 months. Patients receiving any antihyperglycemia medication other than metformin and insulin (e.g., GLP-1RA, dipeptidyl peptidase-4 inhibitor, sulfonylurea, meglitinide, sodium–glucose cotransporter 2 inhibitor, or thiazolidinedione) within 30 days before screening were excluded. Additional criteria at randomization were HbA1c ≥7.0% to ≤9.0% (≥53 to ≤75 mmol/mol) and fasting plasma glucose (FPG) <280 mg/dL (15.5 mmol/L) 1 week earlier.
Dosing and Dose Titration
The albiglutide (Tanzeum; GlaxoSmithKline, Research Triangle Park, NC) starting dose (subcutaneous injection) was 30 mg weekly and was uptitrated at week 4 to 50 mg weekly for the remaining treatment period.
In the albiglutide + glargine group, the lispro doses at randomization were halved and at week 4 lispro injections were completely discontinued. Lispro could be systematically reintroduced by investigators after week 8 in participants who had self-measured postprandial plasma glucose excursions >180 mg/dL (>10.0 mmol/L), based on mean measurements (taken before lunch, dinner, or bedtime) from the last three available days (at least two consecutive) in the week before the next study visit or telephone contact, using a standardized, stepwise titration algorithm (Supplementary Fig. 2). The mean of measurements (taken before lunch, dinner, or bedtime) from the last three available days (at least two consecutive) in the week before each study visit/telephone contact was used to calculate postprandial glucose (MyGlucoHealth wireless meter and test strips; Entra Health Systems, El Cajon, CA). If measurements from 3 days (at least two consecutive) were not available, the dose adjustment was delayed until the next scheduled study visit/telephone contact unless, in the investigator’s judgment, a dose adjustment was warranted. Participants without the required self-monitoring of plasma glucose (SMPG) measurements were retrained on the requirement for SMPG measurements.
At randomization, participants in the lispro + glargine group continued the dose of lispro from the standardization period but then adjusted it according to a dose titration algorithm (Supplementary Table 1). Participants in both groups continued the glargine dose from standardization, again adjusting it according to a common titration algorithm (Supplementary Table 2). To manage the risk for severe hypoglycemia, study investigators received training to instruct participants to detect, treat, and avoid hypoglycemia with consistent nutritional intake and frequent SMPG. Participants also used electronic diaries to assist patients’ surveillance and monitoring of hypoglycemia and report hypoglycemic events that occurred between study visits. Study investigators had access to information collected in participants’ electronic diaries. In addition, study investigators received notification of extreme SMPG measurements (i.e., SMPG ≤50 mg/dL [≤2.8 mmol/L] and SMPG between 50 and 60 mg/dL [2.8–3.3 mmol/L]), as well as episodes of severe hypoglycemia recorded in the electronic diary.
Daytime hypoglycemia was defined as hypoglycemia events with an onset between 0600 h and 0000 h (inclusive) and nocturnal hypoglycemia was defined as hypoglycemic events with an onset between 0001 h and 0559 h (inclusive). To aid in the correct classification and treatment of hypoglycemic events, patients were instructed to repeat SMPG measurements ≤70 mg/dL (≤3.9 mmol/L). All hypoglycemic events reported by participants were assessed and classified by the investigator as severe (requiring third-party intervention), documented symptomatic (typical symptoms and a plasma glucose concentration ≤70 mg/dL [≤3.9 mmol/L]), asymptomatic (not accompanied by typical symptoms but plasma glucose concentration ≤70 mg/dL [≤3.9 mmol/L]), or probably symptomatic (event during which symptoms typical of hypoglycemia were not accompanied by a plasma glucose determination but were presumably caused by a plasma glucose concentration ≤70 mg/dL [≤3.9 mmol/L]) and recorded as a nonserious or serious AE if appropriate.
Study End Points
The primary efficacy end point was change from baseline in HbA1c at 26 weeks. Secondary end points included the proportion of participants in the albiglutide + glargine group who did not resume lispro; total, basal, and prandial daily insulin doses at week 26; number of weekly insulin injections at weeks 4, 10, 18, and 26; percentage of participants with severe (requiring third-party intervention) or documented (glucose <70 mg/dL [<3.9 mmol/L]) symptomatic hypoglycemia from randomization to week 26; and change from baseline in body weight and FPG at week 26. Prespecified hierarchical testing is described in Supplementary Material. Clinical laboratory-measured plasma glucose (Q2 Solutions; headquarters Morrisville, NC, and central laboratory sites Valencia, CA; San Juan Capistrano, CA; Cypress, CA; Little Rock, AK; Scotland, U.K.; and Singapore) was assayed as FPG and fasting serum glucose (FSG) at screening. At week 26, plasma glucose was assayed as FSG, and FPG values were imputed by regression on the baseline data.
Exploratory end points included the Treatment-Related Impact Measure for Diabetes (TRIM-Diabetes) questionnaire (23) and the Hypoglycemia Fear Survey (HFS)-II (24). Safety assessments included AEs (25), clinical laboratory tests, vital signs, electrocardiograms, and physical examinations. Potential events of pancreatitis were adjudicated by an independent adjudication committee blinded to study treatment.
Statistical Analyses
The planned sample size was 794 participants to test for noninferiority. Assuming a withdrawal rate of 15%, ∼337 participants in each treatment group were expected to complete the 26-week study, resulting in ≥90% power to reject the null hypothesis of inferiority for change from baseline in HbA1c (assuming a noninferiority margin of 0.3% [3 mmol/mol], an expected treatment group difference of 0, and an SD of 1.2% [13 mmol/mol], with a one-sided significance level of 0.025).
The efficacy population consisted of all participants randomized to study treatment. The primary end point analysis used a mixed-effects model with repeated measures (MMRM), with HbA1c changes from baseline at all postbaseline visits as dependent variables; treatment, region, age category, metformin use, visit week, treatment-by-week interaction, and baseline HbA1c-by-week interaction as fixed effects; baseline HbA1c as a continuous covariate; and participant as a random effect. Treatment effect estimates of albiglutide were evaluated within this MMRM model as least squares (LS) means contrasts relative to lispro. Analysis methods for secondary end points are provided in Supplementary Table 3.
The proportions of participants with severe or documented symptomatic hypoglycemia were compared between groups using the nonparametric Cochran-Mantel-Haenszel (CMH) test after adjustment for baseline HbA1c, stratum, age-group, metformin use, and region. The number of severe or documented symptomatic hypoglycemic events was compared between groups using a Poisson regression model, which included treatment effect and covariates (HbA1c, age, metformin use, and region).
The safety population included all those who received one or more doses of randomized study medication. Safety results were summarized using descriptive statistics.
Data and Resource Availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Results
Participants
In all, 402 participants were randomized to the albiglutide + glargine group and 412 to the lispro + glargine group, of whom 86% (87% albiglutide + glargine, 85% lispro + glargine) completed the study (Supplementary Fig. 3). The proportions of participants who withdrew and reasons for withdrawal were similar between groups. Most participants (>86%) were compliant ≥80% of the time to their randomized treatments (albiglutide 99.7% and glargine 91.9% in the albiglutide + glargine group, lispro 86.4% and glargine 88.0% in the lispro + glargine group). Compliance was assessed for albiglutide, lispro, and glargine from the pens dispensed to and returned by study participants.
One participant (albiglutide + glargine group) was not treated and was excluded from the safety population. One participant in that group was dispensed lispro instead of albiglutide but was included in the allocated efficacy population, while being removed to the lispro safety population. The numbers in the safety and efficacy population therefore slightly differ accordingly.
Baseline characteristics were similar between groups (Table 1). The mean duration of diabetes was 15 years, mean HbA1c at screening 8.2% (66 mmol/mol), and mean HbA1c at baseline 7.7% (60 mmol/mol) in both groups.
Table 1.
Baseline demographic and clinical characteristics
| Albiglutide + glargine (n = 402) | Lispro + glargine (n = 412) | |
|---|---|---|
| Age at randomization, mean ± SD, years | 58.0 ± 9.4 | 58.1 ± 9.5 |
| Sex, n (%) | ||
| Female | 228 (56.7) | 214 (51.9) |
| Male | 174 (43.3) | 198 (48.1) |
| Race, n (%) | ||
| White | 284 (70.6) | 312 (75.7) |
| Non-White | 118 (29.4) | 100 (24.3) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 159 (39.6) | 152 (36.9) |
| Not Hispanic/Latino | 243 (60.4) | 260 (63.1) |
| Weight, mean ± SD, kg | 87.8 ± 17.3 | 89.8 ± 17.8 |
| BMI | n = 401 | n = 412 |
| Mean ± SD, kg/m2 | 32.1 ± 4.5 | 32.5 ± 4.7 |
| Baseline HbA1c, mean ± SD, % (mmol/mol), after lead-in insulin optimization | 7.7 ± 0.6 (60 ± 7) | 7.7 ± 0.6 (60 ± 7) |
| Current metformin use, n (%) | ||
| Yes | 269 (66.9) | 280 (68.0) |
| No | 133 (33.1) | 132 (32.0) |
| Baseline clinical FPG | n = 397 | n = 409 |
| Mean ± SD, mg/dL (mmol/L) | 145 ± 46 (8.1 ± 2.6) | 140 ± 48 (7.8 ± 2.7) |
| Duration of diabetes | n = 399 | n = 413 |
| Mean ± SD, years | 15.2 ± 7.7 | 14.7 ± 7.2 |
Blood Glucose Control
At week 26, the LS mean ± SD change from baseline in HbA1c was −1.04 ± 0.04% (−11 ± 0.4 mmol/mol) (reduced to 6.7 ± 0.8% [49 ± 8 mmol/mol]) in the albiglutide + glargine group and −1.10 ± 0.04% (−12.0 ± 0.4 mmol/mol) (reduced to 6.6 ± 0.8% [48 ± 8 mmol/mol]) in the lispro + glargine group (LS mean difference 0.06% [95% CI −0.05 to 0.17], 0.7 mmol/mol [95% CI −0.5 to 1.9]; noninferiority P < 0.0001) (Table 2). Change in HbA1c over time was similar between groups (Fig. 1A).
Table 2.
Summary of efficacy end points
| Albiglutide + glargine (n = 402) | Lispro + glargine (n = 412) | |
|---|---|---|
| HbA1c, % (mmol/mol) | ||
| Participants, n | 345 | 350 |
| At screening* | 8.2 ± 0.6 (66 ± 7) | 8.2 ± 0.6 (66 ± 7) |
| Baseline | 7.8 ± 0.6 (61 ± 7) | 7.7 ± 0.6 (60 ± 7) |
| Week 26 | 6.7 ± 0.8 (49 ± 8) | 6.6 ± 0.8 (48 ± 8) |
| Change from baseline | −1.10 ± 0.8 (−12 ± 9) | −1.12 ± 0.8 (−12 ± 8) |
| Model-adjusted change from baseline, mean ± SE† | −1.04 ± 0.04 (−11 ± 0.4) | −1.10 ± 0.04 (−12 ± 0.4) |
| LS mean difference (95% CI)‡ | ||
| %-units | 0.06 (−0.05 to 0.17)*** | |
| mmol/mol | 0.7 (–0.5 to 1.9) | |
| Clinical FPG, mg/dL (mmol/L)§ | ||
| Participants, n | 345 | 349 |
| Baseline | 144 ± 46 (8.0 ± 2.6) | 139 ± 48 (7.7 ± 2.6) |
| Week 26 | 105 ± 36 (5.8 ± 2.0) | 114 ± 41 (6.3 ± 2.3) |
| Change from baseline | −39 ± 51 (−2.2 ± 2.8) | −25 ± 52 (−1.4 ± 2.9) |
| Model-adjusted change from baseline, mean ± SE† | −36 ± 2.2 (−2.0 ± 0.1) | −26 ± 2.2 (−1.5 ± 0.1) |
| LS mean difference (95% CI)‡ | ||
| mg/dL | −10 (−15 to −5)††† | |
| mmol/L | −0.6 (−0.9 to −0.3) | |
| Total daily insulin dose, units‖ | ||
| Participants, n | 342 | 341 |
| Baseline | 80.3 ± 29.1 | 82.9 ± 32.1 |
| Week 26 | 69.0 ± 33.2 | 130.4 ± 61.1 |
| Model-adjusted total daily insulin dose, mean ± SE† | 70.4 ± 2.2 | 131.2 ± 2.1 |
| LS mean difference (95% CI)‡ | −60.8 (−66.6 to −55.1)‡‡‡ | |
| Total daily basal insulin dose, units‖ | ||
| Participants, n | 342 | 341 |
| Baseline | 41.6 ± 17.3 | 41.6 ± 17.1 |
| Week 26 | 59.3 ± 24.1 | 58.6 ± 25.9 |
| Model-adjusted total daily insulin dose, mean ± SE† | 59.8 ± 1.0 | 59.4 ± 1.0 |
| LS mean difference (95% CI)‡ | 0.4 (−2.3 to 3.0) | |
| Total daily prandial insulin (lispro) dose, units‖ | ||
| Participants, n | 342 | 341 |
| Baseline | 38.7 ± 19.0 | 41.3 ± 21.6 |
| Week 26 | 9.8 ± 17.3 | 71.9 ± 40.1 |
| Model-adjusted daily prandial insulin dose, mean ± SE† | 10.6 ± 1.5 | 72.5 ± 1.5 |
| LS mean difference (95% CI)‡ | −61.8 (−65.9 to −57.8)‡‡‡ | |
| Total number of weekly injections | ||
| Participants, n | 342 | 341 |
| Baseline | 29 ± 1.5 | 28 ± 0.0 |
| Week 26 | 13 ± 7.8 | 28 ± 0.0 |
| Change from baseline | −16 ± 7.9 | 0.0 ± 0.0 |
| Body weight, kg | ||
| Participants, n | 349 | 352 |
| Baseline | 87.7 ± 17.3 | 89.6 ± 18.1 |
| Week 26 | 85.7 ± 17.5 | 92.0 ± 18.6 |
| Change from baseline | −2.0 ± 3.6 | 2.5 ± 4.1 |
| Model-adjusted change from baseline, mean ± SE† | −2.0 ± 0.2 | 2.4 ± 0.2 |
| LS mean difference (95% CI)‡ | −4.4 (−4.9 to −3.8)‡‡‡ | |
| TRIM-Diabetes questionnaire total score¶ | ||
| Participants, n | 347 | 350 |
| Baseline | 72.3 ± 14.1 | 73.5 ± 12.5 |
| Week 26 | 76.7 ± 13.3 | 74.3 ± 13.2 |
| Change from baseline | 4.4 ± 12.3 | 0.7 ± 11.1 |
| Model-adjusted change from baseline, mean ± SE† | 4.3 ± 0.6 | 1.1 ± 0.6 |
| LS mean difference (95% CI)‡ | 3.2 (1.7–4.8)‡‡‡ | |
| HFS-II questionnaire worry subscale total score# | ||
| Participants, n | 348 | 349 |
| Baseline | 16.7 ± 15.6 | 15.3 ± 13.9 |
| Week 26 | 14.2 ± 14.3 | 16.0 ± 13.4 |
| Change from baseline | −2.5 ± 13.6 | 0.7 ± 13.2 |
| Model-adjusted change from baseline, mean ± SE† | −2.6 ± 0.7 | 0.2 ± 0.7 |
| LS mean difference (95% CI)‡ | −2.8 (−4.5 to −1.1)§§§ | |
| Achieving HbA1c <7.0% at week 26 | ||
| Participants, n (%) | 244 (60.7) | 255 (61.9) |
| Odds ratio (95% CI)** | 1.0 (0.7–1.3) | |
| Achieving HbA1c <7.0% without weight gain†† and severe or documented symptomatic hypoglycemia to week 26 | ||
| Participants, n (%) | 64 (15.9) | 16 (3.9) |
| Odds ratio (95% CI)** | 3.8 (2.2–6.5)‖‖‖ | |
Data are means ± SD unless otherwise indicated. Mean baseline value includes only those participants with both baseline and week 26 values.
At screening, n = 389 in the albiglutide + glargine group and n = 403 in the lispro + glargine group.
Based on MMRM model.
For the albiglutide + glargine vs. the lispro + glargine group.
FPG at week 26 is missing for all participants and is imputed with FSG at week 26.
Insulin dose at week 26 is defined as the prescribed insulin dose at week 25.
TRIM-Diabetes total and domain scores range from 0 to 100, with higher scores indicative of better experienced health state (less negative impact).
HFS-II worry subscale ranges from 0 to 72, with higher scores indicative of more worries about low blood glucose.
Difference from lispro + glargine group based on nonparametric CMH test after adjustment for baseline HbA1c category, age category, region, and current use of metformin.
No body weight gain is defined as ≤1 kg increase from baseline.
P < 0.0001 (noninferiority),
P = 0.0004 (superiority),
P < 0.0001 (superiority),
P = 0.0014 (superiority),
P < 0.0001 with the nonparametric CMH test.
Figure 1.
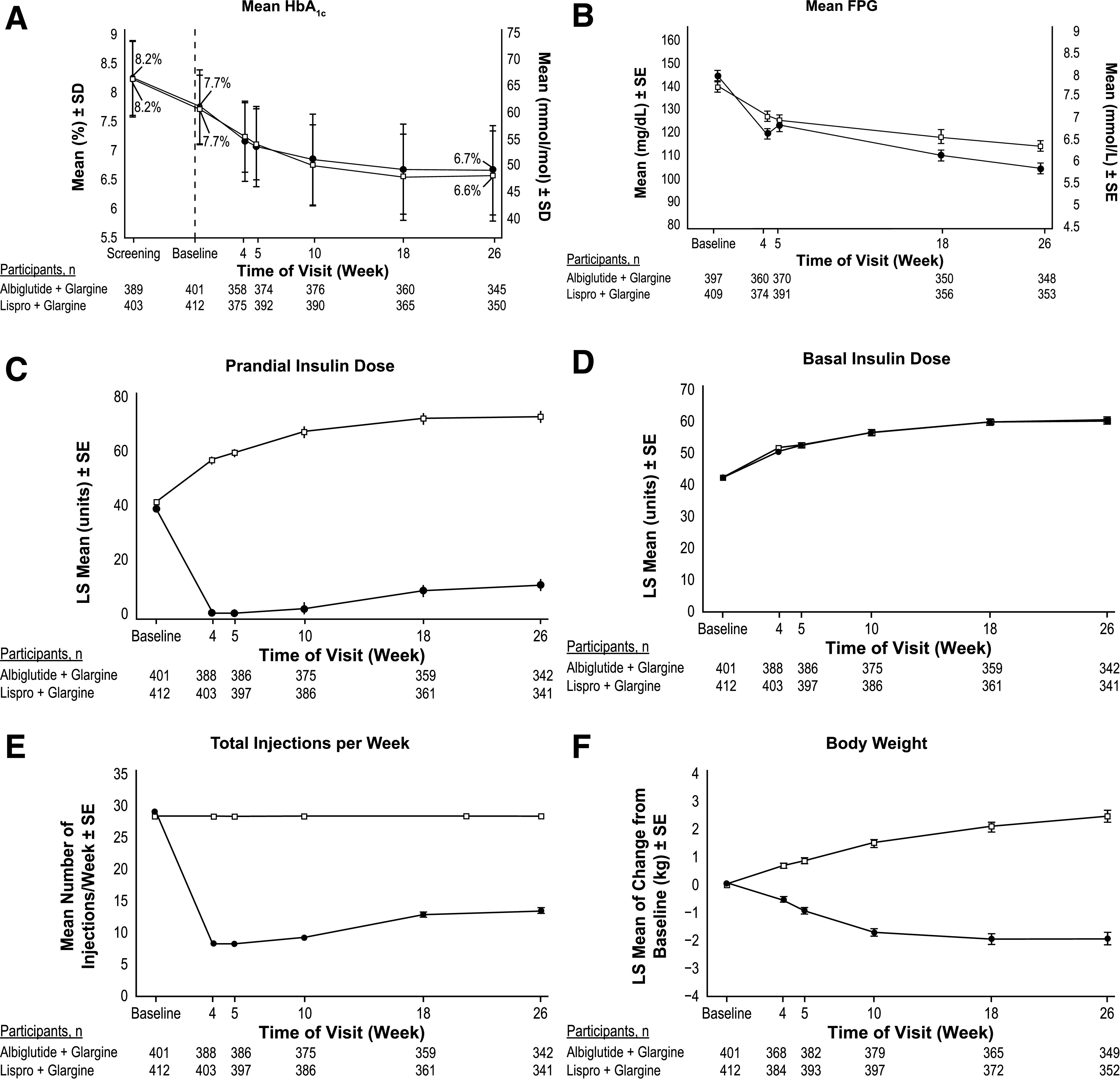
HbA1c, FPG, insulin dose, number of injections, and body weight by study visit (full analysis population). A: Mean HbA1c by study visit from screening to week 26. B: Mean FPG by study visit to week 26. C: Model-adjusted prandial insulin dose by study visit to week 26. D: Model-adjusted basal insulin dose by study visit to week 26. E: Mean number of total injections per week by study visit to week 26. F: Model-adjusted change from baseline in body weight (kg) by study visit to week 26. ●, albiglutide + glargine; □, lispro + glargine.
The proportion of participants achieving HbA1c <7.0% (53 mmol/mol) was similar between groups (61% and 62% at week 26 for the albiglutide + glargine and lispro + glargine groups, respectively) (Table 2).
The LS mean change from baseline in FPG was significantly greater in the albiglutide + glargine than in the lispro + glargine group at week 26 (LS mean difference −10 mg/dL [95% CI −16 to −5], −0.6 mmol/L [95% CI −0.9 to −0.3]) (Table 2).
Medications and Injections
In the albiglutide + glargine group, 54% of participants (218 of 402) were able to totally replace lispro with albiglutide (no reintroduction of lispro) through week 26. The remaining 184 participants (46%) include those who received lispro reintroduction at any time or those for whom data were not available to allow verification of absence of lispro reintroduction through week 26. Overall, 291 participants (72%) did not require lispro reintroduction through week 26 or were able to decrease lispro dose without worsening HbA1c at week 26, while 28% remained on the same or higher dose of lispro or had a lower dose of lispro but worse HbA1c at week 26. There were no differences between individuals who did or did not require the reintroduction of lispro in terms of duration of diabetes, age, weight, BMI, baseline HbA1c, and baseline total insulin dose (data not shown).
Mean prescribed daily prandial insulin dose decreased from 38.7 ± 19.0 units at baseline to 9.8 ± 17.3 units at week 26 in the albiglutide + glargine group and increased from 41.3 ± 21.6 units to 71.9 ± 40.1 units in the lispro + glargine group. Daily basal insulin dose increased to a similar degree in both groups from baseline (41.6 ± 17.3 units for albiglutide + glargine and 41.6 ± 17.1 units for lispro + glargine) to week 26 (59.3 ± 24.1 units for albiglutide + glargine and 58.6 ± 25.9 units for lispro + glargine). Total insulin dose decreased in the albiglutide + glargine group (80.3 ± 29.1 units at baseline and 69.0 ± 33.2 units at week 26) and increased in the lispro + glargine group (82.9 ± 32.1 units at baseline and 130.4 ± 61.1 units at week 26). Changes in prescribed insulin dose with time are shown in Fig. 1C and D. The difference (decrease) in change in LS mean adjusted daily total prescribed insulin in the albiglutide + glargine versus the lispro + glargine groups at week 26 was −61 units and for prandial insulin −62 units (Table 2).
At week 26, the mean ± SD number of injections with albiglutide + glargine was reduced from 29 to 13 per week (reduction of −16 ± 8 per week) but was unchanged at 28 per week in the lispro + glargine group (Table 2 and Fig. 1E). At study end, 62% of the 351 participants who completed the study in the albiglutide + glargine group required no injections of lispro, 9% required one injection of lispro per day, 12% required two injections of lispro per day, and 16% required three injections of lispro per day.
Hypoglycemia and Body Weight
The proportion of participants with severe or documented symptomatic hypoglycemia from baseline to week 26 was lower in the albiglutide + glargine than the lispro + glargine group (57.2% vs. 75.0%, respectively; odds ratio 0.43 [95% CI 0.31–0.60]) (Table 3). The overall on-therapy event rate for severe, documented symptomatic, or asymptomatic hypoglycemia (exposure adjusted) was 13.0 and 32.2 per person-year for the albiglutide + glargine and lispro + glargine groups (event rate ratio 0.43 [95% CI 0.36–0.52]); for on-therapy severe hypoglycemia, the event rate was 0.05 vs. 0.13 per person-year and occurred in 2.3% (albiglutide + glargine) and 5.3% (lispro + glargine) of participants (Table 3). On-therapy daytime severe hypoglycemic events were experienced by 1.5% of participants in the albiglutide + glargine group vs. 3.4% in the lispro + glargine group, while 1.0% of participants in the albiglutide + glargine group experienced an on-therapy nocturnal severe hypoglycemic event vs. 1.5% in the lispro + glargine group. The proportion of participants who experienced an on-therapy documented symptomatic hypoglycemic event was 46.8% (daytime) and 25.3% (nocturnal) in the albiglutide + glargine group and 70.9% (daytime) and 36.8% (nocturnal) in the lispro + glargine group. The proportion of patients who experienced blood glucose levels <56 mg/dL (3.1 mmol/L) was 35.3% in the albiglutide + glargine group and 57.9% in the lispro + glargine group.
Table 3.
Hypoglycemia
| Albiglutide + glargine | Lispro + glargine | |
|---|---|---|
| Hypoglycemia (full analysis population) incidence to week 26 | ||
| n | 402 | 412 |
| Documented symptomatic or severe | ||
| Participants, n (%) | 230 (57.2) | 309 (75.0) |
| Odds ratio (95% CI)* | 0.43 (0.31–0.60)*** | |
| On-therapy hypoglycemia (safety population) exposure-adjusted incidence rate | ||
| n | 400 | 413 |
| Asymptomatic | ||
| Participants, n (%) | 230 (57.5) | 293 (70.9) |
| Events/person-year† | 6.6 | 12.6 |
| Documented symptomatic | ||
| Participants, n (%) | 203 (50.8) | 299 (72.4) |
| Events/person-year† | 6.3 | 19.5 |
| Severe | ||
| Participants, n (%) | 9 (2.3) | 22 (5.3) |
| Events/person-year† | 0.05 | 0.13 |
| All‡ | ||
| Participants, n (%) | 290 (72.5) | 359 (86.9) |
| Events/person-year† | 13.0 | 32.2 |
| Rate ratio (95% CI)§ | 0.43 (0.36–0.52)††† | |
| On-therapy hypoglycemic event by blood glucose level‖ | ||
| n | 400 | 413 |
| <56 mg/dL (<3.1 mmol/L), n (%) | 141 (35.3) | 239 (57.9) |
| ≥56 mg/dL (≥3.1 mmol/L), n (%) | 160 (40.0) | 121 (29.3) |
| Missing data, n (%) | 4 (1.0) | 1 (0.2) |
Difference from lispro + glargine group based on nonparametric CMH test after adjustment for baseline HbA1c category, age category, region, and current use of metformin.
Exposure-adjusted event rate (number of on-therapy severe or documented symptomatic hypoglycemic or asymptomatic events divided by person-years), where person-years is defined as the cumulative study treatment exposure duration (in years) for all participants in the treatment group during the treatment period being summarized.
Severe, documented symptomatic, or asymptomatic hypoglycemia event.
Ratio of LS incidence rate (albiglutide/insulin lispro) from repeated-measure Poisson regression model with offset for the person-year. The model includes treatment effect and interval as factors and HbA1c stratum, age category (<65 vs. ≥65 years), current use of metformin (yes vs. no), and region as covariates. The P value is from a two-sided t test for the difference in rates.
Participants with more than one hypoglycemic event are only counted once in the worst category.
P < 0.0001 with the nonparametric CMH test;
P = 0.0001 with repeated-measures Poisson regression.
Body weight changes from baseline at week 26 differed in direction between the albiglutide + glargine and lispro + glargine groups (LS mean ± SE −2.0 ± 0.2 kg vs. +2.4 ± 0.2 kg; difference −4.4 kg [95% CI −4.9 to −3.8]) (Table 2 and Fig. 1F).
The proportion of participants achieving HbA1c <7.0% (53 mmol/mol) without weight gain and without severe or documented symptomatic hypoglycemia at week 26 was significantly higher (odds ratio 3.8 [95% CI 2.2–6.5]) in the albiglutide + glargine (15.9%) vs. the lispro + glargine (3.9%) group (Table 2).
Patient-Related Outcomes
Participants in the albiglutide + glargine group had improved (higher) TRIM-Diabetes questionnaire scores at week 26 (LS mean difference 3.2 [95% CI 1.7–4.8]) vs. the lispro + glargine group (Table 2). Improvements were seen for all five domains of the TRIM-Diabetes questionnaire (treatment burden, daily life, diabetes management, compliance, and psychological health) in the albiglutide + glargine group (Supplementary Table 4); however, differences compared with the lispro + glargine group were only significant for the diabetes management domain and the compliance domains. Similarly, HFS-II scores improved (LS mean difference −2.8 [95% CI −4.5 to −1.1] in favor of the albiglutide + glargine group (Table 2).
Safety and Tolerability
The incidence of on-therapy AEs (excluding hypoglycemia events) was 65% for albiglutide + glargine and 62% for lispro + glargine. Gastrointestinal events (26% vs. 13%) and injection-site reactions (2% vs. 0.2%) were more common in the albiglutide + glargine group (Supplementary Table 5). There was one event of acute pancreatitis in the albiglutide + glargine group (confirmed by an independent adjudication committee).
The incidence of on-therapy AEs (excluding hypoglycemia events) reported by investigators to be drug related was higher in the albiglutide + glargine group (22.0%) vs. the lispro + glargine group (5.1%), mainly due to gastrointestinal AEs (16.5% albiglutide + glargine vs. 0.5% lispro + glargine).
The incidence of on-therapy serious AEs was 5.8% for albiglutide + glargine and 7.5% for lispro + glargine, including 0% (albiglutide + glargine) and 1.5% (lispro + glargine) serious AEs of severe hypoglycemia. Fourteen participants (3.5%) in the albiglutide + glargine group and 9 (2.2%) in the lispro + glargine group had an on-therapy AE leading to discontinuation of study treatment/study withdrawal. The predominant reasons were nausea (albiglutide + glargine) and hypoglycemia (lispro + glargine). Other reasons included vomiting, cholecystitis, diarrhea, abdominal pain, acute kidney injury, decreased appetite, fatigue, hypersensitivity, malaise, and pancreatitis in the albiglutide + glargine group and congestive cardiac failure in the lispro + glargine group.
No new safety signals were apparent following review of clinical laboratory tests, vital signs, electrocardiograms, and physical examinations.
Conclusions
To our knowledge, this is the first large randomized controlled trial using a treat-to-target design to examine the impact of substitution of a weekly GLP-1RA for prandial insulin on glucose control in type 2 diabetes insufficiently controlled with multiple daily insulin therapy. Many randomized, controlled, insulin treat-to-target studies have achieved average HbA1c of ∼7.0% (53 mmol/mol) (26); however, such glucose control is usually not achieved in the clinical practice setting, as no structured support system is available similar to the structured setting of a randomized controlled trial. Recent basal-prandial insulin clinical trials, with longer-acting basal insulin analogs, resulted in average HbA1c in the 7.0% and 7.2% (53 and 55 mmol/mol) range, but at the expense of major efforts and significant hypoglycemia, mostly daytime and attributed to fast-acting prandial insulin (20,21). In our study, active intervention of the weekly GLP-1RA albiglutide added to optimized basal insulin achieved very good glycemic control (mean HbA1c of 6.7% [49 mmol/mol]) in individuals with long-standing diabetes (14–15 years) similar to what was achieved with optimized basal insulin in the control arm (mean HbA1c of 6.6% [48 mmol/mol]). In the albiglutide + glargine arm, glycemic control was achieved without increase of hypoglycemia risk, with the added benefit of weight loss, and with decreases in prandial injections and doses and total prescribed insulin dose, while basal insulin requirements were unchanged. More than 50% of participants did not need reintroduction of lispro, and the average injection number per week was more than halved. Even in those 46% of participants who had to reintroduce lispro during the study, many achieved HbA1c <7.0% (53 mmol/mol) with a lower insulin dose (only 28% were on the same or higher dose).
In addition, FPG was lower in the albiglutide + glargine arm compared with the lispro + glargine arm, despite nearly identical mean glargine dose in both arms, consistent with observations that long-acting GLP-1RAs can impact both prandial and fasting glucose levels.
Unsurprisingly, considering the larger doses of prandial insulin, hypoglycemia was a significant problem in the lispro + glargine group, affecting 75% of people over 26 weeks, but both the odds ratio for risk of one hypoglycemic event and the hypoglycemia event rate were more than halved (Table 3) with the albiglutide substitution.
In support of these findings, HFS-II scores suggested that switching to a GLP-1RA allowed uptitration of basal insulin without increasing concerns regarding hypoglycemia. The TRIM-Diabetes questionnaire also showed differences between the two treatment groups. As glucose control and trial input were similar, this likely reflects effects of the reduction in injections, decreased hypoglycemia, and/or the better body weight trajectory (mean difference of 4.4 kg) on patient health-related quality of life.
While other larger studies have not addressed switching from prandial insulin, the efficacy of GLP-1RAs in combination with basal insulin has been examined. As examples, exenatide and semaglutide improved glucose control when added to a basal insulin regimen, with the added benefit of weight reduction (11,14). In the HARMONY 6 study, albiglutide added to basal insulin glargine was noninferior to the active control of prandial insulin added to glargine in people with type 2 diabetes uncontrolled with glargine (17). Addition of liraglutide to a multiple daily injection insulin regimen achieved a mean HbA1c of 7.4% (57 mmol/mol), higher than in our study (6.7% [49 mmol/mol]), with no formal reduction in insulin dose (12).
Other studies of GLP-1RA plus insulin combinations earlier in the course of type 2 diabetes, including the fixed-ratio studies (27,28), confirm the synergy of this approach, with noted advantage for hypoglycemia and body weight change (29). Indeed, HbA1c of the same magnitude has been achieved in people on insulin starting fixed-ratio formulations of basal insulin and a GLP-1RA but at earlier stages of diabetes after oral agent failure when only basal insulin would normally be used (26,27).
Limitations of our study include the possibility that the study results reflect the specific titration algorithms used and would differ with less consistent titration protocols used in clinical practice, which is mainly relevant to the findings for the lispro + glargine arm. Further, patients in the lispro + glargine arm may have been more likely to self-monitor blood glucose, which could in turn result in a greater likelihood that hypoglycemic events, particularly asymptomatic events, were detected in these individuals. The patient-reported outcomes measures were not administered to blinded study groups, limiting their interpretation. Our study was 26 weeks in duration, and it is possible that the benefits of the GLP-1RA will be gradually lost and a full insulin replacement regimen again be needed.
Our study provides a systematic evaluation of a novel treatment approach for the management of patients with type 2 diabetes. These findings, together with other data in the literature, provide scientific evidence for a role for GLP-1RAs as a replacement for prandial insulin in patients with type 2 diabetes requiring multiple daily insulin injections to achieve glycemic control and may warrant reassessment of current treatment guidelines. Such an approach might be of particular interest in older individuals for whom individualized HbA1c targets are less stringent, hypoglycemia risk is greater, and simplification of complex treatment regimens is more pressing, and so, conceivably, the percentage of people not requiring prandial insulin injections could be even greater. Further, although no head-to-head studies have been done with albiglutide versus dulaglutide or semaglutide, data from the literature have shown HbA1c reductions with albiglutide in the 0.8%–0.9% range (17,30,31), while studies with dulaglutide and semaglutide achieved HbA1c reductions in the 1.1%–1.8% range and with greater weight loss in most studies (14,15,32–34). Therefore, although the idea is purely speculative, it is conceivable, based on available data, that other weekly GLP-1RAs (i.e., dulaglutide, semaglutide) might have a greater effect than albiglutide for reducing HbA1c and body weight when replacing prandial insulin in patients with type 2 diabetes on multiple daily insulin therapy. Future studies that will test this treatment strategy with these weekly GLP-1RAs are warranted and would be of great interest.
In conclusion, introduction of a once-weekly GLP-1RA with planned cessation of prandial insulin can improve glucose control to near normoglycemia with substantially less insulin and fewer prandial injections, less hypoglycemia, and reduced body weight. More than 50% of people who were previously treated with basal plus prandial insulin were able to achieve glycemic control with continued use of basal insulin alone. These findings highlight the potential to achieve glycemic control with a simplified treatment regimen by adding a weekly GLP-1RA to mitigate the common unwanted effects associated with insulin therapy.
Article Information
Acknowledgments. The authors are grateful for editorial support (assembling tables and figures, collating author comments, copyediting, fact-checking, and referencing: Sarah Hummasti, Nancy Price, and Elizabeth Rosenberg) and graphic services provided by AOIC, LLC and funded by GlaxoSmithKline. The authors thank John Cook, formerly of GlaxoSmithKline, for his assistance with early drafts of the manuscript.
Duality of Interest. This study (NCT02229227) was funded by GlaxoSmithKline (GSK). J.R. has participated in advisory panels for Boehringer Ingelheim Pharmaceuticals, Intarcia Therapeutics, Applied Therapeutics, Janssen Pharmaceuticals, Inc., Eli Lilly and Company, Novo Nordisk, Sanofi, and Oramed Pharmaceuticals and has received research support from GSK, Janssen Pharmaceuticals, Pfizer, Intarcia Therapeutics, Genentech, Merck & Co., Eli Lilly and Company, Novo Nordisk, Sanofi, and Oramed Pharmaceuticals. A.N., J.S., L.E., A.A., and J.D. are employees of and own stock in GSK. M.C.C. and J.M. were employees of GSK during the time of the study. P.H., or institutions with which he is associated, has received funding from the manufacturer of albiglutide, GSK, and from AntriaBio, AstraZeneca, Biocon, Boehringer Ingelheim, Eli Lilly and Company, Hanmi, Janssen, Merck Sharp & Dohme (a subsidiary of Merck & Co.), Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.R., A.N., J.S., A.A., and J.M. contributed to the conception or design of the study and the data analysis or interpretation of the study. L.E., J.D., and P.H. contributed to the data analysis or interpretation of the study. M.C.C. contributed to the conception or design of the study. All authors commented critically and approved the final draft for submission. J.R. and A.A. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT02229227, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.12469121.
M.C.C. is currently affiliated with Eli Lilly, Indianapolis, IN.
J.M. is currently affiliated with Kriya Therapeutics, Durham, NC.
See accompanying article, p. 2333.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 2.Miser WF, Arakaki R, Jiang H, Scism-Bacon J, Anderson PW, Fahrbach JL. Randomized, open-label, parallel-group evaluations of basal-bolus therapy versus insulin lispro premixed therapy in patients with type 2 diabetes mellitus failing to achieve control with starter insulin treatment and continuing oral antihyperglycemic drugs: a noninferiority intensification substudy of the DURABLE trial. Clin Ther 2010;32:896–908 [DOI] [PubMed] [Google Scholar]
- 3.Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 2014;384:2228–2234 [DOI] [PubMed] [Google Scholar]
- 4.Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Ceriello A, Esposito K. Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care 2011;34:510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holst JJ, Vilsbøll T. Combining GLP-1 receptor agonists with insulin: therapeutic rationales and clinical findings. Diabetes Obes Metab 2013;15:3–14 [DOI] [PubMed] [Google Scholar]
- 6.Edelman SV, Weyer C. Unresolved challenges with insulin therapy in type 1 and type 2 diabetes: potential benefit of replacing amylin, a second beta-cell hormone. Diabetes Technol Ther 2002;4:175–189 [DOI] [PubMed] [Google Scholar]
- 7.GlaxoSmithKiline. Highlights of prescribing information: Tanzeum (albiglutide) for injection, for subcutaneous use [Internet], 2014. Research Triangle Park, NC, GlaxoSmithKline. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s019lbl.pdf. Accessed 10 February 2020
- 8. GlaxoSmithKline. Eperzan: summary of product characteristics [Internet], 2014. County Cork, Ireland, GalxoSmithKline. Available from https://www.ema.europa.eu/en/documents/product-information/eperzan-epar-product-information_en.pdf. Accessed 10 February 2020.
- 9.Ahmann A, Rodbard HW, Rosenstock J, et al.; NN2211-3917 Study Group . Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab 2015;17:1056–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103–112 [DOI] [PubMed] [Google Scholar]
- 11.Guja C, Frías JP, Somogyi A, et al. Effect of exenatide QW or placebo, both added to titrated insulin glargine, in uncontrolled type 2 diabetes: the DURATION-7 randomized study. Diabetes Obes Metab 2018;20:1602–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lind M, Hirsch IB, Tuomilehto J, et al. Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: randomised clinical trial (MDI Liraglutide trial). BMJ 2015;351:h5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 2013;36:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab 2018;103:2291–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pozzilli P, Norwood P, Jódar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab 2017;19:1024–1031 [DOI] [PubMed] [Google Scholar]
- 16.Diamant M, Nauck MA, Shaginian R, et al.; 4B Study Group . Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care 2014;37:2763–2773 [DOI] [PubMed] [Google Scholar]
- 17.Rosenstock J, Fonseca VA, Gross JL, et al.; Harmony 6 Study Group . Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care 2014;37:2317–2325 [DOI] [PubMed] [Google Scholar]
- 18.Mathieu C, Rodbard HW, Cariou B, et al.; BEGIN: VICTOZA ADD-ON (NN1250-3948) study group . A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab 2014;16:636–644 [DOI] [PubMed] [Google Scholar]
- 19.Abrahamson MJ, Peters A. Intensification of insulin therapy in patients with type 2 diabetes mellitus: an algorithm for basal-bolus therapy. Ann Med 2012;44:836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garber AJ, King AB, Del Prato S, et al.; NN1250-3582 (BEGIN BB T2D) Trial Investigators . Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012;379:1498–1507 [DOI] [PubMed] [Google Scholar]
- 21.Riddle MC, Rosenstock J, Vlajnic A, Gao L. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal-bolus up to three prandial injections. Diabetes Obes Metab 2014;16:396–402 [DOI] [PubMed] [Google Scholar]
- 22.Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 2008;31:20–25 [DOI] [PubMed] [Google Scholar]
- 23.Brod M, Hammer M, Christensen T, Lessard S, Bushnell DM. Understanding and assessing the impact of treatment in diabetes: the Treatment-Related Impact Measures for Diabetes and Devices (TRIM-Diabetes and TRIM-Diabetes Device). Health Qual Life Outcomes 2009;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency Assessment report for GLP-1-based therapies [Internet], 2013. Available from http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/08/WC500147026.pdf. Accessed 15 May 2018.
- 26.Garber AJ. Treat-to-target trials: uses, interpretation and review of concepts. Diabetes Obes Metab 2014;16:193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gough SC, Bode B, Woo V, et al.; NN9068-3697 (DUAL-I) trial investigators . Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014;2:885–893 [DOI] [PubMed] [Google Scholar]
- 28.Rosenstock J, Diamant M, Aroda VR, et al.; LixiLan PoC Study Group . Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof-of-concept randomized trial. Diabetes Care 2016;39:1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes 2015;9:15–22 [DOI] [PubMed] [Google Scholar]
- 30.Ahrén B, Johnson SL, Stewart M, et al.; HARMONY 3 Study Group . HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 2014;37:2141–2148 [DOI] [PubMed] [Google Scholar]
- 31.Reusch J, Stewart MW, Perkins CM, et al. Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonist albiglutide (HARMONY 1 trial): 52-week primary endpoint results from a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes mellitus not controlled on pioglitazone, with or without metformin. Diabetes Obes Metab 2014;16:1257–1264 [DOI] [PubMed] [Google Scholar]
- 32.Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017;5:341–354 [DOI] [PubMed] [Google Scholar]
- 33.Pratley RE, Aroda VR, Lingvay I, et al.; SUSTAIN 7 investigators . Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 2018;6:275–286 [DOI] [PubMed] [Google Scholar]
- 34.Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014;37:2159–2167 [DOI] [PubMed] [Google Scholar]


