Real-time continuous glucose monitoring (rtCGM) is increasingly used in patients with type 1 diabetes because it provides real-time data with low glucose alarms in place to alert individuals or their carers to developing hypoglycemia. This technology may be especially useful for those with impaired awareness of hypoglycemia (IAH). However, there is limited reported evidence on the accuracy of these devices in the hypoglycemic range in these patients under controlled conditions (1). In an ongoing clinical study of people with type 1 diabetes and IAH we compared data collected from rtCGM with time-matched arterialized venous (AV) blood analyzed using a bedside plasma glucose analyzer and a standard blood glucose meter (used in self-monitoring of blood glucose [SMBG]) under experimental hypoglycemic conditions.
Participants with type 1 diabetes and IAH were recruited to a parallel-group study during which they underwent a 90-min hyperinsulinemic-hypoglycemic (45 mg/dL) clamp. Throughout the clamp, AV blood samples were obtained every 5 min via a retrograde cannula inserted into the nondominant hand that had been placed into a heated hand box. Each AV sample was tested using a standard blood glucose meter (Contour Meter; Ascensia Diabetes Care UK Limited, Newbury, U.K.) before being centrifuged at 5500 rpm and plasma glucose then measured using a bedside plasma glucose analyzer (Biosen C-Line GP+; EKF Diagnostics, Cardiff, U.K.). Each participant had an rtCGM device (Dexcom G6; Dexcom, San Diego, CA) in place that had been fitted at least 48 h preceding the study, and this monitoring was maintained throughout the hypoglycemic clamp.
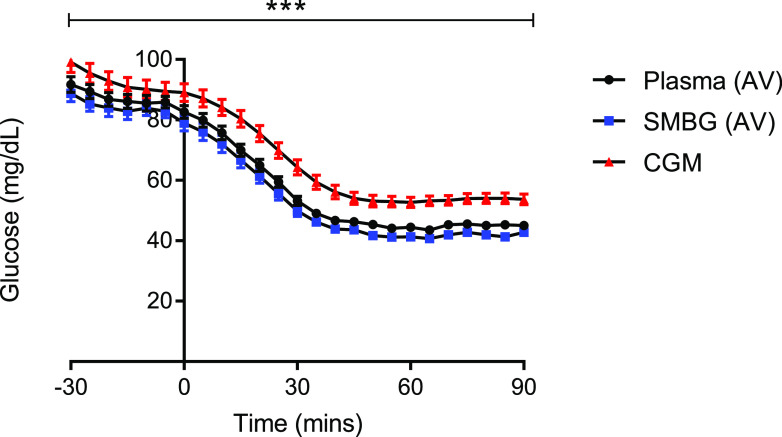
Fifteen hyperinsulinemic-hypoglycemic clamp studies with complete data sets for all three glucose readings obtained were analyzed. Mean (SEM) glucose at euglycemia (plasma; SMBG, CGM) was 91.7 (2.6), 88.3 (2.8), and 99.1 (3.4) mg/dL and during stable hypoglycemia was 45.0 (0.5), 43.2 (0.8), and 53.7 (1.8) mg/dL. Of 105 readings analyzed during the hypoglycemic period, only 46.7% of rtCGM readings were <54 mg/dL. In comparison with AV plasma glucose, we found SMBG of whole AV blood to report 3% lower at euglycemia and 5% lower at hypoglycemia. In contrast, we found rtCGM to report 8% higher at euglycemia and 19% higher at hypoglycemia (Fig. 1). A generalized estimated equation was applied to adjust for baseline and time; this confirmed that, overall, rtCGM overestimates glucose compared with AV plasma (P < 0.05). In addition, in a comparison of methods and time periods throughout the hypoglycemic clamp, rtCGM readings are significantly higher (P < 0.001).
Figure 1.
Glucose data (mean ± SEM) during hyperinsulinemic-hypoglycemic clamps. Black circles represent AV plasma glucose; blue squares, AV blood tested via SMBG; red triangles, CGM. ***P < 0.001.
The hyperinsulinemic-hypoglycemic clamp is the gold standard technique used to assess the impact of hypoglycemia on various aspects of the counterregulatory response including cognitive function. AV glucose provides a close approximation of arterial glucose, a difference of 1.8 mg/dL (2). The glycemic threshold for impaired cognitive function in people without diabetes is <50.5 mg/dL (3), and in people with type 1 diabetes the risk of severe hypoglycemia increases by up to fourfold in those who do not recognize when their blood glucose falls to <54 mg/dL (4). The International Hypoglycaemia Study Group proposed that a blood glucose concentration <54 mg/dL is low enough to be considered serious, clinically important hypoglycemia and should be avoided (5). These observed findings show that when tested under hypoglycemic conditions, rtCGM reports significantly higher glucose readings compared with AV plasma and whole blood using SMBG. rtCGM remains a technology that can be of great benefit to people who struggle with IAH, but clinicians should be aware that it may underestimate the degree and recognition of hypoglycemia, therefore delaying treatment and the possible restoration of hypoglycemia awareness.
Article Information
Funding. C.M.F. was awarded the Sir George Alberti Fellowship by Diabetes UK to carry out this research (17/0005591). Funding was also provided by JDRF (3-SRA-2017-485-S-B).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.M.F. contributed to research design, conducted all experiments, acquired and analyzed data, and drafted the manuscript. S.M.H. and A.D.M. contributed to research design, analysis of data, and revision of the manuscript. R.J.M. contributed to research design and data analysis and drafted the manuscript. All authors approved the final version of the manuscript to be published. R.J.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. ISRCTN15373978, www.isrctn.org
References
- 1.Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann L. Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetologia 2017;60:2319–2328 [DOI] [PubMed] [Google Scholar]
- 2.Liu D, Moberg E, Kollind M, Lins PE, Adamson U, Macdonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia 1992;35:287–290 [DOI] [PubMed] [Google Scholar]
- 3.Frier BM, Heller S, McCrimmon RJ (Eds.). Hypoglycaemia in Clinical Diabetes. 3rd ed. Hoboken, NJ, Wiley-Blackwell, 2014, p. 49 [Google Scholar]
- 4.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 1994;344:283–287 [DOI] [PubMed] [Google Scholar]
- 5.International Hypoglycaemia Study Group Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017;40:155–157 [DOI] [PubMed] [Google Scholar]