Abstract
OBJECTIVE
We examined changes in glucose-lowering medication spending and quantified the magnitude of factors that are contributing to these changes.
RESEARCH DESIGN AND METHODS
Using the Medical Expenditure Panel Survey, we estimated the change in spending on glucose-lowering medications during 2005–2007 and 2015–2017 among adults aged ≥18 years with diabetes. We decomposed the increase in total spending by medication groups: for insulin, by human and analog; and for noninsulin, by metformin, older, newer, and combination medications. For each group, we quantified the contributions by the number of users and cost-per-user. Costs were in 2017 U.S. dollars.
RESULTS
National spending on glucose-lowering medications increased by $40.6 billion (240%), of which insulin and noninsulin medications contributed $28.6 billion (169%) and $12.0 billion (71%), respectively. For insulin, the increase was mainly associated with higher expenditures from analogs (156%). For noninsulin, the increase was a net effect of higher cost for newer medications (+88%) and decreased cost for older medications (−34%). Most of the increase in insulin spending came from the increase in cost-per-user. However, the increase in the number of users contributed more than cost-per-user in the rise of most noninsulin groups.
CONCLUSIONS
The increase in national spending on glucose-lowering medications during the past decade was mostly associated with the increased costs for insulin, analogs in particular, and newer noninsulin medicines, and cost-per-user had a larger effect than the number of users. Understanding the factors contributing to the increase helps identify ways to curb the growth in costs.
Introduction
Diabetes imposes a substantial economic burden on the national health care system in the U.S. It was the most costly condition among common health conditions in 2013 (1). The direct medical costs attributable to diabetes doubled from 2007 to 2017, from $116 billion to $237 billion (2,3). Of all the service components of total medical costs for diabetes, the incremental expenditure on medications increased at a higher rate than other components, and its share increased from 27% in 1987 to 41% in 2011 (4). The financial burden of glucose-lowering medications becomes a great concern to patients, payors, and policymakers and can possibly cause adverse health outcomes for patients and negatively affect the health care system.
Many factors could have influenced the rising national spending on glucose-lowering medications. First, the number of medicines available for diabetes increased over time, especially after 2005 (5). By 2011, there were 13 classes of glucose-lowering medicines, and several new medicine classes were in development (6). Second, the prices of newer oral medications and newer forms of insulin also went up, increasing higher than the old ones. For example, the list price per tablet of dipeptidyl peptidase 4 inhibitors (DPP-4) increased from $6.67 to $8.92 from 2006 to 2013, and the average list price per milliliter of insulin increased from $4.34 to $12.92 from 2002 to 2013 (7). Third, the number of people taking glucose-lowering medications also increased. In 2003, 13.2 million adults filled prescriptions for glucose-lowering medications from commercial pharmacies. The number increased to 18.8 million in 2012 (8).
However, how these factors contributed to the increase in national spending on glucose-lowering medications is not well understood. Our study’s goals were to 1) estimate the increase in national spending for glucose-lowering medications from 2005–2007 to 2015–2017 in total and by medication groups and 2) quantify the contribution of each medication group, the number of users, and cost-per-user to the increase. Exploring the magnitude of contributions for each factor helps identify targeted interventions to slow down the rising cost of glucose-lowering medications.
Research Design and Methods
Data Source and Study Sample
Data were from the Medical Expenditure Panel Survey (MEPS), a nationally representative survey for the civilian noninstitutionalized population in the U.S.(9). The MEPS contains rich information on health conditions, health care service use, and health costs. To identify persons with diabetes, we used the MEPS full-year consolidated files, which contained person-level data within a calendar year. Physician’s diagnoses of diabetes were self-reported by participants. Each person was then linked to his or her use records of prescribed medicines.
Data on prescription medications were collected from both in-person interviews and pharmacies’ purchasing records (10). Survey participants reported all prescribed medicines they purchased. With written permission to release their pharmacy records, further information on medications, such as medication name, number of refills, national medication code, and costs, was collected directly from their pharmacies. Medication costs reflected the actual payment received for each refill, which was the sum of out-of-pocket (OOP) payments and payments made by private and public health insurances (10).
The study sample consisted of adults aged ≥18 years with diabetes who used glucose-lowering medications. To increase the sample size, we pooled data from three consecutive years during 2005–2007 and during 2015–2017 and properly weighted all estimates to account for the survey design so that estimates represent the average yearly values in each period.
Medication Classification
Glucose-lowering agents were categorized into medication groups by using the Multum Lexicon therapeutic class codes in MEPS. We first grouped glucose-lowering medicines into insulin and noninsulin. Among insulin, we identified analog insulin and human insulin using the generic (or biosimilar) names of the medications. Among noninsulin, we grouped agents into metformin, older medications (sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, and meglitinides), newer medications (DPP-4, amylin analog, glucagon-like peptide 1 receptor agonists [GLP-1], and sodium–glucose cotransporter 2 inhibitors [SGLT2]), and combination medications. A combination medication is a single pill with a fixed-dose combination of two or more active pharmaceutical ingredients. We separated metformin from other noninsulin medications because it is the most used and recommended first-line medication for the treatment of type 2 diabetes (T2D) (11).
Estimating National Costs and Increases
We estimated the national spending on glucose-lowering medications, in total and by medication group, for two periods, 2005–2007 and 2015–2017. The annual national cost was calculated in the following steps. First, we estimated the proportion of users for each of the medication groups from MEPS data. Second, we derived the number of users for each medication group by applying the proportion of users to the U.S. resident population estimates from the U.S. Census Bureau for people aged ≥18 years (12). Third, we calculated the cost-per-user for each medication group from MEPS. Finally, we multiplied the number of users and costs-per-user to estimate the expenditures for medication groups and added them together to obtain the total national glucose-lowering medication spending. The increase in the costs of glucose-lowering medications was the percentage change between 2005–2007 and 2015–2017. All costs were inflated to 2017 U.S. dollars using the gross domestic product price index (13).
Breaking Down the Increase in Medication Spending
To examine the magnitude of each factor leading to an increase in total spending, we used a full decomposition method, which is commonly used to assess spending growth between two periods (14). The contribution to cost growth of a given factor was assessed by determining how much would have changed if only one factor changed while other factors remained constant. Thus, the contribution or share of the given factor represents a percentage point increase in total medication cost (see the Supplementary Material for details). Using the decomposition method, we evaluated the magnitude, in percentage points, of each factor to explain the increase in total spending. Specifically, we separated the growth in cost for each medication group into the contributions of the number of users, cost-per-user, and their combined effect. The contribution of one medication group to the total medication cost was the sum of the effects of the three components within the medication group.
To understand who bore the increase in medication costs during the study period, we broke down cost-per-user by the source of payment for OOP, Medicare, Medicaid, private insurance, and others (Veterans Health Administration, TRICARE, Indian Health Service, workers’ compensation, and other miscellaneous sources). We estimated both the amount and proportion paid by each payment source and used a t test to examine the statistical significance of the difference between the two time points for each source.
Results
The study population included >5,000 individuals in each study period. Supplementary Table 1 provides sample characteristics. Compared with individuals in 2005–2007, those in 2015–2017 were older, more likely to be Hispanic than non-Hispanic, less likely to be married, more likely to be high school graduates or have a college education, and more likely to have public insurance.
Changes in National Spending, the Number of Users, and Cost-per-User
During 2005–2007 and 2015–2017, the annual national cost for glucose-lowering medications increased from $16.9 billion to $57.6 billion, representing a 240% increase (Table 1). Although the costs for both insulin and noninsulin medicines increased considerably, total insulin spending increased sixfold, whereas total noninsulin spending doubled. Because of the larger cost growth in insulin, the total cost for insulin surpassed the total cost for noninsulin during 2015–2017. Within the insulin category, the increase in the total cost of analog insulin was seven times the increase in human insulin. Among noninsulin medicines, total costs for newer medications increased by 17-fold, whereas total costs for older medications decreased by 80%.
Table 1.
Changes in the total cost, the number of users, and cost-per-user for glucose-lowering medicines from 2005–2007 to 2015–2017 (costs are in 2017 U.S. dollars)
| Total cost (in millions) | Number of users (in thousands) | Cost-per-user | ||||
|---|---|---|---|---|---|---|
| 2005–2007 | 2015–2017 | 2005–2007 | 2015–2017 | 2005–2007 | 2015–2017 | |
| All medications | 16,944 | 57,557 | 15,318 | 21,106 | 1,106 | 2,727 |
| Insulin | 4,723 | 33,323 | 4,333 | 7,303 | 1,090 | 4,562 |
| Human | 1,692 | 3,846 | 3,325 | 2,239 | 509 | 1,718 |
| Analog | 3,031 | 29,476 | 1,957 | 5,859 | 1,549 | 5,031 |
| Noninsulin | 12,221 | 24,234 | 13,262 | 17,997 | 922 | 1,346 |
| Metformin | 2,957 | 3,286 | 7,969 | 13,690 | 371 | 240 |
| Older noninsulin* | 7,299 | 1,466 | 9,090 | 7,049 | 803 | 208 |
| Newer noninsulin† | 864 | 15,840 | 657 | 4,322 | 1,316 | 3,665 |
| Combinations‡ | 1,101 | 3,643 | 1,641 | 1,474 | 671 | 2,471 |
Older noninsulin medications include sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, and meglitinides.
Newer noninsulin medications include DPP-4, amylin analog, GLP-1, and SGLT2.
A combination medication is a single pill with a fixed-dose combination of two or more active pharmaceutical ingredients.
In addition, both the number of users and cost-per-user increased, although at different rates. For insulin, the cost-per-user increased more than the number of users. For noninsulin medicines, both the number of users and cost-per-user increased by ∼40%. Within the insulin category, while more patients used insulin in general, the number of users for human insulin decreased. Among noninsulin medicines, newer medicines experienced an increase in both the number of users and cost-per-user, while metformin had more users but lower cost-per-user.
Contribution by Medication Groups
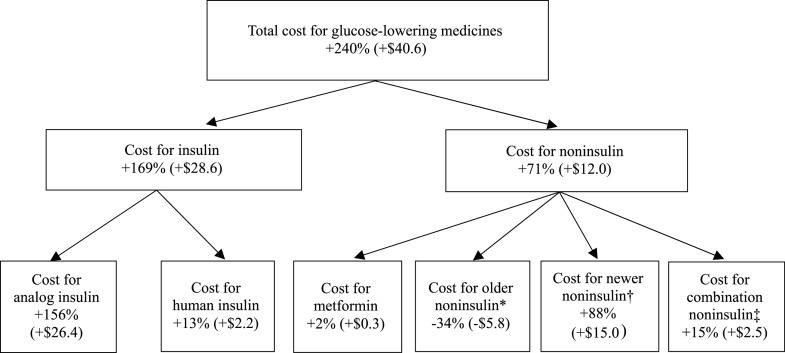
Of the $40.6 billion (240%) increase in total medication spending, two-thirds was from insulin (Fig. 1). Within the insulin category, 156 percentage points of the 169 percentage-point increase in the cost of all insulins was from analog insulin. Within the noninsulin category, the $12.0 billion (71 percentage points) increase in total medication cost was the net result of a 34 percentage-point decrease in older medication spending, an 88 percentage-point increase in newer medicines, and a 15 percentage-point increase in combination medications. Metformin had a small effect on total cost change.
Figure 1.
Contribution (in percentage points and dollar values in 2017 U.S. dollars, billions) of medication groups to the increase in total glucose-lowering medication spending, 2005–2007 to 2015–2017. *Older noninsulin medications include sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, and meglitinides. †Newer noninsulin medications include DPP-4, amylin analog, GLP-1, and SGLT2. ‡A combination medication is a single pill with a fixed-dose combination of two or more active pharmaceutical ingredients.
Contribution by the Number of Users and Cost-per-User
The contributions of the number of users and cost-per-user differed between insulin and noninsulin medicines (Table 2). For insulin, the cost-per-user had a larger effect on spending than the number of users for all insulin groups. In particular, the contribution of the number of users was negative to human insulin. For noninsulin medicines, cost-per-user had a slightly larger effect on spending than the number of users overall. However, the effect of the cost-per-user was negative for metformin and older noninsulin medicines. For newer noninsulin medicines, which contributed the majority of the spending increase for noninsulin medicines overall, the effect of the number of users was three times that of cost-per-user.
Table 2.
Contribution (in percentage points) of the number of users and cost-per-user to the increase in glucose-lowering medication spending, 2005–2007 to 2015–2017
| Number of users | Cost-per-user | Combined effect* | |
|---|---|---|---|
| Insulin | 19 | 89 | 61 |
| Human | −3 | 24 | −8 |
| Analog | 36 | 40 | 80 |
| Noninsulin | 26 | 33 | 12 |
| Metformin | 13 | −6 | −4 |
| Older noninsulin† | −10 | −32 | 7 |
| Newer noninsulin‡ | 28 | 9 | 51 |
| Combinations§ | −1 | 17 | −2 |
Combined effect represents the interaction of the changes in number of users and cost-per-user.
Older noninsulin medications include sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, and meglitinides.
Newer noninsulin medications include DPP-4, amylin analog, GLP-1, and SGLT2.
A combination medication is a single pill with a fixed-dose combination of two or more active pharmaceutical ingredients.
Source of Payment
Changes in the cost-per-user by payment source are presented in Table 3. Most of the increase in medication costs between 2005–2007 and 2015–2017 was borne by payors, especially by Medicare. In contrast, the amount of OOP payment did not change substantially. Furthermore, the proportion of the cost-per-user paid by OOP fell significantly for all medication groups except analog insulin.
Table 3.
Changes in amount and proportion paid by different sources for the average annual cost-per-user
| 2005–2007 | 2015–2017 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OOP | Medicare | Medicaid | Private insurance | Other | OOP | Medicare | Medicaid | Private insurance | Other | |
| Amount (2017 US$) | ||||||||||
| All medication | 347 | 236 | 98 | 357 | 68 | 249* | 1,094* | 223* | 978* | 183* |
| Insulin | 342 | 232 | 112 | 324 | 80 | 364* | 1,979* | 445* | 1,442* | 332* |
| Human | 215 | 98 | 52 | 90 | 53 | 187* | 1,019* | 136* | 251 | 125* |
| Analog | 392 | 347 | 159 | 563 | 87 | 382* | 2,077* | 503* | 1,702* | 367* |
| Noninsulin | 290 | 197 | 76 | 306 | 53 | 145* | 480* | 81 | 561* | 80* |
| Metformin | 140 | 61 | 22 | 121 | 26 | 25* | 71 | 20 | 110 | 13 |
| Older noninsulin† | 237 | 189 | 80 | 250 | 48 | 35* | 81* | 11* | 61* | 20* |
| Newer noninsulin‡ | 289 | 306 | 14 | 696 | 12 | 354* | 1,418* | 213* | 1,507* | 174* |
| Combinations§ | 229 | 128 | 59 | 226 | 30 | 332 | 652* | 122 | 1,119* | 246* |
| Proportion (%) | ||||||||||
| All medication | 31.4 | 21.3 | 8.9 | 32.3 | 6.1 | 9.1* | 39.9* | 8.1* | 35.6* | 6.7* |
| Insulin | 31.4 | 21.3 | 10.3 | 29.7 | 7.3 | 8.1* | 43.8* | 9.9* | 31.9* | 7.4* |
| Human | 42.3 | 19.3 | 10.2 | 17.7 | 10.4 | 11.3* | 61.5* | 8.2* | 15.1* | 7.5 |
| Analog | 25.3 | 22.4 | 10.3 | 36.4 | 5.6 | 7.6 | 41.1* | 10.0* | 33.7* | 7.3* |
| Noninsulin | 31.5 | 21.4 | 8.2 | 33.2 | 5.7 | 10.7* | 35.5* | 6.0* | 41.4* | 5.9* |
| Metformin | 37.8 | 16.5 | 5.9 | 32.7 | 7.0 | 10.6* | 30.1* | 8.5* | 46.6* | 5.5* |
| Older noninsulin | 29.5 | 23.5 | 10.0 | 31.1 | 6.0 | 16.7* | 38.6* | 5.2* | 29.0* | 9.5* |
| Newer noninsulin | 22.1 | 23.4 | 1.1 | 53.3 | 0.9 | 9.6* | 38.4* | 5.8* | 40.9* | 4.7* |
| Combinations | 34.1 | 19.0 | 8.8 | 33.6 | 4.5 | 12.7* | 25.0* | 4.7* | 42.9 | 9.4* |
Significantly different (P < 0.05) compared with 2005–2007.
Older noninsulin medications include sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, and meglitinides.
Newer noninsulin medications include DPP-4, amylin analog, GLP1, and SGLT2.
A combination medication is a single pill with a fixed-dose combination of two or more active pharmaceutical ingredients.
Conclusions
From 2005–2007 to 2015–2017, the national total annual cost for glucose-lowering medicines more than tripled; insulin added mostly to the increase. The main contributing factor of the total spending increase for insulin was a shift to analogs and an increase in costs-per-user. Furthermore, increased use of newer noninsulin medicines had a large effect on increases in total noninsulin medication spending.
Our estimates of multifold increases in total glucose-lowering medication spending, especially total insulin spending, are consistent with previous studies that documented a rising trend of total costs for glucose-lowering medicines during the past two decades. Previous studies found that the total cost for insulin tripled from 1997 to 2007 (15), and the per capita cost for insulin tripled from 2002 to 2013 in the U.S. (7). With the decomposition method, our study extends existing knowledge by quantifying that three-quarters of the increase in total medication cost from 2005–2007 to 2015–2017 was associated with insulin, specifically analog insulin.
For insulin, our estimates showed that cost-per-user accounted for a larger share of the spending increase than the number of users. Previous studies have found that the unit list price of insulin grew fast, and it related to an exponential increase in Medicaid reimbursement and patients’ OOP cost (7,16,17). Our estimates further showed that the national spending on insulin increased considerably and that cost-per-user was a big contributing factor in insulin spending. We also found that a change in medication choice (i.e., decreased use of human insulin and increased use of analog insulin) may have played a role in the spending increase. Previous studies suggested such a shift as one of the reasons for the rapid growth in insulin spending (17–19). We quantified that the contribution of the share of analog insulin was 12 times the contribution of the share of human insulin to the increase in overall insulin spending. Within analogs, the contribution of basal insulin was slightly larger than the contribution of rapid-acting insulin (Supplementary Table 2).
For noninsulin medicines, the number of users had a larger effect than the cost-per-user for most noninsulin medication groups. There are many possible reasons. First, the increase in the number of users could be a natural consequence of the increase in both the prevalence of diabetes and the proportion of patients with diabetes taking noninsulin medicines. From 2006 to 2015, the prevalence of diagnosed diabetes increased from 5.6% (16 million) to 9.3% (23 million) (12,20). Alongside this trend, the total number of prescriptions from commercial pharmacies for noninsulin medicines increased by one-third, from 89 million to 121 million, between 2003 and 2012 (8). Second, the benefit of metformin is better understood, and its use is more common among patients with T2D in recent years. As the first-line medication for T2D recommended by the American Diabetes Association, the use of metformin doubled from 2003 to 2012 (8). Third, newer noninsulin medicines, including DPP-4, GLP-1, and SGLT2, are being used more frequently nowadays than following their first introduction to the market (8).
The high cost of glucose-lowering medicines could lead to adverse health outcomes. In the U.S., nearly a quarter of patients with diabetes encounter cost-related insulin underuse (17). Studies have shown that a reduction in medication adherence results in poor glycemic control and increased hospitalization (21). The effect of medication costs on patients extends beyond health consequences, because many insulin underusers also report going into financial debt or cutting back on living expenses to pay for their medications (22).
Addressing the affordability of insulin is a complex issue. Since we found that medication choice and cost-per-user were important contributing factors to insulin cost, health care providers may consider the use of less expensive insulins when medically appropriate. The American Diabetes Association recommended that health care providers could consider prescribing human insulin instead of analogs for most patients with T2D who have a low risk of hypoglycemia (11,23). One study found that substitution of analogs with human insulin reduced insulin costs considerably among Medicare beneficiaries in 2 years (24). Another study found that switching from extended-release insulin to immediate-release insulin when they are therapeutically equivalent could also reduce insulin costs (25). Certainly, a medication regimen should be made primarily based on clinical benefits, and such a decision should not be focusing exclusively on cost consideration.
The rising cost of insulin is also at the center of policy discussion. Previous studies have proposed various potential solutions such as increasing competition by introducing more generic (or biosimilar) forms of insulin to the market and regulating the pricing mechanism through enhancing the transparency of the insulin market and simplifying the supply chain (26,27). Policies aimed at addressing these issues could reduce insulin prices considerably.
For noninsulin medications, although newer medicines, such as SGLT2 inhibitors and GLP-1 receptor agonists, have been associated with cost increases, they may have clinical advantages. Recent cardiovascular trials suggested cardiovascular benefits of SGLT2 inhibitors and GLP-1 receptor agonists in patients with T2D and cardiovascular disease or chronic kidney disease (28–30). While the availability of newer medications increases treatment choices, the decision of the regimen needs to consider both the potential benefits and also costs of medications, especially for patients with high OOP payments.
Another noteworthy solution to the rising medication costs lies in the efficient communication between physicians and patients. Physicians make their prescription decisions by considering more of the clinical benefits of medications than the cost of medications (31). Patients, even those with financial difficulties to pay for their medications, hesitate to discuss cost issues with their providers (22). Transparency about the cost burden of medication selection in discussions between physicians and patients could have profound implications for lowering medication costs (22,27).
Compared with the U.S., many other high-income countries have much lower medication costs. One study found that, on average, the U.S. spent four times more on noninsulin medications for persons with diabetes in 2015 than other high-income countries, such as Australia, Canada, France, and the U.K. (32). One of the reasons for the difference is that medication prices are highly regulated in these countries. For example, New Zealand maintains relatively low medication prices via negotiated and competitive national supply contracts (32). The U.K. entails critical reviews of the cost-effectiveness of new medications before the approval of these medications for public reimbursement. Some other countries adopt a single-payor system that has stronger consolidated bargaining power at medication prices compared with payors in the U.S. (32). These policies have effectively curbed the rising medication costs in these countries. In contrast, the U.S. has no nationwide policies to constrain medication costs. However, some states have begun to implement relevant policies. Colorado, for example, was the first state to pass a bill to cap the monthly copay of insulin at $100 in 2019 (33). We expect that more states will enact similar policies to shield patients from rising medication costs.
Future studies are needed to explore the in-depth causes and implications of rising medication costs. For example, future studies can investigate the underlying factors that have caused the increasing cost-per-user, such as how much of the increase was due to adherence or dose, to changes in the unit price of medications, and to a switch from cheaper to more expensive medications. Moreover, it is also interesting to see whether using newer agents at a higher cost leads to better glucose control and a reduction in diabetes complications or hospitalization costs. At the national level, there is no clear evidence that glucose control has been improved over time (34,35). Rates of acute myocardial infarction and stroke in persons with diabetes have been stable since 2010 after a long-time improvement, while the rate of diabetes-related lower-extremity amputation increased after 2010 (36,37). Studies are needed to examine whether the use of newer glucose-lowering medications is cost-effective. In addition, future studies can explore who bears the financial burden of the increased medication spending and what is the implication to patients, payors, and the health care system. Our crude analysis on the source of payment for cost-per-user showed that the increase in spending on glucose-lowering medications between 2005–2007 and 2015–2017 was mainly paid by payors, while the OOP spending changed little. However, there might be variations in OOP spending across subpopulations. For example, persons without insurance, most likely from low-income families, may face a high financial burden as a result of increased medication costs.
Our study has several limitations. First, costs were measured as payments in the analyses, which are payments for each refill of medication and aggregated at the person level. The payment reflected the total amount paid by insurers and patients, and it might differ from the amount received by drug manufacturers due to discounts and rebates. The degree of the gap is unknown at the national level due to the lack of information on discounts and rebates for each medication. A study that used data for a broad range of branded medications found that the net prices (i.e., list prices subtracted discounts) still increased substantially between 2007 and 2018 (38). Future studies can refine our estimates if data on discounts and rebates become available. Second, diabetes diagnoses were self-reported. However, MEPS data are proven to be a good source because disease diagnoses were confirmed by associated medical events (39). Third, the decomposition method does not reveal a causal relationship between driving factors and rising spending. Fourth, the full decomposition method did not remove the effect of population changes as we broke down total medication spending rather than per capita medication spending (14,40).
During 2005–2007 and 2015–2017, the total cost for glucose-lowering medications more than tripled. The main contributing factors to such an increase were more users, a higher cost-per-user for analog insulin, and more users of newer noninsulin medicines. The current study provides data to the recent national discussion on why medication therapy for diabetes management is so expensive. Our findings are useful to guide policies for targeted interventions to reduce medication costs for diabetes.
Article Information
Acknowledgments. The authors thank Michael Weeks and Clarice G. Conley at the Centers for Disease Control and Prevention for their editorial assistance with this article.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. X.Z. designed the research, analyzed data, interpreted results, and drafted the manuscript. S.S.S. and H.S. provided important intellectual content to the manuscript. P.Z. designed the research and revised the manuscript. X.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention.
See accompanying article, p. 2330.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12493493.
References
- 1.Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996-2013. JAMA 2016;316:2627–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhuo X, Zhang P, Kahn HS, Bardenheier BH, Li R, Gregg EW. Change in medical spending attributable to diabetes: national data from 1987 to 2011. Diabetes Care 2015;38:581–587 [DOI] [PubMed] [Google Scholar]
- 5.White JR., Jr A brief history of the development of diabetes medications. Diabetes Spectr 2014;27:82–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen QT, Thomas KT, Lyons KB, Nguyen LD, Plodkowski RA. Current therapies and emerging drugs in the pipeline for type 2 diabetes. Am Health Drug Benefits 2011;4:303–311 [PMC free article] [PubMed] [Google Scholar]
- 7.Hua X, Carvalho N, Tew M, Huang ES, Herman WH, Clarke P. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. JAMA 2016;315:1400–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care 2014;37:1367–1374 [DOI] [PubMed] [Google Scholar]
- 9.Agency for Healthcare Research and Quality . Medical Expenditure Panel Survey (MEPS) [Internet], August 2018. Available from: https://www.ahrq.gov/data/meps.html. Accessed 12 November 2019. [DOI] [PubMed] [Google Scholar]
- 10.Moeller JF, Stagnitti MN, Horan E, et al. Outpatient prescription drugs: data collection and editing in the 1996 medical expenditure panel survey (HC-010A). In MEPS Methodology Report. Rockville, MD, Agency for Healthcare Research and Quality, 2001 [Google Scholar]
- 11.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S90–S102 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017. Atlanta, GA, Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017 [Google Scholar]
- 13.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res 2018;53:175–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorpe KE. Treated disease prevalence and spending per treated case drove most of the growth in health care spending in 1987-2009. Health Aff (Millwood) 2013;32:851–858 [DOI] [PubMed] [Google Scholar]
- 15.Sarpong E, Miller GE. Trends in the Pharmaceutical Treatment of Diabetes, 2007: A Comparison of Utilization and Expenditures, 1997 to 2007. Rockville, MD, Agency for Healthcare Research and Quality, 2010 [Google Scholar]
- 16.Luo J, Avorn J, Kesselheim AS. Trends in Medicaid reimbursements for insulin from 1991 through 2014. JAMA Intern Med 2015;175:1681–1686 [DOI] [PubMed] [Google Scholar]
- 17.Lipska KJ, Ross JS, Van Houten HK, Beran D, Yudkin JS, Shah ND. Use and out-of-pocket costs of insulin for type 2 diabetes mellitus from 2000 through 2010. JAMA 2014;311:2331–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beran D, Ewen M, Laing R. Constraints and challenges in access to insulin: a global perspective. Lancet Diabetes Endocrinol 2016;4:275–285 [DOI] [PubMed] [Google Scholar]
- 19.Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care 2017;40:468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 21.Khunti K, Seidu S, Kunutsor S, Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care 2017;40:1588–1596 [DOI] [PubMed] [Google Scholar]
- 22.Piette JD, Heisler M, Wagner TH. Problems paying out-of-pocket medication costs among older adults with diabetes. Diabetes Care 2004;27:384–391 [DOI] [PubMed] [Google Scholar]
- 23.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018;61:2461–2498 [DOI] [PubMed] [Google Scholar]
- 24.Luo J, Khan NF, Manetti T, et al. Implementation of a health plan program for switching from analogue to human insulin and glycemic control among Medicare beneficiaries with type 2 diabetes. JAMA 2019;321:374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumarsono A, Sumarsono N, Das SR, Vaduganathan M, Agrawal D, Pandey A. Economic burden associated with extended-release vs immediate-release drug formulations among Medicare Part D and Medicaid beneficiaries. JAMA Netw Open 2020;3:e200181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo J, Kesselheim AS. Evolution of insulin patents and market exclusivities in the USA. Lancet Diabetes Endocrinol 2015;3:835–837 [DOI] [PubMed] [Google Scholar]
- 27.Cefalu WT, Dawes DE, Gavlak G, et al.; Insulin Access and Affordability Working Group . Insulin access and affordability working group: conclusions and recommendations. Diabetes Care 2018;41:1299–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 29.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 31.Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med 2007;4:e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan SG, Good CB, Leopold C, Kaltenboeck A, Bach PB, Wagner A. An analysis of expenditures on primary care prescription drugs in the United States versus ten comparable countries. Health Policy 2018;122:1012–1017 [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. American Diabetes Association Applauds Colorado Governor and State Legislature for Passing HB 1216: Reduce Insulin Prices Bill [Internet], 22 May 2019. Available from: https://www.diabetes.org/newsroom/press-releases/2019/colorado-reduce-insulin-prices-bill. Accessed 12 November 2019
- 34.Carls G, Huynh J, Tuttle E, Yee J, Edelman SVJDT. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther 2017;8:863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang M. Trends in diabetes management among US adults: 1999–2016. J Gen Intern Med 2020;35:1427–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geiss LS, Li Y, Hora I, Albright A, D Rolka, Gregg EW. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care 2019;42:50–54 [DOI] [PubMed] [Google Scholar]
- 37.Gregg EW, Hora I, Benoit SRJJ. Resurgence in diabetes-related complications. JAMA 2019;321:1867–1868 [DOI] [PubMed] [Google Scholar]
- 38.Hernandez I, San-Juan-Rodriguez A, Good CB, Gellad WFJJ. Changes in list prices, net prices, and discounts for branded drugs in the US, 2007-2018. JAMA 2020;323:854–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machlin SR, Soni A. Health care expenditures for adults with multiple treated chronic conditions: estimates from the Medical Expenditure Panel Survey, 2009. Prev Chronic Dis 2013;10:E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starr M, Dominiak L, Aizcorbe A. Decomposing growth in spending finds annual cost of treatment contributed most to spending growth, 1980-2006. Health Aff (Millwood) 2014;33:823–831 [DOI] [PubMed] [Google Scholar]