Abstract
OBJECTIVE
To explore the meal response of circulating succinate in patients with obesity and type 2 diabetes undergoing bariatric surgery and to examine the role of gastrointestinal glucose sensing in succinate dynamics in healthy subjects.
RESEARCH DESIGN AND METHODS
Cohort I comprised 45 patients with morbid obesity and type 2 diabetes (BMI 39.4 ± 1.9 kg/m2) undergoing metabolic surgery. Cohort II was a confirmatory cohort of 13 patients (BMI 39.3 ± 1.4 kg/m2) undergoing gastric bypass surgery. Cohort III comprised 15 healthy subjects (BMI 26.4 ± 0.5 kg/m2). Cohorts I and II completed a 2-h mixed-meal tolerance test (MTT) before the intervention and at 1 year of follow-up, and cohort II also completed a 3-h lipid test (LT). Cohort III underwent a 3-h oral glucose tolerance test (OGTT) and an isoglycemic intravenous glucose infusion (IIGI) study.
RESULTS
In cohort I, succinate response to MTT at follow-up was greater than before the intervention (P < 0.0001). This response was confirmed in cohort II with a greater increase after 1 year of surgery (P = 0.009). By contrast, LT did not elicit a succinate response. Changes in succinate response were associated with changes in the area under the curve of glucose (r = 0.417, P < 0.0001) and insulin (r = 0.204, P = 0.002). In cohort III, glycemia, per se, stimulated a plasma succinate response (P = 0.0004), but its response was greater in the OGTT (P = 0.02; OGTT versus IIGI).
CONCLUSIONS
The meal-related response of circulating succinate in patients with obesity and type 2 diabetes is recovered after metabolic surgery.
Introduction
A mismatch between nutrient availability and cellular energy requirements is a key contributing factor to the development of obesity and type 2 diabetes mellitus. Dynamic exchanges in intra- and extracellular metabolites are crucial to adequately integrate and coordinate biological networks in cells, particularly the concentration of nutrients and intermediary metabolites (1,2).
There is a wealth of evidence to indicate that succinate is a pleiotropic metabolite functioning not only as an energy intermediary but also as a signaling molecule, both in the cytosol and extracellularly by engaging its cognate receptor succinate receptor 1 (SUCNR1) (1,3,4). In the context of energy homeostasis, various signaling roles have been ascribed to succinate, including those of an antilipolytic factor (5), a potent activator of brown adipose tissue thermogenesis (6), and a regulator of intestinal gluconeogenesis (7,8). Succinate has also been shown to control the resolution of inflammation, a physiological circuit broken in obesity. Indeed, macrophage-specific deficiency of SUCNR1 in mice stimulates inflammation, glucose intolerance, and cellular metabolic stress (9).
Elevated levels of fasting plasma succinate have been mainly related to pathological processes (10–13), including obesity and type 2 diabetes (12,14). By contrast, a reduction in circulating levels of succinate after bariatric surgery is positively associated with the rate of remission of type 2 diabetes (14). Accordingly, obesity might be associated with succinate resistance, as has been shown for other hormones such as insulin or leptin (15), favoring a vicious cycle of succinate resistance-hypersuccinatemia, at least with regards to its effects on the resolution of inflammation (9). Fascinatingly, circulating succinate levels are not only increased in pathology, and it has been known for many years that succinate levels are elevated in some physiological processes such as exercise (16). But, despite much progress, the physiological function of succinate in energy balance and its involvement in the physiopathology of obesity and associated comorbidities is unclear.
The main source of circulating succinate remains enigmatic, although our recent evidence points to the intestine as an important contributor to blood levels (12). To further examine this idea, in the current study, we explored plasma succinate dynamics after food ingestion in patients with morbid obesity and type 2 diabetes before and after bariatric surgery. We also determined whether the succinate response is dependent on glucose and/or lipid sensing, as well as the contribution of glucose sensing through the gastrointestinal tract in healthy subjects.
Research Design and Methods
Cohort I
Cohort I comprised 45 patients with morbid obesity and type 2 diabetes who were submitted to bariatric surgery in the context of a randomized controlled trial (http://www.isrctn.com/ISRCTN14104758). Methodological aspects and the main characteristics of the patients have been published else‐ where (17). In brief, the patients (30 women, 15 men, age 49 ± 8 years, BMI 39.4 ± 1.9 kg/m2, and HbA1c 7.8 ± 1.9% [62.0 ± 3.3 mmol/mol]) were consecutively recruited for bariatric surgery at the Department of Endocrinology of Bellvitge University Hospital. Patients were randomly assigned (1:1:1) to three subgroups (n = 15) and subjected to one of the following bariatric procedures: laparoscopic greater curvature plication, Roux-en-Y gastric bypass and sleeve gastrectomy. Before surgery and at 1 year after surgery, patients underwent an anthropometric, clinical, and routine biochemical evaluation and a 2-h mixed-meal tolerance test (MTT).
Cohort II
This group included 13 patients with morbid obesity and type 2 diabetes (9 women, 4 men, age 53 ± 7 years, BMI 39.3 ± 1.4 kg/m2) undergoing metabolic surgery at Bellvitge University Hospital between June 2016 and June 2017 (Table 1). Inclusion/exclusion criteria were the same as those for cohort I. All patients underwent Roux-en-Y gastric bypass. The mean weight loss at the end of follow-up was 33.8% (range 23.8–42.3 kg) of the initial weight. Pharmacological treatment was stopped at least 3 days before the functional tests, except insulin treatment, which was stopped 12 h before tests. As in cohort I, patients from cohort II underwent a complete anthropometric, clinical, and routine biochemical evaluation and a 2-h MTT before surgery and at 1 year after surgery. Patients also underwent a 3-h lipid test (LT) during the same periods.
Table 1.
Cohort II: main anthropometric and metabolic variables
| Variables | Baseline | Follow-up at 12 months | P value* |
|---|---|---|---|
| n (female/male) | 13 (9/4) | 13 (9/4) | — |
| Age (years) | 53 ± 7 | 54 ± 7 | — |
| Type 2 diabetes treatment (insulin/others) | 13 (4/9) | 13 (1/12) | — |
| BMI (kg/m2) | 39.3 ± 1.4 | 25.8 ± 2.1 | <0.0001 |
| Waist (cm) | 124.9 ± 16.4 | 92.4 ± 11.4 | <0.0001 |
| Fasting glucose (mmol/L) | 8.9 (6.9–11.0) | 5.1 (4.5–6.1) | 0.0007 |
| 2-h glucose (mmol/L) | 14.5 ± 5.2 | 7.0 ± 3.7 | 0.0005 |
| HbA1c (%) | 7.3 (6.7–8.0) | 5.6 (4.6–6.0) | 0.0002 |
| HbA1c (mmol/mol) | 56.3 (49.7–64.5) | 37.7 (26.3–41.6) | 0.0002 |
| Total cholesterol (mmol/L) | 4.9 ± 1.0 | 4.1 ± 0.7 | 0.002 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.4 | 1.3 ± 0.3 | NS |
| LDL cholesterol (mmol/L) | 2.9 ± 0.9 | 2.3 ± 0.6 | NS |
| TGs (mmol/L) | 1.7 (1.1–3.7) | 1.4 (0.7–1.5) | 0.003 |
| Fasting succinate (µmol/L) | 79.7 ± 28.0 | 51.0 ± 15.3 | 0.003 |
| Fasting insulin (pmol/L) | 116.0 (63.5–275.5) | 39.0 (34.5–55.0) | 0.001 |
| Fasting C-peptide (nmol/L) | 1.47 ± 0.97 | 0.52 ± 0.22 | 0.007 |
| Fasting GLP-1 (pmol/L) | 54.6 (42.1–72.8) | 32.8 (18.5–39.6) | 0.008 |
| TG index | 5.15 ± 0.12 | 4.57 ± 0.08 | <0.0001 |
| Insulinogenic index | 0.3 (0.1–0.6) | 0.9 (0.3–1.3) | NS |
Data are presented as mean ± SD or median (25th–75th percentiles), as appropriate.
P values for the normal distributed variables were calculated using paired t test; for the nonnormal distributed variables, Wilcoxon signed rank test was used.
Cohort III
This group included 15 healthy subjects (11 women, 4 men, age 34 ± 12 years, BMI 26.4 ± 1.9 kg/m2) consecutively recruited at the Hospital Universitari Joan XXIII. Inclusion criteria were BMI ≥19.9 and ≤29.9 kg/m2, absence of acute or chronic systemic disease, absence of pharmacological treatment, and weight stability during the three previous months before entry into the study (Table 2).
Table 2.
Cohort III: main anthropometric and metabolic variables
| Variables | OGTT | IIGI | P value* |
|---|---|---|---|
| n (female/male) | 15 (11/4) | 15 (11/4) | — |
| Age (years) | 34 ± 12 | — | — |
| BMI (kg/m2) | 26.4 ± 1.9 | — | — |
| Waist (cm) | 86.9 ± 8.3 | — | — |
| Fat mass (%) | 30.7 ± 6.3 | — | — |
| Fasting glucose (mmol/L) | 5.3 ± 0.3 | 5.3 ± 0.4 | NS |
| 2 h glucose (mmol/L) | 6.8 ± 1.2 | 6.9 ± 1.0 | NS |
| HbA1c (%) | 5.1 ± 0.2 | — | — |
| HbA1c (mmol/mol) | 32.0 ± 0.7 | — | — |
| Total cholesterol (mmol/L) | 4.1 ± 0.9 | — | — |
| HDL cholesterol (mmol/L) | 1.4 ± 0.2 | — | — |
| LDL cholesterol (mmol/L) | 2.3 ± 0.7 | — | — |
| TGs (mmol/L) | 0.7 (0.6–1.0) | — | — |
| Fasting succinate (µmol/L) | 41.3 ± 14.4 | 42.1 ± 4.2 | NS |
| Fasting insulin (pmol/L) | 47.5 ± 17.5 | 43.1 ± 3.8 | NS |
| Fasting C-peptide (nmol/L) | 0.4 (0.3–0.4) | 0.3 (0.2–0.4) | NS |
| Fasting GLP-1 (pmol/L) | 26.8 ± 8.7 | 24.4 ± 2.2 | NS |
| TG index | 4.4 ± 0.1 | — | — |
| OGIS index (mL/min/m2) | 414.0 ± 53.8 | — | — |
| Insulinogenic index | 1.0 ± 0.4 | — | — |
Data are presented as mean ± SD or median (25th–75th percentiles), as appropriate. OGIS, oral glucose insulin sensitivity index.
P values for the normal distributed variables were calculated using paired t test; for the nonnormal distributed variables, Wilcoxon signed rank test was used.
Subjects underwent a 3-h standard oral glucose tolerance test (OGTT) and, on a separate occasion, a 3-h isoglycemic intravenous glucose infusion (IIGI) study using an ad hoc algorithm to precisely reproduce the glycemic curve observed during the OGTT (isoglycemic protocol).
Ethical Disclosure
All study protocols were conducted according to the principles of the Declaration of Helsinki and approved by the corresponding local ethics committees. All subjects received a comprehensive explanation of the protocol and signed the informed consent before entry into the studies.
Metabolic Assessments
Metabolic studies (MTT, LT, OGTT, and IIGI) were performed in the morning (starting between 7 and 9 a.m.) after an overnight fast, with no food or drink (except for water) after 8 p.m. of the preceding day. After a medical history record and body composition assessment, an intravenous line was established in the antecubital vein, and after 15–30 min of rest, the test was started. Specifically, for the IIGI study, two intravenous lines were used: one in the antecubital vein for the glucose infusion and the other in the cephalic vein (wrist) of the same arm for blood sampling.
Meal Tolerance Test
Patients ingested a standardized liquid meal beverage (cohort I: 15.9% proteins, 53.8% carbohydrates, and 30.3% lipids [202 kcal]; Edanec, Abbott laboratories, and cohort II: 16% proteins, 49% carbohydrates, and 30% lipids [320 kcal]; Isosource, Nestle Health Science) over 5 min. Blood was sampled before meal ingestion (time 0 min) and at 15, 30, 60, and 120 min after meal ingestion (17).
Lipid Test
Patients were prepared as in the MMT protocol. Blood samples were drawn at fasting state (time 0 min) and at 60, 120, and 180 min after lipid ingestion. The LT was performed using an oral lipid solution ingested over 5 min, containing 50 g of fat in 100 mL of solution, of which 30% was saturated, 49% was monounsaturated, and 21% was polyunsaturated (18).
OGTT and IIGI Tests
Each volunteer participated in two studies with a 7–15-day interval between each. The first study was a 3-h OGTT (75 g glucose), and plasma glucose was measured every 10 min during the test. The OGTT glucose time curve was then reconstructed in the second study, i.e., the IIGI study, using an ad hoc algorithm to determine the variable infusion rate of a 20% glucose solution (19). Blood samples for metabolites other than glucose were drawn at −30, 0, 10, 20, 30, 60, 90, 120, 150, and 180 min after glucose ingestion or infusion was started.
Determinations
Plasmatic lipid, hepatic and renal profiles were determined by standard enzymatic methods. Plasma glucose was determined by the glucose oxidase method (ADVIA Centaur; Siemens Healthcare, Erlangen, Germany and GM-9; Analox, London, U.K.) Plasma insulin and C-peptide levels were determined by an immunochemiluminometric assay (ADVIA Centaur). Total plasma glucagon-like peptide 1 (GLP-1) levels were determined by radioimmunoassay (GLP-1T-36HK) or by ELISA (EZGLP1T-36 K) in cohorts I and II/III, respectively (both from Merck KGaa, Darmstadt, Germany) (17). Plasma succinate was determined in plasma filtrates (10 KDa) using a fluorometric assay (EnzyChrom Succinate Assay Kit; BioAssay Systems, Hayward, CA) (9,12,14).
Data Analysis
Body fat mass of patients (cohort I and II) was estimated using the Clínica Universidad de Navarra-body adiposity estimator (CUN-BAE) equation (20) or was analyzed by bioelectrical impedance (Tanita Europe BV, Amsterdam, the Netherlands) (cohort III). We validated the use of the CUN-BAE index for body fat percentage/adiposity in Cohort II by DEXA (Hologic QDR 4500; Hologic Inc., Waltham, MA). Fat mass estimated by the CUN-BAE equation correlated with that measured by DEXA (r = 0.913, P < 0.0001). Insulin resistance was estimated using the product of fasting plasma glucose (FPG) and triglyceride (TG) index (Ln[TG(mg/dL)*FPG(mg/dL)]/2), or using the oral glucose insulin sensitivity index (21). The insulinogenic index was calculated using the equation (Insulin[μU/mL] 30’ − 0’)/(Glucose[mg/dL] 30’ − 0’). Area under the time concentration curve (AUC) was calculated using the trapezoidal rule. Succinate response was calculated as a fold increase of the fasting values. The percentage change of a variable between the baseline and follow-up periods was calculated as follows: Δ% = ([follow up − baseline]/baseline)*100.
Statistical Analysis
All data were tested for normality using the Shapiro-Wilk test. Data are presented as percentage and mean and SD for normally distributed quantitative variables, or median and 25th–75th percentiles (interquartile range [IQR]) for nonnormally distributed quantitative variables. Intragroup responses were compared by paired t test or Wilcoxon signed-rank test when necessary. The time course (parameter response curves) data were evaluated by ANOVA for repeated measures; P values show the interaction between treatment and time. Correlations between quantitative variables were calculated using Pearson’s or Spearman’s test, when necessary. Succinate response was depicted as fold increase from basal values (normalized to 1). Multiple linear regression analysis was used to determine the variables associated with succinate dynamics. All variables significant in univariate analysis were included in the model. Statistical analyses were carried out using SPSS software version 19 (IBM Corp., Armonk, NY).
Results
Dynamic Regulation of Circulating Succinate After Food Ingestion Is Dependent on Metabolic Status
The main anthropometric and clinical characteristics of cohort I have been described previously (14,17), together with the associations between fasting succinate levels and metabolic variables before surgery (14). Notably, we found a positive association between fasting plasma succinate and TG index as a measure of insulin resistance (r = 0.479, P = 0.002), whereas a negative association was observed with the insulinogenic index (r = −0.363, P = 0.02). Also, consistent with our previous study (14), fasting plasma succinate levels were reduced by 32.5% at 1 year of follow-up (P = 0.001) (Fig. 1A). Follow-up fasting plasma levels of succinate were associated with weight (r = 0.386, P = 0.01), FPG (r = 0.390, P = 0.01), HbA1c percentage (r = 0.374, P = 0.02), fasting plasma TGs (r = 0.444, P = 0.005), and TG index (r = 0.480, P = 0.002).
Figure 1.
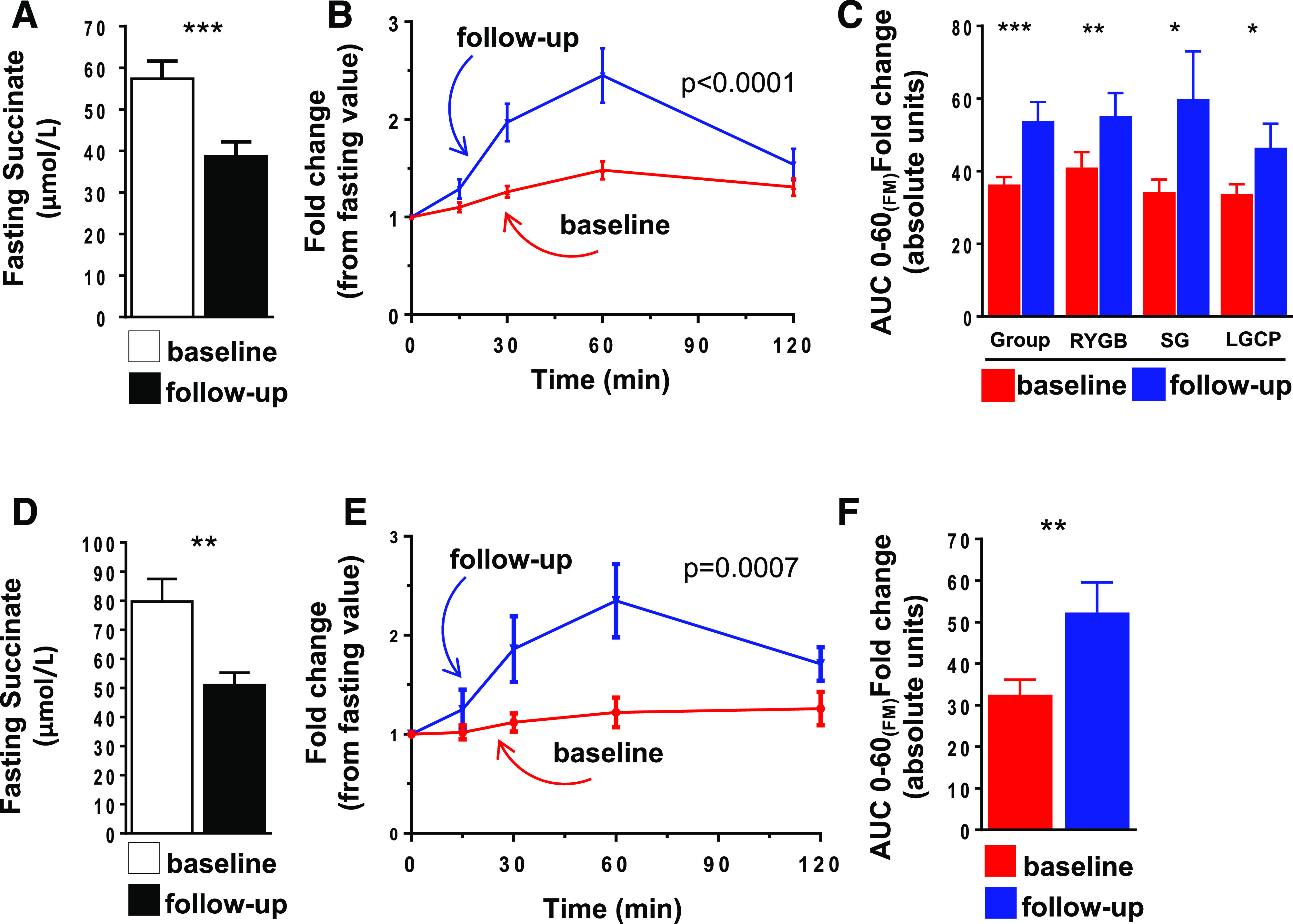
Succinate response to a MTT. A and D: Fasting values of succinate before and 1 year after bariatric surgery for cohort I (A) and II (D). B and E: Time curves of plasma succinate response during an MTT (fold increase over basal values) for cohort I (B) and II (E). C and F: AUC of the succinate time curves normalized for fat mass (kg) for cohort I (C) and II (F). Data are mean ± SEM. Comparisons were tested using the Wilcoxon signed rank test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001), and time curves were compared using repeated measures ANOVA (P values refer to the interaction between treatment and time). FM, fat mass; LGCP, laparoscopic greater curvature plication; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Of note, an examination of plasma succinate dynamics during an MTT revealed a different pattern before and after surgery. At baseline, nutrient intake resulted in a small but significant increase in plasma succinate of 1.48 ± 0.09-fold over basal levels at 60 min (P = 0.003). By contrast, a repeat of the MTT 1 year after surgery revealed a 2.44 ± 0.28-fold increase in succinate over basal levels (P < 0.0001) (Fig. 1B). The normalization of the AUC fold change of the succinate response by fat mass (kg) confirmed a more pronounced succinate response after surgery, which was independent of the surgical technique (Fig. 1C). Notably, the percentage change in plasma succinate levels after surgery was associated with the percentage change in plasma glucose (r = 0.417, P < 0.0001) and insulin (r = 0.204, P = 0.002) during the MTT.
We then performed a multiple regression analysis controlling for age, sex, and change in BMI. Change in AUC of glucose (β = −0.365, P = 0.02) and AUC of insulin (β = 0.323, P = 0.03) appeared as the main determinants of succinate variability. The inclusion of the type of surgical treatment to the model did not change the results.
We sought to confirm the meal-related response of succinate in a second independent cohort (main anthropometric and metabolic variables of cohort II are described in Table 1, and the MTT metabolic response is shown in Supplementary Fig. 1A). In line with the data of cohort I (14), fasting succinate levels were reduced by 36.0% in cohort II after surgery (Fig. 1D). Mirroring the results from cohort I, the MTT (0–60 min) showed an increase of succinate 1.22 ± 0.15-fold (P = NS) before surgery and 2.35 ± 0.37-fold (P = 0.004) in the follow-up analysis (Fig. 1E). Again, the normalization of the AUC fold change of succinate by fat mass revealed a more pronounced succinate response (P = 0.008) at follow-up (Fig. 1F). Of note, in contrast to what was observed in response to an MTT, the hyperlipidemia resulting from the LT did not elicit a succinate response (Supplementary Fig. 1B).
Meal-Related Response of Succinate Is Dependent on Intestinal Glucose Sensing
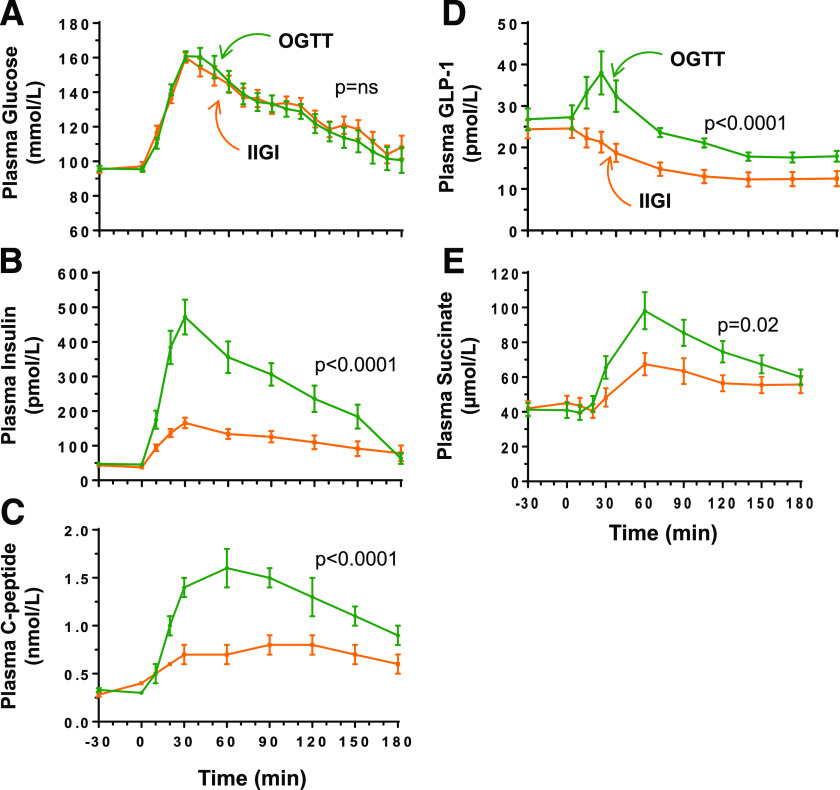
To determine if the nutritional-related succinate response depends on glucose sensing by the gastrointestinal tract or if it is also induced by intravenous glucose infusion, we analyzed succinate dynamics in a cohort of healthy subjects without obesity (cohort III, anthropometric and metabolic characteristics of subjects are shown in Table 2) after an oral and isoglycemic variable intravenous administration of glucose (OGTT and IIGI, respectively).
Plasma glucose curves were superimposable during the tests (P = NS) confirming quite similar peripheral glycemia (Fig. 2A). As expected, insulinemia was almost threefold greater in the oral test than with intravenous glucose stimulation (P < 0.0001) (Fig. 2B). A similar response was observed for C-peptide (P < 0.0001, IIGI versus OGTT) (Fig. 2C), revealing that gastrointestinal factors account for ∼44% of the total insulin response during the OGTT, as expected (19). Also, GLP-1 time curve analysis demonstrated distinct patterns depending on the route of glucose administration (Fig. 2D), with oral glucose promptly stimulating GLP-1 release, as previously described (19).
Figure 2.
Cohort III, metabolic response to an OGTT and an IIGI study. A: The overlay of plasma glucose curves during OGTT and IIGI. B–E: The response of plasma insulin (B), C-peptide (C), GLP-1 (D), and succinate (E) during the OGTT and IIGI. Data are mean ± SEM. Time curves were compared using repeated measures ANOVA (P values refer to the interaction between treatment and time).
Intriguingly, the succinate response to an oral or intravenous glucose administration differed. Both routes of glucose administration elicited a plasmatic response of succinate with a peak at 60 min (Fig. 2E). In the OGTT, the succinate response was 2.4 ± 0.9-fold higher than the fasting value (P < 0.0001), whereas it increased by 1.5 ± 0.4-fold in the IIGI (P = 0.0004). Accordingly, the AUC for succinate (3 h) was higher in the OGTT than in the IIGI (13,020.7 ± 1,059.8 vs. 10,140.5 ± 900.4 µmol/L, respectively, P = 0.0004).
Conclusions
To our knowledge, this is the first description of the nutritional modulation of plasma succinate by luminal nutrients, as contrasted with the traditional paradigm of circulating succinate as a pathological metabolic marker (10–12,22,23). Moreover, we demonstrate that the nutritional-related response of succinate is partly dependent on glucose sensing by the intestine and is associated with the metabolic status of the individual, pointing to an integrated mechanism underpinning these dynamic changes.
Beyond its role as an energy source in the tricarboxylic acid (TCA) cycle, succinate is a positive regulator of both intestinal gluconeogenesis (8) and adipose tissue thermogenesis (6). We and others previously demonstrated that increased plasma levels of succinate are associated with metabolic abnormalities, such as hy-pertension, obesity, and type 2 diabetes (11,12). Moreover, recovery from hyperglycemia and body weight gain is associated with a reduction in fasting plasma succinate, both by lifestyle changes and bariatric surgery (12,14). Consistent with previous data, we found a clear association between fasting succinate, BMI, HbA1c, FPG, and plasma TGs, supporting the notion of elevated circulating succinate as a biomarker of a poor metabolic status (9,12,14,24).
When we analyzed the dynamics of circulating succinate in response to a nutritional challenge in patients with morbid obesity and type 2 diabetes, before and after bariatric surgery, we found a similar pattern of results to those of plasma insulin and GLP-1 (17,25,26). In two independent surgical cohorts, we found that the patients had high levels of fasting succinate at baseline (before surgery) and a mostly flat succinate response to a meal test. A study by Sadagopan et al. (11) reported no differences between fasting or postprandial plasma succinate levels in healthy control subjects or patients with diabetes. By contrast, a recent metabolomic analysis in healthy postmenopausal women reported a similar succinate response to a mixed meal that observed in our study (24). In our surgical cohorts, weight loss and metabolic improvement promoted by bariatric surgery stimulated a decrease in the fasting levels of succinate and triggered a recovery in nutrient stimulation, with a normal bell-shaped succinate response curve in response to an MTT similar to that observed for glucose, insulin and GLP-1 (17,25,26). Remarkably, carbohydrates seemed to be uniquely responsible for the succinate response as the LT had no effect on succinate at baseline or follow-up.
The glucose tests in healthy subjects have shed some light on the potential mechanism underpinning the novel meal-related succinate response. Accordingly, the time curve of succinate response was clearly higher in the OGTT than in the IIGI and is similar to that observed for insulin, C-peptide, and GLP-1. The results indicate the relevance of glucose transit through the intestinal tract for postprandial succinate dynamics, pointing to the intestine as a relevant source of circulating succinate after feeding.
In keeping with this notion, our previous studies demonstrated the close association between circulating succinate and the gut microbiota (12,27). Nevertheless, further studies are required to validate this relationship and alternative sources should not be ruled out, particularly in the context of obesity. For example, it has been described in human adipose tissue explants that hyperglycemia and hypoxia exert a synergistic effect on succinate production (13). Thus, changes in adiposity could explain the differences in succinate response observed between subjects with morbid obesity before and after bariatric surgery. However, the profile of the succinate response was unchanged when the AUC of succinate response was normalized for fat mass (before and after surgery). Similar results were observed with the normalization by lean mass (data not shown). Consequently, it is possible that in obesity, circulating succinate originating from both adipose tissue and intestinal microbiota provokes a condition of chronically elevated succinate, suppressing succinate dynamics induced by a nutritional challenge. In fact, weight loss after bariatric interventions improves adipose tissue inflammation (28), gut permeability (29) and dysbiosis, and modifies the levels of TCA cycle intermediary metabolites (30). Hence, it is tempting to speculate that after weight loss, succinate levels decrease and the evident dynamic response in healthy subjects is recovered, restoring succinate sensitivity, which is a plausible marker of metabolic health status. However, further investigation would be required to determine whether the recovery of succinate response in obese patients with diabe-tes after metabolic surgery is dependent on weight loss, or conversely, it could be detected in early stages where metabolic improvement does not fully rely on weight loss.
Assessing the physiological significance of nutrient-related succinate dynamics is a key challenge that needs to be addressed in the future. In this context, the new concept of energy metabolites as signaling molecules with extracellular functions beyond energy is gaining traction (22,23). On the basis of the results presented here, it is not unreasonable to suspect that succinate might function similarly to other microbiota-derived metabolites (e.g., short-chain fatty acids) as a paracrine and autocrine signal in metabolic tissues, such as adipose tissue (1). Indeed, succinate has been described as an inhibitor of lipolysis in adipocytes via activation of SUCNR1 (5,31). It is generally acknowledged that peripheral SUCNR1 remains inactive under healthy conditions and would be activated only by the accumulation of succinate in pathological states. The data presented here and elsewhere describing higher circulating succinate levels after exercise (16) points to a new role for this metabolite in physiological metabolic homeostasis.
In conclusion, our data reveal a meal-related response of circulating succinate that is influenced by the metabolic status of the subject and is dependent on glucose sensing by the gastrointestinal tract. This response is blunted in patients with morbid obesity and type 2 diabetes and is recovered after weight loss. This nutritional modulation of plasma succinate in healthy states goes against the general perception of circulating succinate as an exclusively surrogate marker of hypoxia, tissue damage, and inflammation. Further research is needed to establish the physiological role of postprandial succinate and to fully understand the effect of loss of succinate dynamics in the pathogenesis of diabetes and obesity.
Article Information
Acknowledgments. The authors thank the BioBank-IISPV (PT17/0015/0029) integrated into the Spanish National Biobanks Network for its collaboration and Judit Borras Mauri, Alba Guasch Sintes (Unitat d'Estudis Clinics – Institut d’Investigació Sanitària Pere Virgili), and Zoila Nathalie Mora Cevallos (Master Degree in Nutrition and Metabolism – Rovira I Virgili University [URV]) who participated in the human recruitment and helped to conduct the metabolic tests. The authors thank the study participants.
Funding. This study was supported by grants from the Ministerio de Ciencia e Innovación (PI18/00516 to A.M., PI14/01997 and PI17/01556 to N.V., PI14/00228 and PI17/0153 to J.V., SAF2015-65019R and RTI2018-093919-B-I00 to S.F.-V., and CB07708/0012) and cofinanced by the European Regional Development Fund (ERDF). The Spanish Biomedical Research Center in Diabetes and Associated Metabolic Disorders is an initiative of the Instituto de Salud Carlos III. B.A. is the recipient of a Martí Franquès Postdoctoral Fellowship Program 2018 – URV (MINECO/AEI/FEDER/UE), Spain, and S.F.-V. is the recipient of the Miguel Servet tenure-track program (CP10/00438 and CPII16/00008) from the Fondo de Investigación Sanitaria, cofinanced by the ERDF.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.A., J.V., and S.F.-V. conceived, designed, and supervised the research project and wrote the manuscript. B.A., V.C.-M., G.L., M.M.R., and A.C. participated in sample collection and statistical analysis and wrote the manuscript. L.M. participated in patient screening and study execution. M.T.-P. and M.M.R. performed sample analysis. A.M., N.V., and S.P. provided scientific discussion and revised the manuscript. A.C., S.P., and N.V. participated in human recruitment and helped to conduct the metabolic tests. S.F.-V. and J.V. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. ISRCTN14104758, www.isrctn.org
This article contains supplementary material online at https://doi.org/10.2337/figshare.12621884.
References
- 1.Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW. GPCR-mediated signaling of metabolites. Cell Metab 2017;25:777–796 [DOI] [PubMed] [Google Scholar]
- 2.Ho JE, Larson MG, Vasan RS, et al. . Metabolite profiles during oral glucose challenge. Diabetes 2013;62:2689–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilissen J, Jouret F, Pirotte B, Hanson J. Insight into SUCNR1 (GPR91) structure and function. Pharmacol Ther 2016;159:56–65 [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 2020;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell 2008;135:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills EL, Pierce KA, Jedrychowski MP, et al. . Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018;560:102–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K, Liao M, Zhou N, et al. . Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep 2019;26:222–235.e5 [DOI] [PubMed] [Google Scholar]
- 8.De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab 2016;24:151–157 [DOI] [PubMed] [Google Scholar]
- 9.Keiran N, Ceperuelo-Mallafré V, Calvo E, et al. . SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat Immunol 2019;20:581–592 [DOI] [PubMed] [Google Scholar]
- 10.DʼAlessandro A, Moore HB, Moore EE, et al. . Plasma succinate is a predictor of mortality in critically injured patients. J Trauma Acute Care Surg 2017;83:491–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadagopan N, Li W, Roberds SL, et al. . Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am J Hypertens 2007;20:1209–1215 [DOI] [PubMed] [Google Scholar]
- 12.Serena C, Ceperuelo-Mallafré V, Keiran N, et al. . Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J 2018;12:1642–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Diepen JA, Robben JH, Hooiveld GJ, et al. . SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced inflammation and diabetes. Diabetologia 2017;60:1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceperuelo-Mallafré V, Llauradó G, Keiran N, et al. . Preoperative circulating succinate levels as a biomarker for diabetes remission after bariatric surgery [published correction appears in Diabetes Care 2019;42:2347] Diabetes Care 2019;42:1956–1965 [DOI] [PubMed] [Google Scholar]
- 15.Mitchell A, Lazar MJB. Principles of hormone action. In Williams Textbook of Endocrinology. 13th ed Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, Eds. Philadelphia, Elsevier Inc., 2016, p. 18–48 [Google Scholar]
- 16.Hochachka PW, Dressendorfer RH. Succinate accumulation in man during exercise. Eur J Appl Physiol Occup Physiol 1976;35:235–242 [DOI] [PubMed] [Google Scholar]
- 17.Casajoana A, Pujol J, Garcia A, et al. . Predictive value of gut peptides in T2D remission: randomized controlled trial comparing metabolic gastric bypass, sleeve gastrectomy and greater curvature plication. Obes Surg 2017;27:2235–2245 [DOI] [PubMed] [Google Scholar]
- 18.Clemente-Postigo M, Queipo-Ortuño MI, Murri M, et al. . Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J Lipid Res 2012;53:973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muscelli E, Mari A, Natali A, et al. . Impact of incretin hormones on β-cell function in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab 2006;291:E1144–E1150 [DOI] [PubMed] [Google Scholar]
- 20.Gómez-Ambrosi J, Silva C, Catalán V, et al. . Clinical usefulness of a new equation for estimating body fat. Diabetes Care 2012;35:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001;24:539–548 [DOI] [PubMed] [Google Scholar]
- 22.Murphy MP, O’Neill LAJ. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell 2018;174:780–784 [DOI] [PubMed] [Google Scholar]
- 23.Bénit P, Letouzé E, Rak M, et al. . Unsuspected task for an old team: succinate, fumarate and other Krebs cycle acids in metabolic remodeling. Biochim Biophys Acta 2014;1837:1330–1337 [DOI] [PubMed] [Google Scholar]
- 24.Shrestha A, Müllner E, Poutanen K, Mykkänen H, Moazzami AA. Metabolic changes in serum metabolome in response to a meal. Eur J Nutr 2017;56:671–681 [DOI] [PubMed] [Google Scholar]
- 25.Astiarraga B, Gastaldelli A, Muscelli E, et al. . Biliopancreatic diversion in nonobese patients with type 2 diabetes: impact and mechanisms. J Clin Endocrinol Metab 2013;98:2765–2773 [DOI] [PubMed] [Google Scholar]
- 26.Camastra S, Muscelli E, Gastaldelli A, et al. . Long-term effects of bariatric surgery on meal disposal and β-cell function in diabetic and nondiabetic patients. Diabetes 2013;62:3709–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández-Veledo S, Vendrell J. Gut microbiota-derived succinate: friend or foe in human metabolic diseases? Rev Endocr Metab Disord 2019;20:439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camastra S, Vitali A, Anselmino M, et al. . Muscle and adipose tissue morphology, insulin sensitivity and beta-cell function in diabetic and nondiabetic obese patients: effects of bariatric surgery [published correction appears in Sci Rep 2018;8:8177] Sci Rep 2017;7:9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casselbrant A, Elias E, Fändriks L, Wallenius V. Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 2015;11:45–53 [DOI] [PubMed] [Google Scholar]
- 30.Tulipani S, Griffin J, Palau-Rodriguez M, et al. . Metabolomics-guided insights on bariatric surgery versus behavioral interventions for weight loss. Obesity (Silver Spring) 2016;24:2451–2466 [DOI] [PubMed] [Google Scholar]
- 31.McCreath KJ, Espada S, Gálvez BG, et al. . Targeted disruption of the SUCNR1 metabolic receptor leads to dichotomous effects on obesity. Diabetes 2015;64:1154–1167 [DOI] [PubMed] [Google Scholar]