Abstract
Background
The prognostic significance of cardiac radiation dose in esophageal cancer after definitive concurrent chemoradiotherapy (CCRT) remains largely unknown. We aimed to investigate the association between cardiac dose-volume parameters and overall survival (OS) in esophageal squamous cell carcinoma (ESCC) after definitive CCRT.
Methods
One hundred and twenty-one ESCC patients undergoing definitive CCRT with intensity modulated radiotherapy technique between 2008 and 2018 were reviewed. Cardiac dose-volume parameters were calculated. Survival of patients and cumulative incidence of adverse events were estimated by the Kaplan–Meier method and compared between groups by the log-rank test. The prognostic significance of cardiac dose-volume parameters was determined with multivariate Cox proportional hazards regression analysis.
Results
Median follow-up was 16.2 months (range, 4.3–109.3). Median OS was 18.4 months. Heart V5, V10, and V20 were independent prognostic factors of OS. Median OS was longer for patients with heart V5 ≤ 94.3% (24.7 vs. 16.3 months, p = 0.0025), heart V10 ≤ 86.4% (24.8 vs. 16.9 months, p = 0.0041), and heart V20 ≤ 76.9% (20.0 vs. 17.2 months, p = 0.047). Lower cumulative incidence of symptomatic cardiac adverse events was observed among patients with heart V5 ≤ 94.3% (p = 0.017), heart V10 ≤ 86.4% (p = 0.02), and heart V20 ≤ 76.9% (p = 0.0057). Patients without symptomatic cardiac adverse events had a higher 3-year OS rate (33.8% vs. 0%, p = 0.03).
Conclusions
Cardiac radiation dose inversely correlated with survival in ESCC after definitive CCRT. Radiation dose to the heart should be minimized.
Keywords: Esophageal cancer, Chemoradiotherapy, Intensity modulated radiotherapy, Cardiac radiation dose
Introduction
Esophageal cancer is the sixth leading cause of cancer-related death globally [1]. Definitive concurrent chemoradiotherapy (CCRT) without surgery is one of treatment options for locally advanced esophageal cancer [2–4]. As the outcome of esophageal cancer after definitive CCRT was unsatisfactory, it is important to find the prognostic factors and improve the treatment.
Radiation dose to the heart was a prognostic factor of non-small cell lung cancer treated with definitive CCRT [5–7]. Similarly, cardiac radiation dose was shown to correlate with overall survival (OS) in a large group of esophageal cancer patients undergoing CCRT with or without surgery [8]. However, the prognostic significance of cardiac radiation dose remains to be elucidated specifically in esophageal cancer after definitive CCRT without surgery.
In the present study, we analyzed a single-institution cohort of esophageal squamous cell carcinoma (ESCC) patients receiving definitive CCRT with intensity modulated radiotherapy (IMRT) technique. The association between cardiac dose-volume parameters and survival was examined.
Methods
Patients and study design
This study was approved by the institutional review board of our hospital. Patients with primary ESCC treated by definitive CCRT at our institution between 2008 and 2018 were retrospectively reviewed. The study patient flow diagram was shown in the Additional file 1: Fig. S1. Patients were recruited on the basis of criteria as follows: newly pathologically confirmed ESCC without distant metastasis, no past history of thoracic radiotherapy, CCRT via IMRT and conventional fractionation with dose ≥50 Gy, and follow-up after CCRT ≥3 months. The cases with distant metastasis, past history of thoracic radiotherapy, radiotherapy alone, two- or three-dimensional radiotherapy, radiation dose < 50 Gy, or follow-up < 3 months were excluded. The pre-treatment evaluation of esophageal cancer included esophagogastroduodenoscopy, endoscopic ultrasonography, computed tomography (CT) of the chest and abdomen, and bone scan. Positron emission tomography-computed tomography was performed in cases with indeterminate results of CT or bone scan. The clinical stage was classified according to the seventh edition of the American Joint Committee on Cancer staging system.
Definitive concurrent chemoradiotherapy
All patients received definitive CCRT for esophageal cancer with IMRT technique, as previously described [9]. Briefly, the gross tumor volume (GTV) consisted of GTV of the primary (GTVp) and GTV of lymph nodes (GTVn). The clinical target volume (CTV) 1 included GTVp with a 5-cm craniocaudal and 1-cm radial margin along the esophagus, and GTVn with a 1-cm margin. The CTV 2 included GTVp with a 2-cm craniocaudal and 1-cm radial margin along the esophagus, and GTVn with a 1-cm margin. The planning target volume (PTV) was generated by expanding 1 cm around the GTV and CTV in all directions. Elective nodal irradiation was omitted. CTV 1 and 2 with the relevant PTV were sequentially treated to 36 and 50–50.4 Gy, respectively. Thereafter, GTV with the relevant PTV was boosted up to 66.6 Gy if dose constraints of the organs at risk could be met. Normal tissue dose constraints included Dmax < 50 Gy for spinal cord, V50 < 33% for heart, V20 < 33% for lung, Dmax < 55 Gy for stomach, and V35 < 50% for liver. During radiation treatment, concurrent chemotherapy and supportive therapy were given.
Dosimetric analysis
The organs at risk were delineated on each axial slice of simulation CT scan [10, 11]. For heart, the superior aspect began from the level of the inferior border of the pulmonary artery passing the midline and extended inferiorly to the cardiac apex. Dose volume histogram of organs at risk was subsequently generated using the treatment planning system. We calculated the following dose-volume parameters of the heart and lung: mean dose and the percent volumes receiving doses ≥5 Gy (V5), ≥ 10 Gy (V10), ≥ 20 Gy (V20), ≥ 30 Gy (V30), ≥ 40 Gy (V40), and ≥ 50 Gy (V50).
Evaluation of symptomatic cardiac adverse events
Follow-up evaluations included clinical examinations, esophagogastroduodenoscopy, and CT scan of the chest and abdomen at 1 month after CCRT and then every 3–6 months. In addition, electrocardiography, echocardiogram, and other cardiovascular evaluations were arranged as clinically indicated. To identify symptomatic cardiac adverse events, clinical symptoms and signs, CT images, electrocardiograms, echocardiograms, and managements for cardiovascular diseases were reviewed.
Statistical analysis
The data cutoff date was June 26, 2019. OS was calculated from the start of IMRT to the date of death or last follow-up. The time to cardiac adverse events was defined as the interval from the beginning of IMRT to the occurrence of events. Survival of patients and cumulative incidence of cardiac events were estimated by the Kaplan-Meier method and compared between groups by the log-rank test. The factors associated with OS were checked with univariate analysis. The independent prognostic factors of OS were examined by multivariate Cox proportional hazards regression analyses in 42 models in which clinical variables with a trend in univariate analysis (p < 0.1), one cardiac dose-volume parameter, and one pulmonary parameter were taken into consideration. A p-value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 22.0 software and R version 3.5.1 for Windows.
Results
Patients’ characteristics
Of the 204 patients reviewed, 121 patients matched the recruitment criteria while 83 patients were excluded from the analysis with reasons as follows: stage IV (n = 21), radiation dose < 50 Gy (n = 23), post-CCRT follow-up < 3 months (n = 32), histology other than squamous cell carcinoma (n = 5), and use of 3-dimensional conformal radiotherapy (n = 23). Table 1 summarized demographic and clinical characteristics of the 121 patients, including five women and 116 men. Upper or middle esophagus was involved by the tumor in 104 (86.0%) patients. Furthermore, six (5.0%) patients had a history of cardiovascular diseases (1 coronary artery disease, 3 congestive heart failure, 1 aortic valve infectious endocarditis after valve replacement, and 1 arrhythmia) at baseline. The six patients were considered medically fit for CCRT under the suggestion of our institutional multidisciplinary esophageal cancer team.
Table 1.
Demographic and clinical characteristics of patients at baseline
| Characteristic | No. of patients (%) |
|---|---|
| Age (years) | |
| Median (Range) | 56 (34–81) |
| ≤ 56 | 61 (50.4) |
| > 56 | 60 (49.6) |
| Gender | |
| Male | 116 (95.9) |
| Female | 5 (4.1) |
| Body mass index (kg/m2) | |
| Median (Range) | 21.3 (15.5–30.0) |
| ≤ 21.3 | 61 (50.4) |
| > 21.3 | 60 (49.6) |
| Body surface area (m2) | |
| Median (Range) | 1.65 (1.3–2.1) |
| ≤ 1.65 | 63 (52.1) |
| > 1.65 | 58 (47.9) |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 11 (9.1) |
| 1 | 95 (78.5) |
| 2 | 14 (11.6) |
| 3 | 1 (0.8) |
| Stage | |
| I | 2 (1.7) |
| II | 8 (6.6) |
| III | 111 (91.7) |
| Tumor location | |
| U | 51 (42.1) |
| M | 30 (24.8) |
| L | 17 (14.0) |
| U + M (from U to M) | 9 (7.4) |
| U + M + L (from U to L) | 1 (0.8) |
| M + L (from M to L) | 13 (10.7) |
| Smoking | |
| Yes | 109 (90.1) |
| No | 12 (9.9) |
| Alcohol | |
| Yes | 111 (91.7) |
| No | 10 (8.3) |
| Hypertension | |
| Yes | 24 (19.8) |
| No | 97 (80.2) |
| Diabetes | |
| Yes | 15 (12.4) |
| No | 106 (87.6) |
| Cardiovascular disease | |
| Yes | 6 (5.0) |
| No | 115 (95.0) |
| Heart volume (ml) | |
| ≤ 592 | 61 (50.4) |
| > 592 | 60 (49.6) |
| Chemotherapy regimen | |
| Fluoropyrimidine-based | 113 (93.4) |
| Taxane-based | 4 (3.3) |
| Others | 4 (3.3) |
| Radiation dose (Gy) | |
| Median (range) | 61.2 (50–66.6) |
| ≤ 61.2 | 68 (56.2) |
| > 61.2 | 53 (43.8) |
| PTV prescribed to 36 Gy (ml) | |
| Median (Range) | 780.4 (97.1–1799.5) |
| PTV prescribed to 50 Gy (ml) | |
| Median (Range) | 640.0 (26.0–1761.2) |
Abbreviations: L Lower thoracic esophagus, M Middle thoracic esophagus, PTV Planning target volume, U Upper thoracic and cervical esophagus
Treatment
The utilized chemotherapy regimens and radiation doses were summarized in the Additional file 2: Table S1. The median radiation dose was 61.2 Gy (range, 50–66.6 Gy). The radiation doses were not different between patients with or without pre-existing cardiovascular diseases (p = 0.613). Fluoropyrimidine-based chemotherapy regimens were used in 113 (93.4%) patients. Most patients received either cisplatin (25 mg/m2) plus fluorouracil (1000 mg/m2) given intravenously every week or cisplatin (20 mg/m2 daily, on day 1–4) plus fluorouracil (800 mg/m2 daily, on day 1–4) given intravenously every 4 weeks. Other regimens were utilized at the discretion of physicians. Furthermore, during CCRT, enteral nutrition support was given via nasogastric, percutaneous endoscopic gastrostomy, and feeding jejunostomy tubes in eight (6.6%), 11 (9.1%), and 17 (14.0%) patients, respectively. Medications for emesis or pain as well as intravenous hydration were given as clinically indicated.
Clinical characteristics associated with overall survival
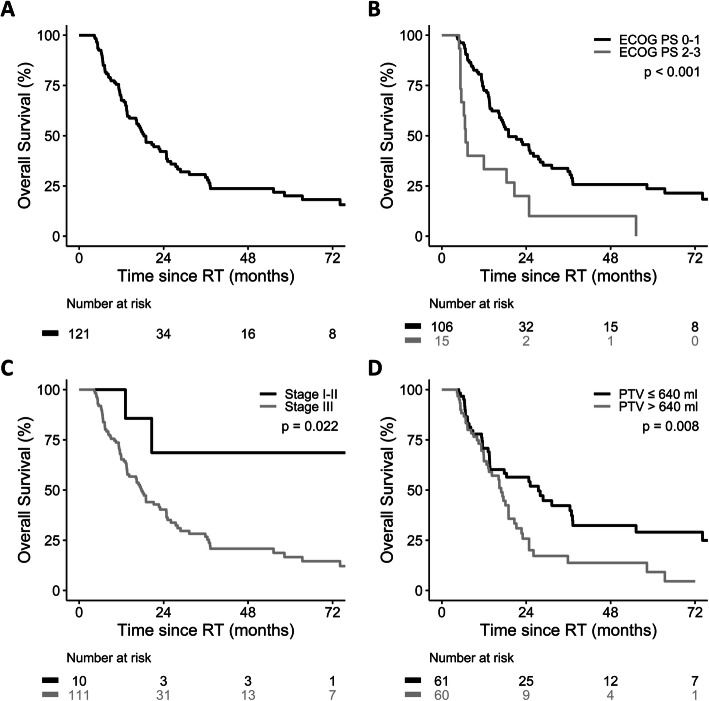
The median follow-up was 16.2 (range, 4.3–109.3) and 21.2 months (range, 5.8–104.6) for the whole cohort and the surviving patients, respectively. The median OS of the whole cohort was 18.4 months (Fig. 1a). In univariate analysis, body mass index, body surface area, Eastern Cooperative Oncology Group (ECOG) performance status, stage, chemotherapy regimens, and the volume of PTV prescribed to 50 Gy were associated with OS, but the pre-existing cardiovascular diseases was not correlated with OS (p = 0.87, Table 2). Moreover, ECOG performance status, stage, chemotherapy regimens, and the volume of PTV prescribed to 36 Gy and 50 Gy were independent prognostic factors of OS by multivariate analysis (Additional files 3, 4, 5, 6, 7, 8 and 9: Table S2–8). The median OS was longer for patients with ECOG performance status 0–1 (19.0 vs. 6.8 months, p < 0.001; Fig. 1b), stage I-II (not reached vs. 17.7 months, p = 0.022; Fig. 1c), fluoropyrimidine-based chemotherapy (19.0 vs. 11.2 months, p = 0.0016), and volume of PTV prescribed to 50 Gy ≤ 640 ml (27.4 vs. 16.9 months, p = 0.008; Fig. 1d). On the other hand, the median volume of overlap between PTV and the heart was 25.1 ml. The median OS was not statistically different between patients with the overlapping volume > 25.1 ml and ≤ 25.1 ml (18.4 vs. 18.1 months, p = 0.192).
Fig. 1.
Overall survival a whole cohort, b by ECOG performance status, c by stage, and d by volume of PTV prescribed to 50 Gy
Table 2.
Univariate analysis of clinical variables associated with overall survival
| Variable | Univariate analysis | |
|---|---|---|
| HR (95% CI) | P value | |
| Age (≤ 56 vs. > 56) | 0.986 (0.639–1.522) | 0.951 |
| Gender (female vs. male) | 0.710 (0.224–2.254) | 0.561 |
| Body mass index (kg/m2) (≤ 21.3 vs. > 21.3) | 1.699 (1.096–2.634) | 0.018 |
| Body surface area (m2) (≤1.65 vs. > 1.65) | 1.614 (1.042–2.502) | 0.032 |
| ECOG performance status (0–1 vs. 2–3) | 0.376 (0.210–0.672) | 0.001 |
| Stage (I&II vs. III) | 0.223 (0.055–0.910) | 0.036 |
| Tumor location (U involved vs. others) | 0.733 (0.473–1.136) | 0.165 |
| Smoking (no vs. yes) | 0.744 (0.358–1.547) | 0.428 |
| Alcohol (no vs. yes) | 0.529 (0.214–1.310) | 0.169 |
| Hypertension (no vs. yes) | 1.002 (0.587–1.710) | 0.994 |
| Diabetes (no vs. yes) | 0.624 (0.335–1.160) | 0.136 |
| Cardiovascular disease (no vs. yes) | 0.919 (0.336–2.518) | 0.870 |
| Heart volume (ml) (≤592 vs > 592) | 1.468 (0.947–2.276) | 0.086 |
| Chemotherapy regimen (F vs. NF) | 0.318 (0.150–0.673) | 0.003 |
| Radiation dose (Gy) (≤ 61.2 vs. > 61.2) | 1.451 (0.924–2.280) | 0.106 |
| PTV prescribed to 36 Gy (ml) (continuous) | 1.001 (1.000–1.001) | 0.055 |
| PTV prescribed to 50 Gy (ml) (continuous) | 1.001 (1.000–1.002) | 0.004 |
Abbreviations: ECOG Eastern Cooperative Oncology Group, F Fluoropyrimidine-based, NF Not fluoropyrimidine-based, PTV Planning target volume, U Upper thoracic and cervical esophagus
Dose-volume parameters associated with overall survival
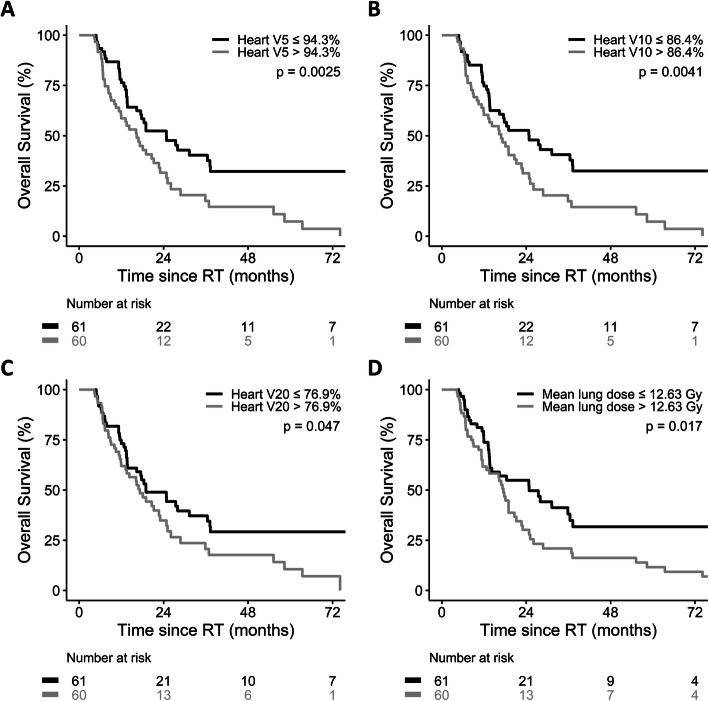
Heart V5, V10, and V20 consistently served as independent prognostic factors of OS under consideration of individual pulmonary dose-volume parameters (Table 3 and Additional file 3, 4, 5, 6, 7, 8 and 9: Table S2–8). The median heart V5, V10, and V20 were 94.3, 86.4, and 76.9%, respectively. A longer median OS was observed among patients with heart V5 ≤ 94.3% (24.7 vs. 16.3 months, p = 0.0025; Fig. 2a), heart V10 ≤ 86.4% (24.8 vs. 16.9 months, p = 0.0041; Fig. 2b), and heart V20 ≤ 76.9% (19.0 vs. 17.2 months, p = 0.047; Fig. 2c). In addition, mean lung dose was consistently shown to be a prognostic factor of OS in analytic models including different cardiac dose-volume parameters (Additional file 3, 4, 5, 6, 7, 8, 9 and 10: Table S2–9). Patients with mean lung dose ≤12.63 Gy had a superior median OS (24.8 vs. 17.5 months, p = 0.017; Fig. 2d).
Table 3.
Multivariate analysis for heart dose-volume parameters and overall survival under consideration of different lung parameters
| Heart | Mean lung dose | Lung V5 | Lung V10 | Lung V20 | Lung V30 | Lung V40 |
|---|---|---|---|---|---|---|
| HR (95% CI) P value | ||||||
| Mean dose | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| (1.000–1.000) | (1.000–1.000) | (1.000–1.000) | (1.000–1.000) | (1.000–1.000) | (1.000–1.000) | |
| 0.052 | 0.068 | 0.034 | 0.035 | 0.022 | 0.020 | |
| V5 | 1.011 | 1.011 | 1.012 | 1.012 | 1.012 | 1.012 |
| (1.001–1.020) | (1.001–1.021) | (1.002–1.022) | (1.002–1.021) | (1.003–1.022) | (1.003–1.022) | |
| 0.029 | 0.032 | 0.016 | 0.016 | 0.010 | 0.009 | |
| V10 | 1.010 | 1.010 | 1.011 | 1.011 | 1.011 | 1.012 |
| (1.001–1.019) | (1.001–1.020) | (1.002–1.020) | (1.002–1.020) | (1.003–1.020) | (1.003–1.021) | |
| 0.032 | 0.039 | 0.020 | 0.020 | 0.012 | 0.010 | |
| V20 | 1.010 | 1.010 | 1.011 | 1.011 | 1.011 | 1.012 |
| (1.001–1.019) | (1.001–1.020) | (1.002–1.020) | (1.002–1.020) | (1.003–1.020) | (1.003–1.021) | |
| 0.029 | 0.038 | 0.019 | 0.020 | 0.011 | 0.009 | |
| V30 | 1.010 | 1.009 | 1.011 | 1.010 | 1.011 | 1.011 |
| (1.000–1.020) | (0.999–1.020) | (1.001–1.020) | (1.001–1.020) | (1.002–1.021) | (1.002–1.021) | |
| 0.048 | 0.068 | 0.035 | 0.036 | 0.021 | 0.016 | |
| V40 | 1.011 | 1.011 | 1.012 | 1.012 | 1.012 | 1.013 |
| (1.001–1.022) | (1.000–1.022) | (1.001–1.023) | (1.001–1.022) | (1.002–1.023) | (1.002–1.023) | |
| 0.034 | 0.053 | 0.028 | 0.028 | 0.019 | 0.017 | |
| V50 | 1.014 | 1.012 | 1.014 | 1.014 | 1.014 | 1.014 |
| (1.000–1.027) | (0.999–1.026) | (1.001–1.028) | (1.001–1.028) | (1.001–1.028) | (1.001–1.028) | |
| 0.046 | 0.077 | 0.042 | 0.038 | 0.035 | 0.040 | |
Fig. 2.
Overall survival by a heart V5, b heart V10, c heart V20, and d mean lung dose
Dose-volume parameters associated with symptomatic cardiac adverse events
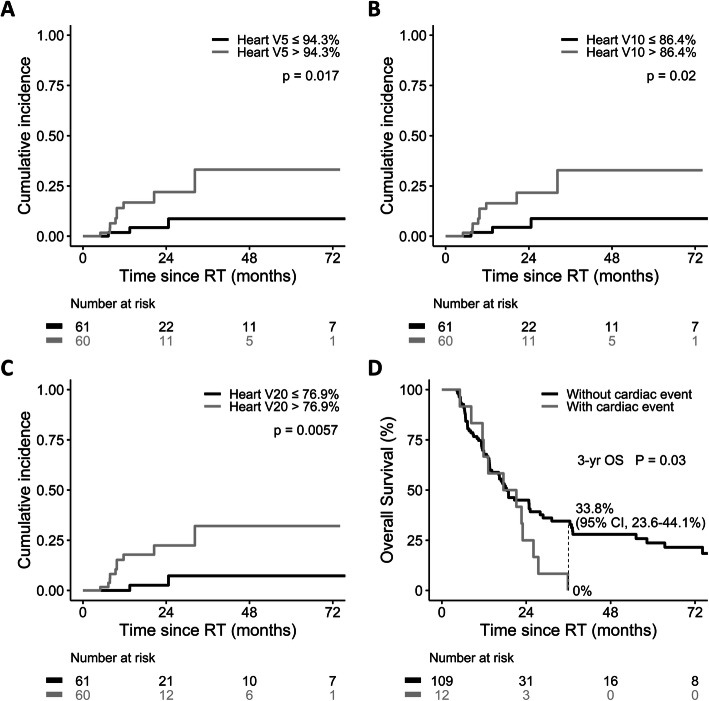
There were 12 symptomatic CCRT-related cardiac adverse events, including ischemic heart disease in one, arrhythmia in three, and pericardial effusion in eight patients. The median interval from the start of IMRT to development of cardiac events was 9.8 months. The pre-existing cardiovascular diseases were not associated with the cumulative incidence of cardiac adverse events after CCRT (p = 0.459). Lower cumulative incidence of symptomatic cardiac adverse events was found among patients with heart V5 ≤ 94.3% (p = 0.017; Fig. 3a), heart V10 ≤ 86.4% (p = 0.02; Fig. 3b), and heart V20 ≤ 76.9% (p = 0.0057; Fig. 3c). Moreover, patients without symptomatic cardiac adverse events had a higher 3-year OS rate (33.8% vs. 0%, p = 0.03; Fig. 3d). There was a trend toward better survival at 2 years in patients without symptomatic cardiac complications (44.3% vs. 25.0%, p = 0.23).
Fig. 3.
Cumulative incidence of symptomatic cardiac adverse events by a heart V5, b heart V10, and c heart V20. d Overall survival by symptomatic cardiac adverse events
Cancer-specific survival and cause of death
The causes of deaths were shown in Table 4. Fifty-eight (47.9%) patients died of esophageal cancer with or without other etiologies. The median esophageal cancer-specific survival was 24.7 months. As shown in the Additional file 11: Fig. S2, a longer median esophageal cancer-specific survival was observed among patients with heart V5 ≤ 94.3% (36.4 vs. 21.2 months, p = 0.042), heart V10 ≤ 86.4% (36.4 vs. 19.0 months, p = 0.032), and volume of PTV prescribed to 50 Gy ≤ 640 ml (37.2 vs. 19.0 months, p = 0.005). In addition, there was a trend toward better esophageal cancer-specific survival in cases with heart V20 ≤ 76.9% (p = 0.2). But the association between the tumor location and esophageal cancer-specific survival was not found (p = 0.67).
Table 4.
Cause of death
| Cause of death | No. of deaths (%) (n = 83) |
|---|---|
| Cases with symptomatic cardiac adverse events (n = 12) | |
| Disease progression + Cardiac event + Infection | 1 (1.2) |
| Disease progression + Cardiac event | 3 (3.6) |
| Disease progression + Infection | 2 (2.4) |
| Disease progression | 3 (3.6) |
| Cardiac event | 1 (1.2) |
| Infection | 1 (1.2) |
| Second primary malignancy | 1 (1.2) |
| Cases without symptomatic cardiac adverse events (n = 71) | |
| Disease progression | 32 (38.6) |
| Disease progression + Infection | 15 (18.1) |
| Disease progression + Second primary malignancy | 2 (2.4) |
| Infection | 15 (18.1) |
| Second primary malignancy | 6 (7.2) |
| Unknown | 1 (1.2) |
Discussion
The present study analyzed 121 ESCC patients undergoing definitive CCRT with IMRT technique. We identified heart V5, V10, V20, and mean lung dose as independent predictors of prognosis by multivariate analysis. Cardiac radiation dose was correlated with the incidence of symptomatic cardiac adverse events which were inversely associated with survival.
Cardiac dose-volume parameters were found to be independent prognostic factors in a large group of esophageal cancer patients undergoing CCRT [8]. Notably, several key factors differentiated our data from the previously published one. To begin with, the present study specifically analyzed patients treated with definitive CCRT without surgery while the previous research included patients receiving CCRT with or without surgery. In addition, patients with adenocarcinoma and ESCC were combined for investigation in the previous report. The current cohort only included patients with ESCC. To the best of authors’ knowledge, we were the first to show the prognostic significance of cardiac dose-volume parameters in ESCC after definitive CCRT with IMRT technique. Finally, we systematically performed the multivariate analyses with models incorporating different lung and heart dose-volume parameters. The prognostic significance of the cardiac dose was consistently confirmed under individual consideration of different lung dose-volume parameters in the present study.
In line with the large comprehensive research evaluating the association of cardiac dosimetric factors and the outcomes in esophageal cancer after CCRT with or without surgery [8], heart V20 was shown to influence the survival of ESCC treated with definitive CCRT in the present study. However, heart V5 and V10 which acted as independent prognostic factors in the current cohort were not found to independently influence OS in the previously published report. These discrepant results were possibly derived from the different cancer histologies and treatments between studies as the current cohort only reviewed ESCC patients receiving definitive CCRT without surgery while the previous research included patients with adenocarcinoma and ESCC treated by CCRT with or without operation [8]. In addition, heart V30, V40, and V50 were not reported as independent predictors of OS in the present study because the statistical significance was not achieved in multivariate analytic models in which lung V5 was included. But the existing trend suggested that this negative result might be attributed to the limited sample size. Further investigations with expanded cases are warranted in the future. Moreover, the current study found the inferior survival in patients with large PTV which was a surrogate marker of the tumor burden. This data coincided with the evidence that high tumor burden was associated with poor prognosis in cancers treated with definitive CCRT [12–15].
The previous research did not find the association between cardiac radiation dose and survival in the subset analysis of esophageal cancer patients undergoing definitive CCRT [8]. Notably, the present study showed that lower cardiac radiation dose predicted the superior survival in ESCC treated by definitive CCRT. Our finding is novel and needs external validation in independent cohorts. On the other hand, radiation-related heart disease is not only a well-known late effect of low dose irradiation in non-cancer subjects, but also a recognized adverse event of radiotherapy in cancer patients [16]. Symptomatic cardiotoxicity has been reported in esophageal cancer patients undergoing definitive CCRT. High cardiac radiation dose was identified as a risk factor of cardiac complications [9, 17–19]. In the current study, the prevalence of symptomatic cardiac adverse events was 9.92% which was comparable to 5.88–18.97% reported in the literature. Consistent with prior researches of lung and esophageal cancer treated with CCRT [6, 8], we found patients receiving higher radiation dose to the heart had more symptomatic cardiac adverse events which were in turn correlated with a worse survival. The inferior survival might partly result from the mortality which was directly caused by the cardiac complications. Furthermore, the cardiotoxicity which existed after CCRT possibly reduced patients’ tolerance to the salvage treatment upon cancer progression and thereby could result in the unfavorable outcomes. Collectively, our data supported the causal relationship between cardiac radiation dose, cardiac adverse events, and poor survival among ESCC patients receiving definitive CCRT and indicated the importance of sparing the heart from radiation.
Our study was limited by its retrospective research design and all potential inherent biases. In addition, we did dosimetric analyses based on planning CT scan without consideration of cardiac physiological motion. Errors of estimation would exist under such circumstance. Although cardiac synchronized radiotherapy has been developed [20], it is not routinely used in CCRT for esophageal cancer. Better protection of the heart and more precise estimation of cardiac radiation dose would be possible with cardiac synchronized radiotherapy in the future. Moreover, the present study evaluated the dose-volume parameters of the whole heart. The prognostic significance of radiation dose to specific cardiac chambers remains an interesting issue to be investigated in esophageal cancer after CCRT. Finally, the present study only included ESCC patients undergoing definitive CCRT without surgery. The results could not be generalized to patients diagnosed with adenocarcinoma or treated by neoadjuvant CCRT plus operation. But on the positive side, we provided a specific information for ESCC patients receiving definitive CCRT. Further validation with independent cohorts is warranted.
Conclusions
We were the first to report that the high cardiac radiation dose predicted the inferior outcome of ESCC patients undergoing definitive concurrent chemotherapy and IMRT. Radiation dose to the heart should be minimized.
Supplementary information
Additional file 1: Figure S1. Study patient flow diagram.
Additional file 2: Table S1. Summary of the Chemotherapy Regimens and Radiation Doses.
Additional file 3: Table S2. Multivariate Analysis for Mean Heart Dose and Overall Survival.
Additional file 4: Table S3. Multivariate Analysis for Heart V5 and Overall Survival.
Additional file 5: Table S4. Multivariate Analysis for Heart V10 and Overall Survival.
Additional file 6: Table S5. Multivariate Analysis for Heart V20 and Overall Survival.
Additional file 7: Table S6. Multivariate Analysis for Heart V30 and Overall Survival.
Additional file 8: Table S7. Multivariate Analysis for Heart V40 and Overall Survival.
Additional file 9: Table S8. Multivariate Analysis for Heart V50 and Overall Survival.
Additional file 10: Table S9. Multivariate Analysis for Lung Dose-volume Parameters and Overall Survival under Consideration of Different Heart Parameters.
Additional file 11: Figure S2. Cancer-specific survival by A) heart V5, B) heart V10, C) heart V20, and D) volume of PTV prescribed to 50 Gy.
Acknowledgements
Not applicable.
Abbreviations
- CCRT
Concurrent chemoradiotherapy
- CT
Computed tomography
- CTV
Clinical target volume
- ECOG
Eastern Cooperative Oncology Group
- ESCC
Esophageal squamous cell carcinoma
- GTV
Gross tumor volume
- GTVn
Gross tumor volume of lymph nodes
- GTVp
Gross tumor volume of the primary
- IMRT
Intensity modulated radiotherapy
- OS
Overall survival
- PTV
Planning target volume
Authors’ contributions
THP and FCL participated in the design. THP, WLC, WWL, YLT, YTY, TJC, and FCL participated in data collection. THP, NJC, JSMC, CYL, and FCL participated in data analysis. All authors participated in data interpretation, drafting, and finalizing the report. The authors read and approved the final manuscript.
Funding
This work was supported by National Cheng Kung University Hospital of Taiwan [NCKUH-10902063 to FCL] and the Ministry of Science and Technology of Taiwan [MOST 105–2314-B-006-045-MY2 to FCL].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of National Cheng Kung University Hospital (reference number, A-ER-107-349). The informed consent was waived because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tzu-Hui Pao, Email: e16283233@hotmail.com.
Wei-Lun Chang, Email: changwl6@gmail.com.
Nai-Jung Chiang, Email: njchiang@nhri.org.tw.
Jeffrey Shu-Ming Chang, Email: jeffreychang@nhri.edu.tw.
Chia-Ying Lin, Email: gracelinchiaying@msn.com.
Wu-Wei Lai, Email: wwlai@mail.ncku.edu.tw.
Yau-Lin Tseng, Email: tsengyl@mail.ncku.edu.tw.
Yi-Ting Yen, Email: b85401067@gmail.com.
Ta-Jung Chung, Email: tjchung@mail.ncku.edu.tw.
Forn-Chia Lin, Email: fornchia@mail.ncku.edu.tw.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13014-020-01664-7.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 3.Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Galais MP, Raoul JL, Bouché O, Gourgou-Bourgade S, Douillard JY, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15(3):305–314. doi: 10.1016/S1470-2045(14)70028-2. [DOI] [PubMed] [Google Scholar]
- 5.Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speirs CK, DeWees TA, Rehman S, Molotievschi A, Velez MA, Mullen D, et al. Heart dose is an independent Dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12(2):293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 7.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu C, Guo L, Liao Z, Wang Y, Liu X, Zhao S, et al. Heart and lung doses are independent predictors of overall survival in esophageal cancer after chemoradiotherapy. Clin Transl Radiat Oncol. 2019;17:17–23. doi: 10.1016/j.ctro.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pao TH, Chang WL, Chiang NJ, Lin CY, Lai WW, Tseng YL, et al. Pericardial effusion after definitive concurrent chemotherapy and intensity modulated radiotherapy for esophageal cancer. Radiat Oncol. 2020;15(1):48. doi: 10.1186/s13014-020-01498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79(1):10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong FM, Machtay M, Bradley J. Atlases for organs at risk (OARs) in thoracic radiation therapy. Radiation Therapy Oncol Group (RTOG). 2011; https://www.eviq.org.au/getmedia/a4c012a8-d6a7-4d87-93f7-d3f465b49889/RTOG-heart-contouring-atlas.pdf.aspx?ext=.pdf (12 Nov 2018, date last accessed).
- 12.Bradley JD, Ieumwananonthachai N, Purdy JA, Wasserman TH, Lockett MA, Graham MV, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2002;52(1):49–57. doi: 10.1016/S0360-3016(01)01772-2. [DOI] [PubMed] [Google Scholar]
- 13.Strongin A, Yovino S, Taylor R, Wolf J, Cullen K, Zimrin A, et al. Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(5):1823–1830. doi: 10.1016/j.ijrobp.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Lin Y, Cai W, Su T, Wang B, Li J, et al. A new clinical staging system for esophageal cancer to predict survival after definitive chemoradiation or radiotherapy. Dis Esophagus. 2018;31(11). [DOI] [PubMed]
- 15.Lin FC, Chang WL, Chiang NJ, Lin MY, Chung TJ, Pao TH, et al. Radiation dose escalation can improve local disease control and survival among esophageal cancer patients with large primary tumor volume receiving definitive chemoradiotherapy. PLoS One. 2020;15(8):e0237114. doi: 10.1371/journal.pone.0237114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76(3):656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konski A, Li T, Christensen M, Cheng JD, Yu JQ, Crawford K, et al. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol. 2012;104(1):72–77. doi: 10.1016/j.radonc.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi Y, Iijima H, Isohashi F, Tsujii Y, Fujinaga T, Nagai K, et al. The heart's exposure to radiation increases the risk of cardiac toxicity after chemoradiotherapy for superficial esophageal cancer: a retrospective cohort study. BMC Cancer. 2019;19(1):195. doi: 10.1186/s12885-019-5421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogino I, Watanabe S, Iwahashi N, Kosuge M, Sakamaki K, Kunisaki C, et al. Symptomatic radiation-induced cardiac disease in long-term survivors of esophageal cancer. Strahlenther Onkol. 2016;192(6):359–367. doi: 10.1007/s00066-016-0956-1. [DOI] [PubMed] [Google Scholar]
- 20.Poon J, Kohli K, Deyell MW, Schellenberg D, Reinsberg S, Teke T, et al. Technical note: cardiac synchronized volumetric modulated arc therapy for stereotactic arrhythmia radioablation - proof of principle. Med Phys. 2020;47(8):3567–72. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Study patient flow diagram.
Additional file 2: Table S1. Summary of the Chemotherapy Regimens and Radiation Doses.
Additional file 3: Table S2. Multivariate Analysis for Mean Heart Dose and Overall Survival.
Additional file 4: Table S3. Multivariate Analysis for Heart V5 and Overall Survival.
Additional file 5: Table S4. Multivariate Analysis for Heart V10 and Overall Survival.
Additional file 6: Table S5. Multivariate Analysis for Heart V20 and Overall Survival.
Additional file 7: Table S6. Multivariate Analysis for Heart V30 and Overall Survival.
Additional file 8: Table S7. Multivariate Analysis for Heart V40 and Overall Survival.
Additional file 9: Table S8. Multivariate Analysis for Heart V50 and Overall Survival.
Additional file 10: Table S9. Multivariate Analysis for Lung Dose-volume Parameters and Overall Survival under Consideration of Different Heart Parameters.
Additional file 11: Figure S2. Cancer-specific survival by A) heart V5, B) heart V10, C) heart V20, and D) volume of PTV prescribed to 50 Gy.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.