INTRODUCTION
Opioid therapy for chronic pain is common with prevalence estimates around 20%.[26] Due to the development of tolerance and inadequate control of pain, opioid dose escalation is a common decision prescribers have to face for patients on chronic opioid therapy.[14,20] Opioid tolerance is the decreased pharmacologic response of the opioid receptor to the opioid agonist which fuels the need for dose escalation to continue to achieve the desired effects.[9,25] Opioid dose escalation can result in adverse effects such as constipation, dizziness, opioid-induced hyperalgesia, and increased risk of developing substance use disorders.[16,17] These adverse effects paired with tolerance can often create challenges for prescribers.
Lacking objective measures to quantify pain, pain intensity is measured by patient self-report.[36] The Numeric Rating Scale (NRS) is one of the most common ratings of pain intensity.[28] The NRS is a single item that asks patients to rate their pain on an 11-point interval scale with 0 being no pain and 10 reflecting the most severe pain. Other self-reported tools for measuring pain intensity include the visual analog scale, which applies words or faces with the numeric values, and the verbal rating scale, which is composed of phrases describing levels of pain intensity.[15,19]
Unfortunately, limited evidence exists to guide clinician decisions when contemplating opioid dose increases in the chronic pain setting. One trial randomized 135 VA patients to a stable opioid dosing strategy versus a more liberal dose escalation strategy and found no differences in the primary outcomes of pain or functional disability.[27] A retrospective cohort study with 109 patients on chronic opioid therapy for chronic pain within one hospital setting found a lack of association between opioid dose change and pain intensity. However, as the authors state, the small sample size could account for the lack of association.[4] A follow-up study evaluated the impact of opioid dosing on pain severity and disability finding a slight, but clinically insignificant, improvement in pain severity with dose increases. However, this study was conducted within a community-based, interdisciplinary pain management clinic and may not be generalizable across clinical settings.[6]
The purpose of this study is to provide insights into the influence of opioid dose escalation on NRS pain scores recorded in the electronic medical record among patients on chronic opioid therapy for chronic pain being treated in VA clinical settings. We hypothesize that pain intensity will not clinically differ between patients on chronic opioid therapy who escalate their opioid doses as compared to those maintaining their doses.
METHODS
Data Source
Inpatient, outpatient, demographic, pharmacy, and vital sign files from the Corporate Data Warehouse (CDW) of the Veterans Health Administration (VHA) spanning the fiscal years of 2008–2015 were used. The study was approved by the Central Arkansas Veterans Healthcare System Institutional Review Board.
Subjects
Veterans with chronic, non-cancer pain (CNCP) and receiving chronic opioid therapy for at least two consecutive 180-day periods were identified. Using data from 10/1/2008 to 9/30/2015, CNCP was defined as having at least one diagnosis for arthritis, back pain, neck pain, neuropathic pain, or headache/migraine.[11] Prescription opioids were defined by CN101, the VA Drug Class Code corresponding to ‘Opioid Analgesics.’ Chronic opioid therapy was defined as receiving at least a 90 days’ supply of non-parenteral opioids within a 180 day period without having a ≥30 day gap in opioid coverage.[35]
Study Design, Main Independent Variable, and Exclusion Criteria
Using a retrospective cohort study design, Veterans were classified into 2 mutually exclusive groups based on difference in opioid dose observed in two consecutive 6 month periods: dose escalators and dose maintainers. Dosage change was calculated by dividing the average daily dose in morphine milligram equivalents from the second 180-day period of chronic opioid therapy by the average daily dose from the first 180-day period. Dose escalation was defined as at least a 20% increase in average daily opioid dose, and dose maintenance was defined as less than a 20% change in average daily dose in the second 180-day period.[4] Veterans who experienced a dose decrease of 20% or greater were excluded. The index date was the first day of the second 180-day period in which dose changes were assessed, or, in other words, the first day following the initial 180-day period of chronic opioid therapy. See eFigure 1 for visual representation of the cohort.
Exclusion Criteria
Eleven exclusion criteria were implemented using electronic medical records and administrative data from the 12 months prior to the index date unless otherwise specified: (1) classification as an opioid dose decreaser as previously described, (2) less than 18 years of age, (3) index date before 10–1-2009 or after 10–1-2014 to ensure a year of data before and after the index date, (4) diagnosis of a substance use disorder, opioid-related adverse outcome, or cancer (except for non-melanoma skin cancer), (5) receipt of hospice/palliative care or opioid agonist therapy for opioid use disorder, (6) potentially erroneous opioid prescription records in 180 days before or after the index date [inability to calculate morphine milligram equivalents (MME), average daily dose above 1000 MMEs, or prescription quantity greater than 1000 units], (7) visits with providers outside the VA exceeding the number of visits with VA providers to assist in ensuring use of the VA for most healthcare needs, (8) fewer than 2 visits at least 30 days apart to any VA facility, (9) less than 2 NRS pain scores in the 180 days prior to the index date with at least 1 on or within 90 days prior to the index date, (10) less than 1 NRS pain score in each 90 day period in the 6 month follow-up period (at least 2 pain scores total in the follow-up period), and (11) death in the 180 day period after the index date.
Study Outcome: Pain Intensity
NRS pain scores were obtained from vital sign files from CDW and assessed in the 90 days prior to the index date and over the 180-day period after the index date. Previous work with NRS pain scores by Dobscha et al have shown that patients have clinically-relevant variability in NRS pain scores within a given month.[8] Therefore, the use of multiple NRS pain scores over time provides better estimates of changes in pain intensity. For this study, as with the Dobscha et al study, NRS pain scores were evaluated as 3-month averages.[7]
Minimal clinically important differences (MCIDs) in NRS pain scores are important in interpreting differences between comparison groups. There are several estimates for MCIDs for individuals that range from a 1.4 to 2.0 point change on the NRS.[5,12,22,23,31,33] However, applying individual MCIDs to a group average is believed to obscure meaningful responses to treatment.[10] Therefore, differences in pain scores between groups of less than 0.5 were considered as non-meaningful differences, differences of between 0.5 and 1.0 as potentially meaningful, and differences greater than 1.0 as meaningful.
Covariates
Baseline covariates were evaluated in the 12 months prior to the index date. Demographic covariates included sex, race, marital status, and rural-urban commuting area (urban, large rural, isolated small rural).[30] Medical covariates included the enhanced Charlson comorbidity score and diagnoses for CNCP and mental health conditions (schizophrenia, major depressive disorder, post-traumatic stress disorder, anxiety disorders, bipolar disorder, multiple mental health conditions).[3] Medication classes used to treat pain or that increase the risk of opioid-related adverse outcomes when paired with opioids were identified using VA Drug Class Codes. These medication classes included antidepressants, benzodiazepines, skeletal muscle relaxants, other non-opioid analgesics, hypnotics/other non-benzodiazepine sedatives. Any use of these was characterized in the 12-month period prior to the index date. Opioid medication characteristics in the first 180 days of chronic opioid therapy were also evaluated, including schedule, duration of action (long acting, short acting) and average morphine equivalent dose. Health care visits (physical therapy, pain clinic, chiropractic care, medicine/primary care, and mental health visits) were characterized in two ways: 1) any visit in the 12-month period prior to the index date, and 2) the number of days with each healthcare visit type. NRS pain score characteristics were also identified within the first 180-day period of chronic opioid therapy, including average pain score, last pain score, and pain score change (difference between initial and last pain score).
Analysis
Using the covariates defined above, propensity scores were generated for dose escalation. Dose escalators were matched to dose maintainers using a 1:1 greedy matching algorithm without replacement based on the propensity score and index date (within ± 180 days of each other).[2,29] Standardized differences before and after matching assessed the balance of covariates between does escalators and dose maintainers. Matching was repeated until the standardized differences were less than 10% between the two groups.[1] Linear repeated measures models were estimated among the propensity score matched sample.
Change in NRS pain scores were based on the 90-day pre-index period (baseline period) contrasted with the average pain scores in the two consecutive 90 day periods after the index date. To estimate the effect of dose escalation, the main independent variables were a dummy variable for dose escalation status (escalation, maintain), variables to demarcate the time intervals (pre-index, 1st 90 days post-index, 2nd 90 days post-index) and the interaction of the dummy variable for dose escalation status and time interval variables which served as the primary variable of interest. Visit counts for physical therapy, pain clinics, chiropractic care, primary care, and mental health care were the only covariates added to the model. Veterans were balanced with the propensity score on whether they used each type of healthcare service; however, for some types of healthcare services, most were not users. Therefore, visit counts were included for further adjustment. Each Veteran served as a repeated measure since multiple measurements were obtained for each Veteran.
Analyses were conducted using SAS Enterprise Guide 7.1 using a two-sided significance level of 0.05.
Sensitivity Analyses
To explore the robustness of the primary analysis, three alternative analytic approaches were undertaken. First, an adjusted linear repeated measures model regression among the entire sample was estimated using the covariates previously described in addition to the main independent variables. Second, stabilized inverse probability of treatment weights (SIPTW), calculated using publicly available SAS code, were used with the linear repeated measures model.[21] Trimming of Veterans in non-overlapping regions of the propensity score distribution was conducted among the final sample.[32] Third, the model was re-estimated using median pain scores instead of mean pain scores.
RESULTS
Sample Derivation and Characteristics
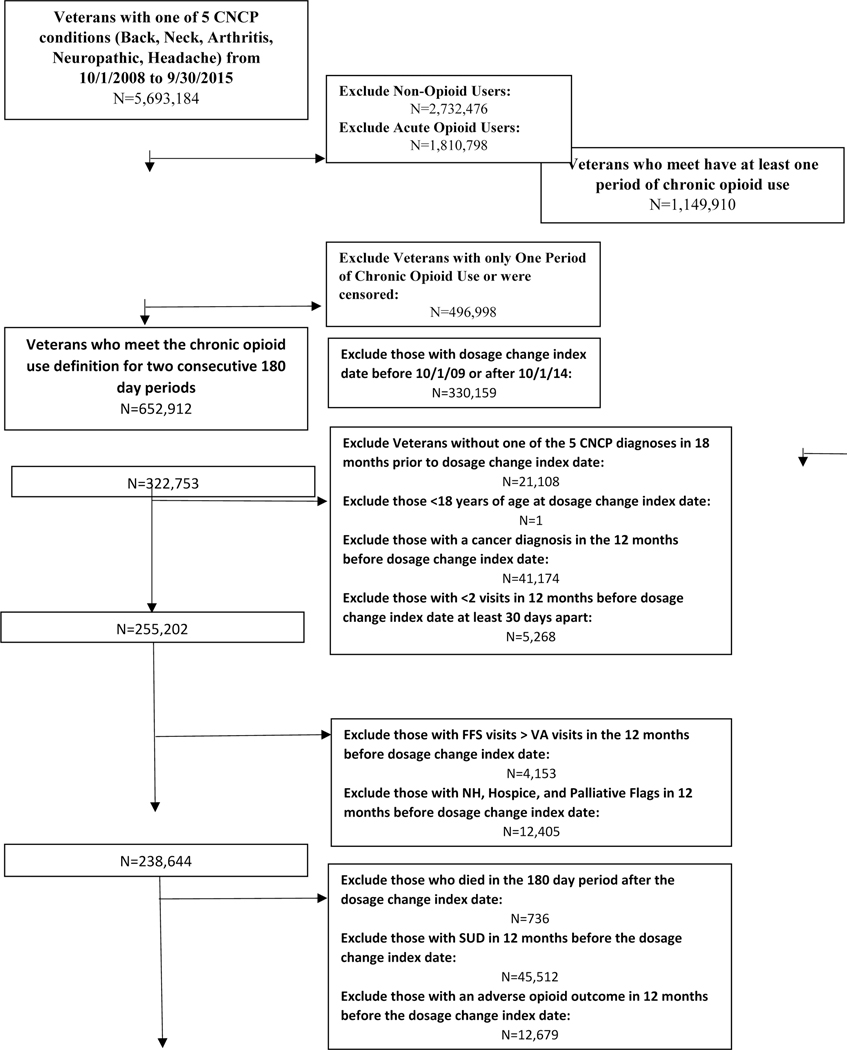
A total of 53,187 Veterans were retained in the final sample, 32,420 (61%) dose maintainers and 20,767 (39%) dose escalators (Figure 1). The majority of both dose escalators and maintainers were urban males between the ages of 50 and 64 (Table 1). The most common pain conditions were the combinations of arthritis and back/neck pain followed by neuropathic pain and other pain conditions. About half the sample were not diagnosed with a mental health condition (46% of dose escalators and 48% of dose maintainers); however, having multiple mental health conditions was common (26% and 24% respectively).
Figure 1:
Derivation of Study Sample
Table 1.
Baseline Demographic Characteristics of Dose Escalators and Maintainers before and after Matching
| Unmatched Sample (N=53,187) | Matched Sample (N=38,716) | |||||
|---|---|---|---|---|---|---|
| Dose Maintainer (N=32,420) | Dose Escalator (N=20,767) | Abs Std Diff☩ | Dose Maintainer (N=19,358) | Dose Escalator (N=19,358) | Abs Std Diff☩ | |
| N (Column %) | N (Column %) | (%) | N (Column %) | N (Column %) | (%) | |
| Race | ||||||
| White | 22715 (70.06) | 14824 (71.38) | 2.9 | 13751 (71.04) | 13729 (70.92) | 0.3 |
| Black | 5343 (16.48) | 3363 (16.19) | 0.8 | 3163 (16.34) | 3202 (16.54) | 0.5 |
| Multiracial | 1127 (3.48) | 636 (3.06) | 2.3 | 579 (2.99) | 607 (3.14) | 0.8 |
| Other | 2294 (7.08) | 1328 (6.39) | 2.7 | 1278 (6.60) | 1242 (6.42) | 0.8 |
| Unknown | 941 (2.90) | 616 (2.97) | 0.4 | 587 (3.03) | 578 (2.99) | 0.3 |
| Age | ||||||
| 58.2 ± 12.82 | 55.6 ± 13.22 | 56.21 ± 12.95 | 56.14 ± 13.06 | |||
| 18–30 | 1097 (3.38) | 1118 (5.38) | 9.8 | 905 (4.68) | 915 (4.73) | 0.2 |
| 31–49 | 5850 (18.04) | 4655 (22.42) | 10.9 | 4198 (21.69) | 4167 (21.53) | 0.4 |
| 50–64 | 16164 (49.86) | 10434 (50.24) | 0.8 | 9706 (50.14) | 9831 (50.79) | 1.3 |
| ≥65 | 9309 (28.71) | 4560 (21.96) | 15.6 | 4549 (23.50) | 4445 (22.96) | 1.3 |
| Gender | ||||||
| Male | 29342 (90.51) | 18715 (90.12) | 1.3 | 17463 (90.21) | 17458 (90.18) | 0.1 |
| Marital Status | ||||||
| Married | 16859 (52.00) | 10309 (49.64) | 4.7 | 9787 (50.56) | 9719 (50.21) | 0.7 |
| Rural-Urban Commuting Area | ||||||
| Urban | 22314 (68.83) | 14534 (69.99) | 2.5 | 13550 (70.00) | 13518 (69.83) | 0.4 |
| Large Rural | 4682 (14.44) | 2993 (14.41) | 0.1 | 2793 (14.43) | 2800 (14.46) | 0.1 |
| Isolated Small Rural | 4628 (14.28) | 2826 (13.61) | 1.9 | 2616 (13.51) | 2656 (13.72) | 0.6 |
| Missing | 796 (2.46) | 414 (1.99) | 3.1 | 399 (2.06) | 384 (1.98) | 0.6 |
| Enhanced Charlson Comorbidity Index | ||||||
| 2.6 ± 1.95 | 2.4 ± 1.95 | 2.45 ± 1.97 | 2.43 ± 1.95 | |||
| 0 | 3481 (10.74) | 2694 (12.97) | 6.9 | 2365 (12.22) | 2435 (12.58) | 1.1 |
| 1 | 7489 (23.10) | 5245 (25.26) | 5.0 | 4830 (24.95) | 4804 (24.82) | 0.3 |
| 2 | 7525 (23.21) | 4777 (23.00) | 0.5 | 4440 (22.94) | 4461 (23.04) | 0.3 |
| 3 | 5605 (17.29) | 3256 (15.68) | 4.3 | 3107 (16.05) | 3105 (16.04) | 0.0 |
| 4 | 3558 (10.97) | 2033 (9.79) | 3.9 | 1930 (9.97) | 1934 (9.99) | 0.1 |
| 5 | 2100 (6.48) | 1146 (5.52) | 4.0 | 1110 (5.73) | 1092 (5.64) | 0.4 |
| ≥6 | 2662 (8.21) | 1616 (7.78) | 1.6 | 1576 (8.14) | 1527 (7.89) | 0.9 |
| Pain Condition | ||||||
| Back and/or Neck Pain Only | 4384 (13.52) | 2929 (14.10) | 1.7 | 2597 (13.42) | 2704 (13.97) | 1.6 |
| Arthritis Only | 6094 (18.80) | 3464 (16.68) | 5.5 | 3260 (16.84) | 3332 (17.21) | 1.0 |
| Headaches Only | 276 (0.85) | 125 (0.60) | 2.9 | 149 (0.77) | 120 (0.62) | 1.8 |
| Neuropathic Pain Only | 550 (1.70) | 296 (1.43) | 2.2 | 310 (1.60) | 281 (1.45) | 1.2 |
| Arthritis and Back and/or Neck Pain Only | 9372 (28.91) | 6077 (29.26) | 0.8 | 5693 (29.41) | 5636 (29.11) | 0.7 |
| Arthritis, Back and/or Neck Pain, and Headaches Only | 1932 (5.96) | 1369 (6.59) | 2.6 | 1264 (6.53) | 1247 (6.44) | 0.4 |
| Neuropathic Pain and One or More Others | 7919 (24.43) | 5202 (25.05) | 1.4 | 4864 (25.13) | 4830 (24.95) | 0.4 |
| All Tracer Pain Conditions | 453 (1.40) | 394 (1.90) | 3.9 | 317 (1.64) | 361 (1.86) | 1.7 |
| Other Multiple Pain Conditions | 1440 (4.44) | 911 (4.39) | 0.3 | 904 (4.67) | 847 (4.38) | 1.4 |
| Other Medication Use | ||||||
| Antidepressant Use | 17322 (53.43) | 11735 (56.51) | 6.2 | 10794 (55.76) | 10809 (55.84) | 0.2 |
| Skeletal Muscle Relaxant Use | 11690 (36.06) | 8614 (41.48) | 11.1 | 7768 (40.13) | 7822 (40.41) | 0.6 |
| Benzodiazepine Use | 8768 (27.05) | 5846 (28.15) | 2.5 | 5369 (27.74) | 5408 (27.94) | 0.5 |
| Other Non-Opioid Analgesic Use | 22688 (69.98) | 15254 (73.45) | 7.7 | 14100 (72.84) | 14176 (73.23) | 0.9 |
| Hypnotics and Non-Benzodiazepine Sedative Use | 4871 (15.02) | 3405 (16.40) | 3.8 | 3052 (15.77) | 3123 (16.13) | 1.0 |
| Mental Health Conditions | ||||||
| No Mental Health Conditions | 15876 (48.97) | 9697 (46.69) | 4.6 | 9027 (46.63) | 9149 (47.26) | 1.3 |
| Schizophrenia | 271 (0.84) | 142 (0.68) | 1.8 | 133 (0.69) | 138 (0.71) | 0.3 |
| Major Depressive Disorder | 4332 (13.36) | 3066 (14.76) | 4.0 | 2751 (14.21) | 2803 (14.48) | 0.8 |
| Post-Traumatic Stress Disorder | 1854 (5.72) | 1139 (5.48) | 1.0 | 1105 (5.71) | 1068 (5.52) | 0.8 |
| Bipolar Disorder | 320 (0.99) | 244 (1.17) | 1.8 | 184 (0.95) | 229 (1.18) | 2.3 |
| Anxiety Disorders | 1717 (5.30) | 1069 (5.25) | 0.7 | 1045 (5.40) | 1004 (5.19) | 1.0 |
| Multiple Mental Health Conditions | 8050 (24.83) | 5410 (26.05) | 2.8 | 5113 (26.41) | 4967 (25.66) | 1.7 |
| Percent with Each of the Following Visit Types in the 12 Months before Dosage Change Index Date | ||||||
| Physical Therapy | 10715 (33.05) | 7757 (37.35) | 9.0 | 6865 (35.46) | 7130 (36.83) | 2.9 |
| Pain Clinic | 4697 (14.49) | 3767 (18.14) | 9.9 | 3173 (16.39) | 3328 (17.19) | 2.1 |
| Chiropractic Care | 485 (1.50) | 363 (1.75) | 2.0 | 316 (1.63) | 333 (1.72) | 0.7 |
| Medicine and Primary Care | 32392 (99.91) | 20747 (99.90) | 0.3 | 19343 (99.92) | 19339 (99.90) | 0.7 |
| Mental Health Care | 15801 (48.74) | 10840 (52.20) | 6.9 | 9959 (51.45) | 9957 (51.44) | 0.0 |
| Duration of Action of Opioid Use in First 180 Days | ||||||
| Long-Acting Only | 715 (2.21) | 299 (1.44) | 5.7 | 332 (1.72) | 297 (1.53) | 1.4 |
| Short-Acting Only | 29365 (90.58) | 17792 (85.67) | 15.2 | 17219 (88.95) | 17183 (88.76) | 0.6 |
| Combination of Long and Short-Acting | 2340 (7.22) | 2676 (12.89) | 18.9 | 1807 (9.33) | 1878 (9.70) | 1.3 |
| Schedule of Opioid Use in First 180 Days | ||||||
| Schedule II Only | 18908 (58.32) | 12076 (58.15) | 0.4 | 11601 (59.93) | 11429 (59.04) | 1.8 |
| Schedule III Only | 1070 (3.30) | 457 (2.20) | 6.7 | 448 (2.31) | 457 (2.36) | 0.3 |
| Schedule IV Only | 7087 (21.86) | 2953 (14.22) | 20.0 | 2974 (15.36) | 2946 (15.22) | 0.4 |
| Schedule V Only | 0 (0.00) | 0 (0.00) | 0.0 | 0 (0.00) | 0 (0.00) | 0.0 |
| Use of Multiple Schedules | 5355 (16.52) | 5281 (25.43) | 22.0 | 4335 (22.39) | 4526 (23.38) | 2.4 |
| Duration of Action of Opioid Use in Second 180 Days | ||||||
| Long-Acting Only | 848 (2.62) | 538 (2.59) | -- | 504 (2.60) | 449 (2.32) | -- |
| Short-Acting Only | 29291 (90.35) | 16031 (77.19) | -- | 17310 (89.42) | 15424 (79.68) | -- |
| Combination of Long and Short-Acting | 2281 (7.04) | 4198 (20.21) | -- | 1544 (7.98) | 3485 (18.00) | -- |
| Schedule of Opioid Use in Second 180 Days | ||||||
| Schedule II Only | 20259 (62.49) | 13687 (65.91) | -- | 12810 (66.17) | 12701 (65.61) | -- |
| Schedule III Only | 1040 (3.21) | 248 (1.19) | -- | 485 (2.51) | 245 (1.27) | -- |
| Schedule IV Only | 6771 (20.89) | 2086 (10.04) | -- | 3199 (16.53) | 2050 (10.59) | -- |
| Schedule V Only | 0 (0.00) | 0 (0.00) | -- | 0 (0.00) | 0 (0.00) | -- |
| Use of Multiple Schedules | 4350 (13.42) | 4746 (22.85) | -- | 2864 (14.79) | 4362 (22.53) | -- |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Average Morphine Equivalent Dose | ||||||
| First 180 Days | 30.33 (41.44) | 27.16 (29.52) | 8.8 | 27.75 (29.29) | 26.84 (29.95) | 3.1 |
| Second 180 Days | 30.61 (41.62) | 45.76 (48.41) | -- | 28.04 (29.53) | 44.82 (48.57) | -- |
| Percent Change in Average Morphine Equivalent Dose | 1.13% | 77.29% | -- | 1.20% | 75.98% | -- |
| Pain Characteristics in 180 Days before Dosage Change Index Date | ||||||
| First Pain Score | 4.73 (3.30) | 5.26 (3.20) | 16.1 | 5.16 (3.22) | 5.17 (3.22) | 0.2 |
| Last Pain Score | 3.96 (3.28) | 4.71 (3.26) | 23.1 | 4.54 (3.24) | 4.58 (3.26) | 1.2 |
| Pain Score Average | 4.23 (2.38) | 4.88 (2.30) | 27.8 | 4.76 (2.31) | 4.76 (2.29) | 0.3 |
| Change in Pain Score | −0.77 (3.84) | −0.54 (3.83) | 6.1 | −0.62 (3.84) | −0.59 (3.86) | 0.9 |
| Service Visits in the 12 Months before Dosage Change Index Date Conditional on Use of the Visit Type | ||||||
| Physical Therapy | 3.96 (5.76) | 3.94 (5.87) | -- | 4.05 (6.08) | 3.94 (5.93) | -- |
| Pain Clinic | 3.27 (3.17) | 3.46 (3.64) | -- | 3.28 (3.21) | 3.45 (3.64) | -- |
| Chiropractic Care | 4.25 (4.62) | 4.36 (4.95) | -- | 4.18 (4.79) | 4.30 (5.05) | -- |
| Medicine and Primary Care | 10.66 (7.49) | 10.97 (7.51) | -- | 10.79 (7.51) | 10.90 (7.50) | -- |
| Mental Health Care | 8.40 (11.79) | (10.54) | -- | 8.49 (11.44) | 8.12 (10.59) | -- |
Abs Std Diff=Absolute Standardized Difference
Opioid medication and pain score characteristics differed between dose escalators and dose maintainers before matching (Table 1). Dose escalators more commonly used a combination of long and short-acting opioids (20% vs 7%) and a combination of opioids with differing schedules (23% vs 13%). Dose maintainers more commonly used short-acting opioids only (90% vs 77%) and schedule IV opioids (22% vs 14%). Dose maintainers also had lower first, last, and average pain scores in the baseline period. After propensity score matching, 19,358 dose escalators matched to dose maintainers (93% of dose escalators and 60% of dose maintainers). The baseline characteristics of the dose escalators and dose maintainers were well balanced after propensity score matching, including nearly identical average pain scores and changes in pain score in the 180 days prior to the index date (Table 1). All standardized differences for the baseline covariates between the two groups were less than 10%. In the 180 day follow up period, dose escalators had higher average MME (45 MME vs. 28 MME) and were more likely to use long acting opioids in combination with a short acting opioid (18% vs. 8%).
NRS Pain Scores
Linear Repeated Measures Models among the Propensity Score Matched Sample
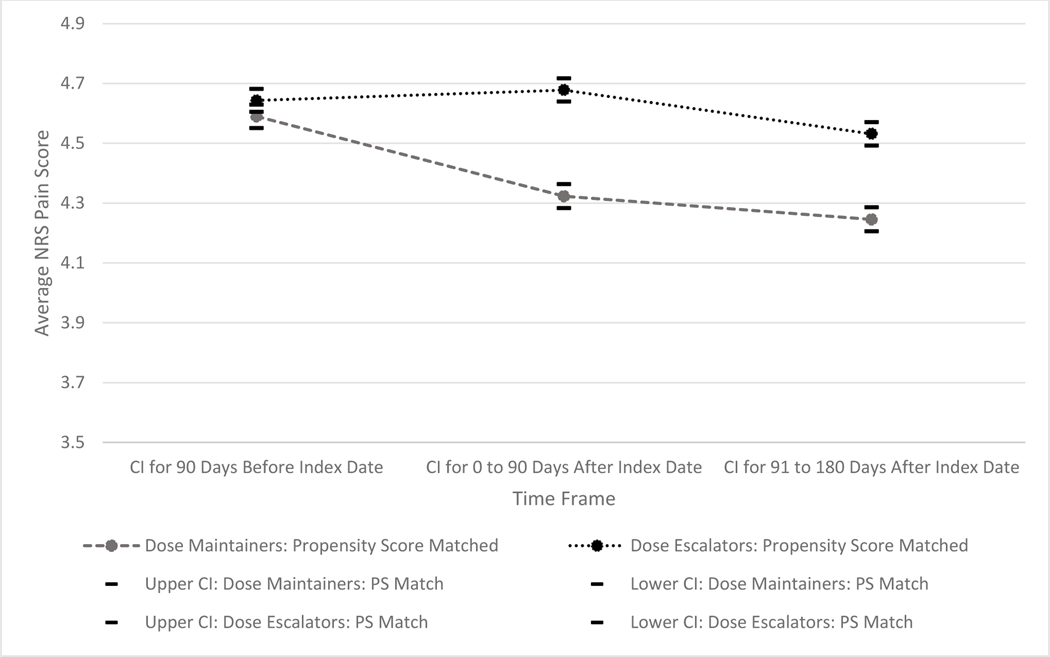
Figure 2 displays the pain scores among dose escalators and dose maintainers in the baseline and two follow up periods. Of note, the scale of the figures represents only a narrow portion of the NRS pain scale since small changes were found, and the confidence intervals were narrow given the large sample size. The linear mixed models estimated among the propensity score matched sample (Figure 2) had similar average pain scores and in the 90-day, pre-index baseline period (Dose Escalators: 4.64, 95%CI: 4.61, 4.68; Dose Maintainers: 4.59, 95%CI: 4.55, 4.63, p=0.0551). Pain score averages among dose escalators did not change significantly from the pre-index baseline period to the first 90 period after the index period (90 Days Prior to the Index date: 4.64, 95%CI: 4.61, 4.68; 0–90 Days After Index Date: 4.68, 95%CI: 4.64, 4.72, p=0.1013); however, pain scores among the dose escalators decreased significantly between the first follow up period and the last follow up period (0–90 Days After Index Date: 4.68, 95%CI: 4.64, 4.72; 91–180 Days After Index Date: 4.53, 95%CI: 4.49, 4.57, p<0.0001). Among the dose maintainers, pain score averages decreased significantly between the pre-index baseline period and the two ensuing follow up periods (Baseline: 4.59, 95%CI: 4.55, 4.63; 0–90 Days After Index Date: 4.32, 95%CI: 4.28, 4.36, p<0.0001; 91–180 Days After Index Date: 4.25, 95%CI: 4.21, 4.29, p=0.0003). Based on the interaction terms, pain scores were persistently higher among dose escalators as compared to dose maintainers at each 90-day period after the index date (p<0.0001). None of these differences between groups in the follow up periods were greater than 0.5.
Figure 2:
Average Pain Scores over Time between Opioid Dose Escalators and Maintainers among the Propensity Score Matched Samples
Sensitivity Analyses
Adjusted and SIPTW Linear Repeated Measures Models
After trimming of the SIPTW, 53,157 of the 53,187 Veterans were included in the SIPTW analysis. The SIPTW analyses provided similar results as the primary, propensity matched sample where average pain scores were similar between dose escalators and maintainers in the baseline pre-index period but maintainers had significantly lower average pain scores in the two follow up periods (eFigure 2; Baseline Differences between Escalators and Maintainers: -0.009, 95%CI: -0.021, 0.003, p=0.1345; 0–90 Days After Index Date Differences between Escalators and Maintainers: -0.046, 95%CI: -0.058, -0.035 p<0.0001; 91–180 Days After Index Date Differences between Escalators and Maintainers: -0.053, 95%CI: -0.065, -0.041, p<0.0001). The adjusted linear repeated measures model using the full sample showed that dose escalators had higher average pain scores in the 90 days before the index date (eFigure 3; Dose Escalators: 4.06, 95%CI: 4.04, 4.07; Dose Maintainers: 3.85, 95%CI: 3.83, 3.87, p<0.0001) but like the previous analyses, dose escalators had statistically significantly higher pain scores in the follow up periods as compared to dose maintainers (Escalators: 0–90 Days After Index Date: 4.04, 95%CI: 4.01, 4.07; Maintainers: 0–90 Days After Index Date: 3.79, 95%CI: 3.77, 3.82, p<0.0001; Escalators: 91–180 Days After Index Date: 3.91, 95%CI: 3.88, 3.94; Maintainers: 91–180 Days After Index Date: 3.75, 95%CI: 3.72, 3.78, p<0.0001).
Linear Repeated Measures Models using Median Pain Scores
For each of the analyses (propensity score match, SIPTW, and adjusted), linear repeated measures models using median pain scores provided similar results as the analyses using average pain scores (eFigure 4a–4c).
DISCUSSION
Pain scores were modestly lower for patients whose chronic opioid doses were maintained compared to those whose doses were increased. In the propensity matched and SIPTW analyses that achieved similar baseline pain scores, the differences between dose maintainers and escalators in the follow up periods appear to be driven by modest decreases in average pain scores over time for dose maintainers compared to steady or slight increases in average pain scores among dose escalators. None of the analyses found lower average or median pain scores among dose escalators compared to those who maintained their doses. It should be noted that despite consistent significant differences in NRS pain scores in the follow up periods, the magnitude of differences were modest, approximately 0.2 on a 0 – 10 scale. These differences in NRS pain scores over time did not reach a potentially meaningful group MCID of 0.5–1.0. [5,12,22,23,31,33] Results provide compelling evidence that increasing opioid doses was not associated with improvements in pain.
These results corroborate emerging evidence regarding opioid dose escalation. Two previous cohort studies found that opioid dose escalation was either not associated with improvements in NRS pain scores or was associated with slight, but clinically insignificant, improvements.[4,6] A retrospective cohort study of older Veterans found that opioid prescriptions are actually associated with a lower likelihood of improvement in NRS scores.[7] Another more recent prospective, cohort study using both Veterans and commercially-insured individuals found that higher opioid doses were also associated with worse, patient-reported pain outcomes.[24] A similar prospective cohort study among middle-aged/senior adults initiating chronic opioid therapy for chronic pain also found worse pain intensity and activity inference among those using higher opioid doses.[34] Collectively these finding suggest that opioid dose escalation does not meaningfully improve pain and clinicians should exercise extreme caution when embarking on a path of increasing opioid doses to manage non-cancer pain.
Given the observational nature of this study, these results should not be interpreted causally. Factors related and unrelated to opioid dose may be responsible for the reported changes in NRS pain scores. For example, changes in the underlying pathology of the painful condition, surgical interventions, improved treatment for mental health disorders or initiation or optimization of other, non-opioid medication regimens for the treatment of pain after the baseline period could account for differences in pain and the decisions to escalate opioid doses; these analyses did not account for these post-index changes in care or pathology. These processes may introduce temporal or time-varying confounding. Time varying confounding could exist between each subsequent NRS pain score and a potential ensuing opioid dosage change. Marginal structure models are a class of models that have the potential to account for time varying confounding but require data describing the temporal sequencing of these events, which were not available in this study. [18] Notably, this study only required that each Veteran have one NRS pain score documented in each 90-day time period. On average 2.7 NRS pain scores were available in each of the 90-day time periods for each subject which made the temporal sequencing between pain scores and opioid doses intractable, particularly given that opioids are often dispensed in 30-day supplies. To partially mitigate the effects of confounding, the propensity matched and SIPTW control for and achieved good balance on a wide range of factors that could influence pain scores including baseline pain scores and, importantly, changes in pain scores that would account, at least partially, for the different pain trajectories observed in the baseline period.
Limitations
Besides potential time-varying confounding, several additional limitations exist. First, validation studies for the NRS were completed through self or researcher/interviewer administration.[12,13] In VA, NRS pain scores are obtained mostly by nurses or healthcare technicians, and therefore, may not have the same level of validity. Second, Veterans, as observed in this sample, are comprised mostly of elderly men receiving care in a closed health system and may not be generalizable to the civilian population. Third, VA data from CDW does not allow for obtaining information on opioid use outside of the VA. We tried to minimize the effect of unmeasured non-VA healthcare use by excluding Veterans with more non-VA visits than VA visits before the index date. We also excluded those who did not have at least 1 NRS pain score in each 90-day period of the 180 days after the index date. Fourth, propensity score methods only adjust for measured confounders. Unmeasured factors that could contribute to the findings are noted above. Lastly, this study reports group averages of individual average and median pain scores observed over 90-day periods which could obscure the proportion of individuals experiencing meaningfully improvements or worsening of self-reported pain.
CONCLUSIONS
The withdrawal of propoxyphene-containing products results in rapid and virtually complete elimination in propoxyphene prescribing in the veterans population, and the policy was associated with decreases in opioid use and chronic opioid use; however, nearly 90% of regular users of propoxyphene switched to alternate opioids, and three quarters continued to use opioids chronically. The use of an alternative opioid likely reduces the risk of propoxyphene-related cardiac conduction disorders; nevertheless, the impact of the withdrawal on the risks of opioid-related misuse, dependence, abuse, and death is likely to be more modest.
When compared to stable chronic opioid doses, increases in opioid dose of 20% or greater were not associated with improvements in NRS pain scores over the 180-day period in which doses increased. Clinicians should carefully evaluate the need for increasing opioid doses, regardless of the current dose. When determining whether to escalate the dose, the decision making process should center less on the potential benefit to improve pain intensity and more on the balance of other potential benefits and harms.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R36DA046717. Dr. Hayes was also supported by the National Institute on Drug Abuse under the Translational Training in Addiction Grant [1T32 DA 022981]. This material is the result of work supported with resources and the use of facilities at the Veterans Health Administration.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflicts of Interest: Dr. Martin receives royalties from TrestleTree LLC for the commercialization of an opioid risk prediction tool, which is unrelated to the current study. Dr. Li is a paid consultant for eMaxHealth Systems for unrelated projects.
REFERENCES
- [1].Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav. Res 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol. Drug Saf 2008;17:1202–1217. doi: 10.1002/pds.1673. [DOI] [PubMed] [Google Scholar]
- [3].Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J. Clin. Epidemiol 2008;61:1234–1240. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- [4].Chen L, Vo T, Seefeld L, Malarick C, Houghton M, Ahmed S, Zhang Y, Cohen A, Retamozo C, St Hilaire K, Zhang V, Mao J. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J. Pain 2013;14:384–92. doi: 10.1016/j.jpain.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila. Pa. 1976) 2005;30:1331–4. Available: http://www.ncbi.nlm.nih.gov/pubmed/15928561 Accessed 18 Aug 2018. [DOI] [PubMed] [Google Scholar]
- [6].DiBenedetto DJ, Wawrzyniak KM, Finkelman M, Kulich RJ, Chen L, Schatman ME, Stone MT, Mao J. Relationships Between Opioid Dosing, Pain Severity, and Disability in a Community-Based Chronic Pain Population: An Exploratory Retrospective Analysis. Pain Med. 2019. doi: 10.1093/pm/pny240. [DOI] [PubMed] [Google Scholar]
- [7].Dobscha SK, Lovejoy TI, Morasco BJ, Kovas AE, Peters DM, Hart K, Williams JL, McFarland BH. Predictors of Improvements in Pain Intensity in a National Cohort of Older Veterans With Chronic Pain. J. Pain 2016;17:824–35. doi: 10.1016/j.jpain.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dobscha SK, Morasco BJ, Kovas AE, Peters DM, Hart K, McFarland BH. Short-term variability in outpatient pain intensity scores in a national sample of older veterans with chronic pain. Pain Med. 2015;16:855–65. doi: 10.1111/pme.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dumas EO, Pollack GM. Opioid tolerance development: a pharmacokinetic/pharmacodynamic perspective. AAPS J. 2008;10:537–51. doi: 10.1208/s12248-008-9056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, Farrar JT, Hertz S, Raja SN, Rappaport BA, Rauschkolb C, Sampaio C. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146:238–244. doi: 10.1016/j.pain.2009.08.019. [DOI] [PubMed] [Google Scholar]
- [11].Edlund MJ, Austen MA, Sullivan MD, Martin BC, Williams JS, Fortney JC, Hudson TJ. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain 2014;155:2337–43. doi: 10.1016/j.pain.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. Available: http://www.ncbi.nlm.nih.gov/pubmed/11690728 Accessed 18 Aug 2018. [DOI] [PubMed] [Google Scholar]
- [13].Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. PAIN® 2011;152:2399–2404. [DOI] [PubMed] [Google Scholar]
- [14].Han H, Kass P, Wilsey B, Li C. Age, gender, and earlier opioid requirement associations with the rate of dose escalation in long-term opioid therapy. J. Opioid Manag 2013;Mar-Apr:129–138. Available: https://www.ncbi.nlm.nih.gov/pubmed/23709322. [DOI] [PubMed] [Google Scholar]
- [15].Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF. Arthritis Care Res. (Hoboken) 2011;63:S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- [16].Hayes CJ, Painter JT. A comprehensive clinical review of opioid-induced allodynia: Discussion of the current evidence and clinical implications. J. Opioid Manag 2017;13:95. doi: 10.5055/jom.2017.0373. [DOI] [PubMed] [Google Scholar]
- [17].Henry SG, Wilsey BL, Melnikow J, Iosif A-M. Dose escalation during the first year of long-term opioid therapy for chronic pain. Pain Med. 2015;16:733–44. doi: 10.1111/pme.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–70. Available: http://www.ncbi.nlm.nih.gov/pubmed/10955409 Accessed 12 Dec 2017. [DOI] [PubMed] [Google Scholar]
- [19].Ho K, Spence J, Murphy MF. Review of Pain-Measurement Tools. Ann. Emerg. Med 1996;27:427–432. doi: 10.1016/S0196-0644(96)70223-8. [DOI] [PubMed] [Google Scholar]
- [20].Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. Sex Differences in Dose Escalation and Overdose Death during Chronic Opioid Therapy: A Population-Based Cohort Study. PLoS One 2015;10:e0134550. doi: 10.1371/journal.pone.0134550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Layton B. Estimation and Use of Propensity Score. 2013. [Google Scholar]
- [22].Mease PJ, Spaeth M, Clauw DJ, Arnold LM, Bradley LA, Russell IJ, Kajdasz DK, Walker DJ, Chappell AS. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res. (Hoboken) 2011;63:821–826. doi: 10.1002/acr.20449. [DOI] [PubMed] [Google Scholar]
- [23].Michener LA, Snyder AR, Leggin BG. Responsiveness of the Numeric Pain Rating Scale in Patients With Shoulder Pain and the Effect of Surgical Status. 2011. Available: https://pdfs.semanticscholar.org/2c6c/2a870cee44c08834192ca673f0a3c9a249a3.pdf Accessed 18 Aug 2018. [DOI] [PubMed]
- [24].Morasco BJ, Yarborough BJ, Smith NX, Dobscha SK, Deyo RA, Perrin NA, Green CA. Higher Prescription Opioid Dose is Associated With Worse Patient-Reported Pain Outcomes and More Health Care Utilization. J. Pain 2017;18:437–445. doi: 10.1016/J.JPAIN.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br. J. Pharmacol 2011;164:1322–34. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nahin RL, Sayer B, Stussman BJ, Feinberg TM. Eighteen-Year Trends in the Prevalence of, and Health Care Use for, Noncancer Pain in the United States: Data from the Medical Expenditure Panel Survey. J. Pain 2019;20:796–809. doi: 10.1016/j.jpain.2019.01.003. [DOI] [PubMed] [Google Scholar]
- [27].Naliboff BD, Wu SM, Schieffer B, Bolus R, Pham Q, Baria A, Aragaki D, Van Vort W, Davis F, Shekelle P. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. J. Pain 2011;12:288–296. [DOI] [PubMed] [Google Scholar]
- [28].Pain Assessment Scales. n.d.
- [29].Parsons LS. Performing a 1:N Case-Control Match on Propensity Score. n.d Available: http://www2.sas.com/proceedings/sugi29/165-29.pdf Accessed 12 Dec 2017.
- [30].Rural Urban Commuting Area Codes Data n.d. Available: http://depts.washington.edu/uwruca/ruca-uses.php Accessed 6 Dec 2017.
- [31].Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur. J. Pain 2004;8:283–291. doi: 10.1016/j.ejpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- [32].Stürmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J. Intern. Med 2014;275:570–580. doi: 10.1111/joim.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tashjian RZ, Hung M, Keener JD, Bowen RC, Mcallister J, Chen W, Ebersole G, Granger EK, Chamberlain AM. Determining the minimal clinically important difference for the American Shoulder and Elbow Surgeons score, Simple Shoulder Test, and visual analog scale (VAS) measuring pain after shoulder arthroplasty. 2017. doi: 10.1016/j.jse.2016.06.007. [DOI] [PubMed] [Google Scholar]
- [34].Turner JA, Shortreed SM, Saunders KW, LeResche L, Von Korff M. Association of levels of opioid use with pain and activity interference among patients initiating chronic opioid therapy: a longitudinal study. Pain 2016;157:849–57. doi: 10.1097/j.pain.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vanderlip ER, Sullivan MD, Edlund MJ, Martin BC, Fortney J, Austen M, Williams JS, Hudson T. National study of discontinuation of long-term opioid therapy among veterans. Pain 2014;155:2673–2679. doi: 10.1016/j.pain.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.[] Younger J, McCue R, Mackey S. Pain outcomes: a brief review of instruments and techniques. Curr. Pain Headache Rep 2009;13:39–43. Available: http://www.ncbi.nlm.nih.gov/pubmed/19126370 Accessed 20 Mar 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.