Abstract
We measured peak oxygen consumption (VO2) in previous recipients of thoracic radiotherapy and assessed the determinants of cardiorespiratory fitness with an emphasis on cardiac and pulmonary function. Cancer survivors who have received thoracic radiotherapy with incidental cardiac involvement often experience impaired cardiorespiratory fitness, as measured by reduced peak VO2, a marker of impaired cardiovascular reserve. We enrolled 25 subjects 1.8 (0.1 to 8.2) years following completion of thoracic radiotherapy with significant heart exposure (at least 10% of heart volume receiving at least 5 Gray). All subjects underwent cardiopulmonary exercise testing, Doppler echocardiography, and circulating biomarkers assessment. The cohort included 16 Caucasians (64%), 15 women (60%) with a median age of 63 (59 to 66) years. The peak VO2 was 16.8 (13.5 to 21.9) ml·kg−1·min−1 or moderately reduced at 62% (50% to 93%) of predicted. The mean cardiac radiation dose was 5.4 (3.7 to 14.7) Gray, and it significantly correlated inversely with peak VO2 (R = −0.445, p = 0.02). Multivariate regression analysis revealed the diastolic functional reserve index and the N-terminal pro-brain natriuretic peptide (NTproBNP) serum levels were independent predictors of peak VO2 ( = +0.813, p <0.01 and = −0.414, p = 0.04, respectively). In conclusion, patients who had received thoracic radiation display a dose-dependent relation between the cardiac radiation dose received and the impairment in peak VO2, the reduction in diastolic functional reserve index, and elevation of NTproBNP.
Radiotherapy is commonly used for the treatment of cancer of the thorax and leads to an improvement in cancer free survival.1 However, patients receiving thoracic radiotherapy experience a dose-dependent increased risk of cardiovascular disease events, including incident heart failure, such that the cancer-related benefits of radiotherapy are, at least in part, offset by an increased risk of cardiovascular disease events, including an increased risk of heart failure.2,3 Many patients with cancer show impairment in cardiorespiratory fitness (CRF) early after radiotherapy, while patients who go on to develop heart failure are generally diagnosed only many years after treatment.4 This may be, at least in part, because commonly-used tools to assess cardiac function (e.g., left-ventricular ejection fraction) are indeed insensitive to minor injury, and hence subtle changes may go unnoticed for many years.5 Patients, including those with cancer, who have impaired CRF defined as reduced peak oxygen consumption (VO2), however, are at increased risk of cardiovascular disease-related mortality and heart failure-related morbidity and mortality6–9 even when left-ventricular ejection fraction is preserved. In the present study, we measured CRF in a cohort of patients who received thoracic radiotherapy for the treatment of cancer with significant cardiac involvement with the goal of assessing the determinants of impaired CRF.
Methods
We conducted a single-center prospective study enrolling breast and lung cancer subjects who had previously underwent irradiation to the chest with a significant incidental cardiac dose. Patients were eligible if they were at least 18 years-old and had previous thoracic radiotherapy with a minimum radiation dose to the heart of at least 5 Gray involving at least 10% of the heart volume. Patients were ineligible if they were unable to provide informed consent, had a contraindication to magnetic resonance imaging or gadolinium contrast (part of a concurrent costudy), had moderate-severe renal impairment (glomerular filtration rate <60 ml/min/1.73 m2), were pregnant or breastfeeding, unable to perform exercise testing, or had a previous history of significant cardiovascular disease such as previous acute myocardial infarction, angina, revascularization, or heart failure.
This study was approved by the Virginia Commonwealth University Institutional Review Board, and all subjects provided informed consent before enrollment. Clinical data were extracted from the patient medical record. Radiation dose calculation was performed based on a volumetric computed tomography data set obtained during a treatment planning session. A single expert radiation oncologist (Elisabeth Weiss) performed quantification of total radiation dose and volumes of heart and lung exposed. The heart and lungs were manually contoured on each computed tomography slice generating 3-dimensional structures using dedicated treatment planning software (Pinnacle, Koninklijke Philips N.V.). After radiation beam definition and target dose calculation, mean cardiac radiation dose (MCRD) and mean lung radiation dose were determined for the whole organ volume as well as using dose-volume histograms to generate %volumes of the heart and lungs receiving at least 5, 10, 20, 30, 40, and 50 Gray, respectively (Figure 1).
Figure 1.
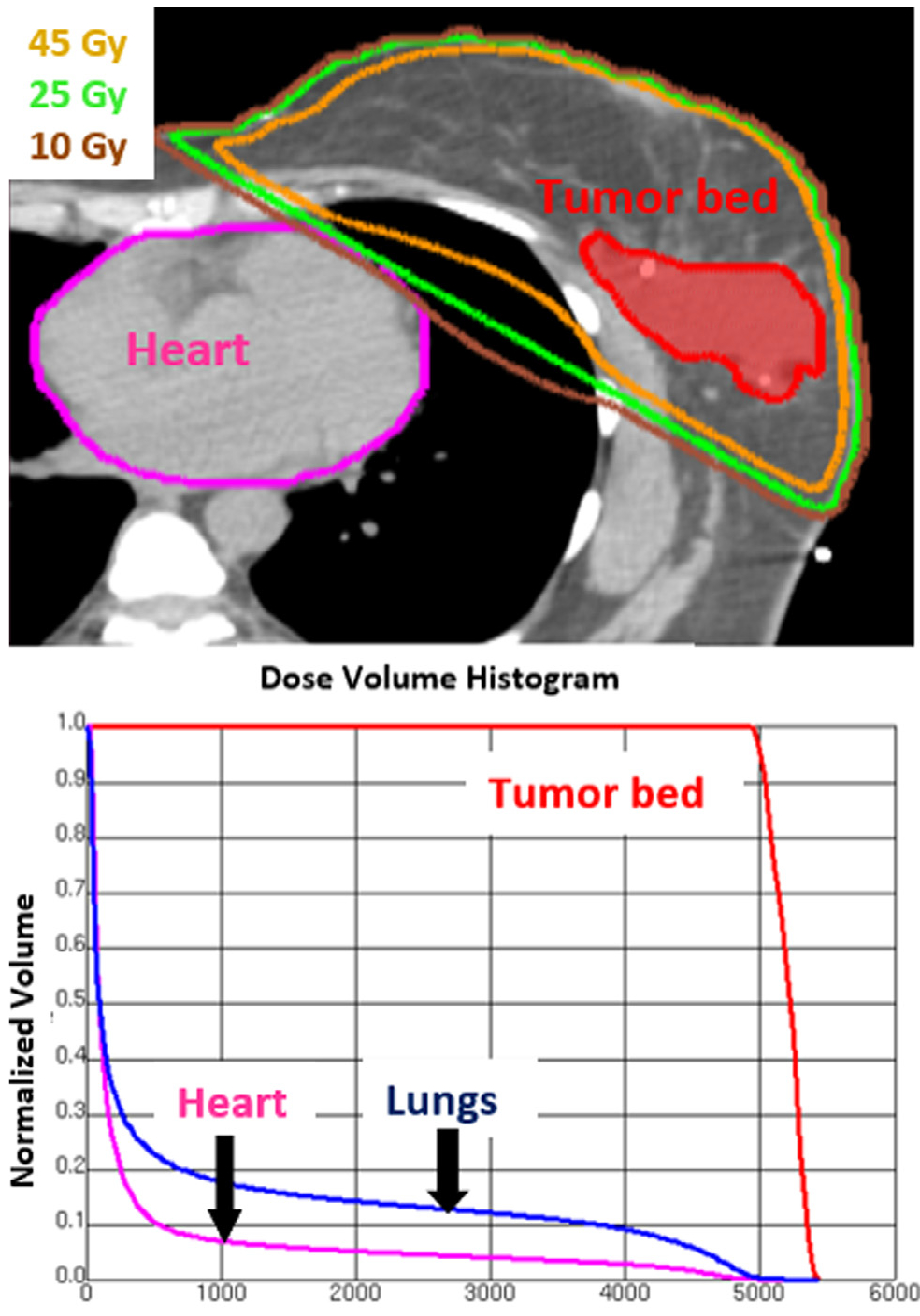
Dose volume histogram used to calculate heart radiotherapy dose. The upper panel shows an axial slice from the pretreatment planning computed tomography scan with tumor bed and heart contoured. Isodose lines for 45 Gray, 25 Gray, and 10 Gray are shown as well. The lower panel shows the associated dose volume histogram. Absolute doses for fractions of the total structure volume are displayed. For example (see heart arrow), 10 Gray are delivered to 7% of the total heart volume.
All patients underwent cardiopulmonary exercise testing, pulmonary function testing, Doppler echocardiography at rest and exercise, and measurement of circulating biomarkers. Patient characterization also included detailed assessments of body composition and physical activity participation.
A physician supervised, symptom-limited cardiopulmonary exercise test was administered to all subjects using a low-level ramping treadmill protocol and according to established guidelines for exercise testing.10 The average value for VO2 during the last 30 seconds of exercise was used to define peak VO2 relative to body weight (ml·kg−1·min−1). Percent of predicted peak VO2 was calculated according to Wasserman et al.11 A peak respiratory exchange ratio <1.0 was used to reflect submaximal effort and/or a noncardiac reason for stopping in the absence of any hemodynamic or electrocardiographic abnormalities. A peak VO2 <83% of predicted values was used to identify exercise intolerance.11 The peak oxygen pulse was defined as the ratio between peak VO2 and peak heart rate in units of ml/beat, and a value ≤85% of predicted was considered abnormal.12 The ventilatory anaerobic threshold was calculated using the dual-methods criteria with a value <95% confidence limits indicating an abnormal response.11 The oxygen uptake efficiency slope was determined from the linear relation of versus the logarithmic transformation of minute ventilation during exercise. The %-predicted oxygen uptake efficiency slope was calculated by comparing the observed with the reference values put forth by Sun13 with a value <89% of predicted considered indicative of an abnormal cardiovascular limitation as proposed by Barron.14
A normal cardiovascular limitation to exercise was defined as a peak VO2 ≥83% of predicted in the setting of a respiratory exchange ratio ≥1.0.15 A priori, an abnormal cardiovascular response to exercise was defined as impaired CRF (i.e., peak VO2 <83%) in the presence of any one of the following observances in the absence of a pulmonary limitation to exercise and a peak respiratory exchange ratio ≥1.0 (1) ventilatory anaerobic threshold <95% confidence limits; (2) oxygen uptake efficiency slope <89% of predicted; (3) peak oxygen pulse <85% of predicted. An indeterminate limitation to exercise was defined as a peak VO2 <83% with a respiratory exchange ratio <1.0 in the absence of any overt cardiopulmonary abnormalities.
All subjects underwent spirometry before exercise according to published standards.16 Peak exercise minute ventilation was compared with the direct maximal voluntary ventilation to assess ventilatory reserve, with a peak ratio of >0.80 indicating a pulmonary limitation to exercise.17 Forehead pulse oximetry was employed to estimate arterial oxygen saturation with values <95% at rest or >5% decrease with exercise also indicative of a pulmonary limitation to exercise.17
Standard 2-dimensional transthoracic echocardiography was performed to measure cardiac structure and function, left-ventricular ejection fraction, transmitral flow velocities (early, late, early to late transmitral flow ratio, and early transmitral flow deceleration time), and tissue Doppler derived early diastolic mitral annular velocity averaged between the lateral and septal annulus according to standard recommendations.18,19 Doppler-derived cardiac output was estimated by measuring the velocity time integral across the left-ventricular outflow tract multiplied by the heart rate. Since estimation of the cross-sectional area of the left-ventricular outflow tract represents a potential source of error, the velocity time integral alone was used as a surrogate for cardiac output measurement.20 The early transmitral flow to early diastolic mitral annular velocity ratio was calculated as an estimate of left-ventricular filling pressures.21 Stress echocardiography was performed to assess the velocity time integral-derived cardiac output, early transmitral flow, lateral early diastolic mitral annular velocity, and early transmitral flow to early diastolic mitral annular velocity ratio at exercise by having the patient seated immediately postexercise and obtaining an apical view in <1-minute. The interval exercise changes in velocity time integral-derived cardiac output, early diastolic mitral annular velocity, and early transmitral flow to early diastolic mitral annular velocity ratio were calculated. The diastolic functional reserve index (DFRI) was defined as the product of resting early diastolic mitral annular velocity multiplied by the delta early diastolic mitral annular velocity during exercise.22 For left-ventricular ejection fraction the normal reference range was considered to be 53% to 73% (mean ± 2-standard deviations).19
A blood sample was obtained before exercise to measure the following circulating biomarkers: (1) high-sensitivity cardiac troponin I (Abbott ARCHITECT); (2) high-sensitivity cardiac troponin T (Roche Elecsys Troponin T Gen 5 STAT); (3) N-terminal pro-brain natriuretic peptide (NTproBNP, [Roche Elecsys proBNP]); (4) Galectin-3 (Abbott ARCHITECT); (5) high-sensitivity C-reactive protein (Siemens Healthcare); (6) soluble suppression of tumorigenicity 2 (Presage, Critical Diagnostics).
Body composition was assessed pre-exercise through body mass index, waist-hip circumference, and single-frequency bioelectrical impedance analysis (RJL Systems, Inc., Clinton Township, Michigan). Physical activity participation was quantified using the International Physical Activity Questionnaire.23
A sample size of at least 19 subjects was estimated to provide 80% power to detect a correlation coefficient >0.60 between CRF and potential determinants (α = 0.05). Continuous data are reported as median and interquartile range or absolute range for potential non-Gaussian distributions. Discrete variables are reported as number and %. The nonparametric Kruskal-Wallis test was used for comparisons between subgroups for exploratory analyses. The Chi-square test was used to compare nominal variables. Univariate analysis between cardiopulmonary exercise test, cardiac-biomarker, and echocardiography variables was performed using Spearman’s rank correlation test. Multivariate analysis using a linear regression model was performed using a stepwise approach including those variables associated with p <0.05 at univariate analysis from prespecified cardiac, pulmonary, and body composition parameters to explore determinants of peak VO2. Signifi-cant univariate predictors were assessed for multicollinearity before placement in the multivariate model. An additional correction for cancer type was performed using a General Linear Model. Statistical analysis was performed using SPSS version 24.0 (IBM Corp, Armonk, New York).
Results
Between August 2016 and November 2017, we screened 106 subjects and enrolled 25 (24%). Reasons for exclusions are reported in the Supplemental Table 1. Of the 25 subjects enrolled, 15 (60%) underwent thoracic radiotherapy for lung cancer, and 10 (40%) for breast cancer. Table 1 describes the clinical characteristics of the subjects enrolled − 13 (52%) had undergone surgery before radiotherapy, and 21 (84%) had received antineoplastic chemotherapy. Six (24%) of the breast cancer patients underwent regimens including anthracycline chemotherapy. The antineoplastic regimens and specific types of chemotherapy, frequency of use, and average doses of the cohort are available in Supplemental Table 2. Seven of the 10 (70%) subjects with breast cancer were on concomitant hormonal modulating therapy at the time of evaluation.
Table 1.
Clinical characteristics of the cohort
| Variable | Entire cohort (n = 25) |
|---|---|
| Age (years) | 63 (59–66) |
| Women | 15 (60%) |
| Caucasian | 16 (64%) |
| African-American | 9 (36%) |
| Cancer type | |
| Lung | 15 (60%) |
| Breast | 10 (40%) |
| Time since cancer diagnosis (years) | 2.4 (0.3–8.6) |
| Time since completion of chemotherapy (years) | 1.5 (0.1–7.4) |
| Prior chemotherapy | 21 (84%) |
| Anthracycline-based chemotherapy | 6 (24%) |
| Time since completion of radiotherapy (years) | 1.8 (0.1–8.2) |
| Cancer stage | Breast cancer n = 10 | Lung cancer n = 15 |
|---|---|---|
| IA | 1 (4%) | 1 (4%) |
| IB | 1 (4%) | |
| II | 1 (4%) | |
| IIA | 1 (4%) | |
| IIB | 1 (4%) | |
| IIIA | 3 (12%) | 10 (40%) |
| IIIB | 2 (8%) | 3 (12%) |
| IIIC | 1 (4%) |
Data are listed as median and (absolute range) or n (%).
Thirteen (52%) patients underwent radiotherapy as primary therapy for cancer, and 12 (48%) underwent adjuvant radiotherapy following surgery. The number of radiotherapy fractions was 30 (24 to 33) with 2.0 Gray per fraction (1.8 to 2.0 Gray) for a prescribed radiotherapy dose of 60.0 Gray (48.0 to 61.7 Gray). Six subjects also underwent additional previous radiotherapy treatments (median of 4 treatments [4 to 22], 10 Gray per fraction [1.8 to 12.0 Gray], total prescribed dose 48.0 Gray [36.0 to 50.4 Gray]). Table 2 lists the total prescribed dose, MCRD, mean lung radiation dose, and mean %heart and %lung volumes that received at least 5, 10, 20, 30, 40, and 50 Gray, respectively reflecting the dose contributions from all radiotherapy treatments for each patient. When separating cancer types (breast vs lung), there was a significant difference in MCRD, and %heart volume receiving at least 5 to 50 Gray (all p’s ≤0.02) with the lung cancer subjects receiving higher cardiac doses than breast cancer subjects.
Table 2.
Prescribed radiotherapy dose, cardiac, and lung dose volumes
| Entire cohort n = 25 | Breast cancer n = 10 | Lung cancer n = 15 | p Value | |
|---|---|---|---|---|
| Total prescribed dose (Gy) | 60.0 (48.0–61.7) | 60.2 (49.5–60.4) | 60.2 (48.0–63.0) | 0.98 |
| Cardiac dose volumes | ||||
| MCRD | 5.4 (3.7–14.7) | 3.7 (2.8–4.3) | 10.3 (5.4–26.5) | <0.001 |
| V5 | 38.0 (15.0–81.0) | 13.5 (11.5–30.0) | 48.0 (36.0–88.0) | <0.01 |
| V10 | 15.0 (8.7–48.5) | 8.2 (2.8–9.0) | 33.0 (16.6–74.0) | <0.001 |
| V20 | 7.0 (1.1–24.0) | 1.6 (0.8–3.5) | 11.0 (7.0–47.0) | <0.01 |
| V30 | 3.0 (0–12.0) | 0.1 (0–2.3) | 7.0 (2.0–33.0) | <0.01 |
| V40 | 1.0 (0–6.0) | 0 (0–1.0) | 3.0 (1.0–18.0) | <0.01 |
| V50 | 0 (0–2.0) | 0 (0–0) | 1.0 (0–10.0) | 0.02 |
| Lung dose volumes | ||||
| MLRD | 9.4 (6.5–15.0) | 6.9 (5.7–10.4) | 13.9 (8.0–16.8) | 0.03 |
| V5 | 42.0 (25.1–60.0) | 27.4 (17.0–39.7) | 57.5 (37.0–66.5) | <0.01 |
| V10 | 29.7 (20.0–36.8) | 20.5 (13.3–29.9) | 35.0 (25.8–47.5) | 0.01 |
| V20 | 18.0 (11.3–26.0) | 13.6 (11.3–20.1) | 22.0 (10.3–28.5) | 0.21 |
| V30 | 13.0 (7.0–18.0) | 9.8 (7.0–13.5) | 17.0 (4.8–20.3) | 0.19 |
| V40 | 9.0 (3.3–13.3) | 5.7 (3.5–9.0) | 11.0 (2.8–15.0) | 0.11 |
| V50 | 2.0 (1.0–8.5) | 1.2 (0–2.0) | 6.5 (1.8–11.8) | <0.01 |
Values are listed as median and (interquartile range). p Values based upon comparison between breast versus lung cancer.
MCRD = mean cardiac radiation dose; V = percent volume of the heart/lung; Gy = Gray units; MLRD = mean lung radiation dose.
Hypertension was the primary established cardiovascular disease risk factor (n = 14 [56%]) followed by sedentary lifestyle (n = 11 [44%]). Supplemental Table 3 lists the prevalence of cardiovascular disease risk factors and cardiovascular medications in the group. Lung disease was the most common noncardiovascular disease related co-morbidity present in 15 (60%) individuals (all lung cancer patients). All subjects were Eastern Cooperative Oncology Group status 0 to 1 with a mean Karnofsky score of 89 ± 7. Fourteen (56%) subjects met body mass index criteria§for overweight (7 [28%]) or obesity (7 [28%]). Anthropometrics of the entire cohort are detailed in Supplemental Table 4.
The median peak respiratory exchange during maximal exercise was 0.98 (0.94 to 1.07), with 11 of 25 (44%) achieving ≥1.0, and 9 of 25 (36%) reaching ≥1.10. The primary reason for test termination was dyspnea (43%), followed by fatigue (30%) or other reasons (27%).
Table 3 provides a comprehensive summary of the analyzed exercise test variables. In univariate analysis, peak VO2 inversely correlated with age (R = 0.40, p = 0.03). Peak VO2 was not significantly different with regards to sex (p = 0.12), race (p = 0.56), or reported physical activity participation (p = 0.28). Peak VO2 was significantly higher in subjects with breast cancer compared with lung cancer (21.0 [17.8 to 23.6] ml·kg−1·min−1 versus 14.6 [12.0 to 17.5] ml·kg−1·min−1, p <0.01) or 93% (77% to 98%) predicted versus 53% (44% to 61%) predicted (p <0.0001). Peak VO2 was not significantly different when comparing those who underwent chemotherapy of any type versus those who did not undergo chemotherapy (p = 0.14). Likewise, peak VO2 was not significantly different in those who underwent anthracycline-based chemotherapy regimens (22.2 [15.5 to 23.6] versus 16.6 [13.7 to 19.9] ml·kg−1·min−1, p = 0.18), and did not correlate with anthracycline dose when treated as a continuous variable (p = 0.16).
Table 3.
Cardiopulmonary exercise test variables
| CPET variables | Entire cohort |
|---|---|
| Relative peak VO2 (mL·kg−1·min−1) | 16.8 (13.5–21.9) |
| Percent-predicted relative peak VO2 (%) | 62 (50–93) |
| Relative peak VO2 <83% predicted | 17 (68%) |
| Oxygen Pulse (ml/beat) | 9.0 (7.5–10.5) |
| Percent-predicted oxygen pulse (%) | 82 (63–97) |
| Oxygen pulse <85% predicted | 13 (52%) |
| Ventilatory anaerobic threshold (mL·kg−1·min−1) | 13.3 (11.4–16.2) |
| Percent-predicted VAT (%) | 55 (43–62) |
| VE/VCO2 slope | 32.5 (28.4–36.5) |
| Oxygen uptake efficiency slope | 1.58 (1.28–2.03) |
| Percent-predicted OUES (%) | 110 (86–141) |
| Peak respiratory exchange ratio | 0.98 (0.94–1.07) |
| Exercise time (minutes) | 9.9 (6.6–12.0) |
| Resting heart rate (bpm) | 73 (68–86) |
| Maximal heart rate (bpm) | 150 (115–165) |
| Percent-predicted APMHR (%) | 93 (78–101) |
| Resting systolic BP (mm Hg) | 124 (112–141) |
| Resting diastolic BP (mm Hg) | 70 (63–82) |
| Max systolic BP (mm Hg) | 174 (157–185) |
| Max diastolic BP (mm Hg) | 70 (70–80) |
| Resting SpO2 (%) | 99 (97–100) |
| Exercise SpO2 (%) | 97 (94–99) |
| Δ SpO2 exercise (%) | 2 (0–4) |
| VE/MVV ratio | 0.74 (0.58–0.80) |
Data are listed as median and (interquartile range) or n (%). CPET = cardiopulmonary exercise test; VO2 = oxygen consumption; VAT = ventilatory anaerobic threshold; VE/VCO2 = minute ventilation to carbon dioxide production; OUES = oxygen uptake efficiency slope; APMHR = age-predicted maximal heart rate; SpO2 = oxygen saturation; VE/MVV = peak minute ventilation/maximal voluntary ventilation ratio.
Of the 25 subjects, 13 (52%) showed no evidence of significant airflow limitation on spirometry, while the remaining subjects showed mild-to-severe grade limitations according to Global Initiative for Chronic Obstructive Lung Disease staging criteria as follows: Stage-1 (Mild) = 1 (4%); Stage-2 (Moderate) = 6 (24%); Stage-3 (Severe) = 4 (16%); Stage-4 (Very Severe) = 1 (4%). Supplemental Table 5 provides a detailed assessment of spirometry values.
Using Doppler echocardiography half of the subjects (n = 14 [56%]) had a left-ventricular ejection fraction ≤52% which is below the lower limit of the normal reference range. Table 4 provides a detailed summary of the Doppler echocardiographic variables of the entire cohort.
Table 4.
Echocardio-Doppler parameters
| Variables | Entire cohort (n = 25) |
|---|---|
| Left-ventricular ejection fraction (%) | 52 (47–61) |
| <40% | 2 (8%) |
| 40%−49% | 5 (20%) |
| ≥50% | 16 (64%) |
| ≥53% | 11 (44%) |
| European Society of Cardiology criteria for diastolic dysfunction* | 11 (44%) |
| European Society of Cardiology criteria for HFpEF† | 5 (20%) |
| Left-ventricular end-diastolic volume Index (ml/m2) | 44 (39–56) |
| Left-ventricular end-systolic volume Index (ml/m2) | 22 (16–26) |
| Stroke volume index (ml/m2) | 23 (19–29) |
| Early transmitral E velocity (cm/sec) | 75.5 (66.0–85.0) |
| Late transmitral A velocity (cm/sec) | 87.0 (80.5–94.3) |
| E/A ratio | 0.85 (0.71–1.00) |
| Left-atrial volume index (ml/m2) | 20.1 (16.6–24.9) |
| Early diastolic mitral annular velocity e’ (cm/sec) | 7.6 (7.0–9.6) |
| Longitudinal systolic strain (cm/sec) | 7.9 (7.1–8.6) |
| Late diastolic myocardial velocity (cm/sec) | 10.3 (8.7–11.9) |
| E velocity deceleration time (ms) | 220 (178–249) |
| Ratio of E to e’ | 9.4 (7.5–13.3) |
| Exercise E velocity (cm/sec) | 87 (75–123) |
| Exercise e’ (cm/sec) | 10.5 (6.8–14.4) |
| Δ Exercise E (cm/sec) | 3.4 (1.0–6.5) |
| Ratio of exercise E to e’ | 7.9 (7.0–13.5) |
| Δ Ratio of exercise E to e’ | −0.6 (−1.8 to 3.0) |
| Diastolic Functional Reserve Index | 27.3 (8.2–68.1) |
| Left-ventricular outflow tract velocity time integral –Rest (cm) | 15.2 (14.4–20.5) |
| Left-ventricular outflow tract velocity time integral –Exercise (cm) | 19.6 (17.8–25.0) |
| Δ Exercise left-ventricular outflow tract velocity time integral | 3.2 (2.3–5.9) |
Data are listed median and (interquartile range). ml/m2 = milliliters per meter squared; cm/sec = centimeters per second; ms = milliseconds.
European Society of Cardiology criteria for diastolic dysfunction = ratio of early transmitral velocity to early diastolic mitral annular velocity >13; mean early diastolic mitral annular velocity <9 cm/sec; or left-atrial volume index >34 ml/m2; or presence of left-ventricular hypertrophy.
European Society of Cardiology Diagnostic algorithm for a diagnosis of heart failure with preserved ejection fraction (HFpEF) of nonacute onset: Exercise intolerance= typical symptom of heart failure; Cardiotoxic radiation = assessment of heart failure probability; heart failure with preserved ejection fraction = left-ventricular ejection fraction ≥50% with N terminal pro-brain natriuretic peptide ≥125 pg/ml and European Society of Cardiology criteria for diastolic dysfunction.
Table 5 indicates the proportion of the cohort with abnormal responses for each blood-based biomarker and the median values for each. Elevated C-reactive protein (≥2 mg/L, n = 16 [64%]) and NTproBNP (≥ 125 pg/ml, n = 14 [56%]) was noted in over half of all subjects.
Table 5.
Cardiac-specific blood-based biomarkers
| Biomarker | Abnormal response | Values |
|---|---|---|
| N-terminal pro-brain natriuretic peptide (pg/ml) | 14 (56%) | 180 (47–344) |
| High-sensitivity C-reactive protein (mg/L) | 16 (64%) | 3.0 (1.7–6.9) |
| Galectin-3 (ng/ml) | 8 (32 %) | 15.4 (12.8–19.8) |
| High-sensitivity cardiac troponin T (ng/L) | 5 (20%) | 9.5 (5.1–13.8) |
| High-sensitivity cardiac troponin I (ng/L) | 3 (12%) | 4.0 (2.0–8.0) |
| Soluble suppression of tumorigenicity-2 (ST2; [ng/ml]) | 8 (32%) | 28.2 (21.9–37.3) |
Data are listed as n (%) or median and (interquartile range). Abnormal response was defined as: N-terminal pro-brain natriuretic peptide ≥125 pg/ml, high-sensitivity C-reactive protein ≥2 mg/L, Galectin-3 >17.8 ng/ml, high-sensitivity cardiac troponin T (Male >22 ng/L, Female >14 ng/L), high-sensitivity cardiac troponin I (Male >14 ng/L, Female >11.1 ng/L), soluble suppression of tumorigenicity-2 ≥35 ng/ml. pg/ml = picograms per milliliter; mg/L = milligrams per liter; ng/L = nanograms per liter; ng/ml = nanograms per milliliter.
Cardiorespiratory fitness was preserved, defined by peak VO2 ≥83% of predicted values, in only 8 (32%) subjects. In the remaining 17 (68%) subjects with a reduced peak VO2, 6 (24%) demonstrated a predominant abnormal cardiovascular limitation, 7 (28%) a pulmonary limitation, and 4 (16%) an indeterminate limitation to exercise. Table 6 details the comparison of groups based upon exercise tolerance and the predominant limitation to exercise.
Table 6.
Comparison of groups based upon limitation to exercise
| Normal exercise eolerance (n = 8, 32%) | Cardiovascular limitation (n = 6, 24%) | Pulmonary limitation (n = 7, 28%) | Indeterminate limitation (n = 4, 16%) | p Value | |
|---|---|---|---|---|---|
| Age (years) | 59.5 (49.5–67.5) | 61.5 (57.8–64.5) | 64.0 (59.0–75.0) | 63.5 (60.8–70.8) | 0.58 |
| Female | 8 (32%)* | 2 (8%) | 2 (8%) | 3 (12%) | 0.02 |
| Breast cancer | 7 (28%)† | 1 (4%) | 0 (0%) | 2 (8%)‡ | <0.01 |
| Lung cancer | 1 (4%) | 5 (20%)§ | 7 (28%)¶ | 2 (8%) | <0.01 |
| Peak oxygen consumption (ml·kg−1·min−1) | 22.8∥ (20.5–25.0) | 15.3 (12.2–17.9) | 14.5 (11.5–16.9) | 14.7 (13.3–18.3) | <0.01 |
| Body mass index (kg/m2) | 27.0 (22.9–30.6) | 22.1 (18.8–31.3) | 26.0 (23.6–27.1) | 42.7 (28.9–47.5) | 0.09 |
| FEV1 (Liters) | 2.35 (2.14–2.72) | 1.95 (1.42–2.53) | 0.99# (0.83–1.86) | 1.88 (1.69–2.31) | <0.01 |
| LVEF (%) | 59 (51–63) | 48 (38–56) | 55 (47–59) | 45 (44–67) | 0.23 |
| Cancer chemotherapy | 7 (28%) | 6 (24%) | 4 (16%) | 4 (16%) | 0.13 |
| MCRD (Gray) | 3.5** (2.7–5.1) | 8.9 (4.2–24.5) | 7.1 (5.2–28.6) | 7.6 (4.3–22.6) | 0.04 |
| MLRD (Gray) | 8.1 (5.4–13.5) | 13.9 (10.9–16.7) | 8.0 (4.5–17.3) | 9.4 (6.1–15.9) | 0.39 |
| Time since radiotherapy (years) | 1.5 (0.7–2.5) | 1.4 (0.2–3.8) | 4.2 (1.5–6.8) | 0.4 (0.2–2.4) | 0.16 |
| Hypertension | 4 (16%) | 4 (16%) | 3 (12%) | 3 (12%) | 0.70 |
| Hyperlipidemia | 4 (16%) | 3 (12%) | 3 (12%) | 4 (16%) | 0.37 |
| Diabetes mellitus | 3 (12%) | 1 (4%) | 1 (4%) | 1 (4%) | 0.73 |
| Obesity | 3 (12%) | 2 (8%) | 0 (0%) | 2 (8%) | 0.27 |
| Smoker | 0 (0%) | 1 (4%) | 4 (16%) | 2 (8%) | 0.08 |
| Sedentary (MET-min/week) | 1689 (1064–4467) | 330 (0–2661) | 604 (0–926) | 594 (426–4452) | 0.12 |
Data are listed as median and (interquartile range) or n (%). NL = normal exercise tolerance; CV = cardiovascular limitation; P = pulmonary limitation; I = indeterminate limitation; mL·kg−1·min−1 = milliliters per kilogram per minute; kg/m2 = kilograms per meter square; FEV1 = forced expiratory volume in 1-second; LVEF = left-ventricular ejection fraction; MCRD = mean cardiac radiation dose; MLRD = mean lung radiation dose; MET-min/week = metabolic equivalents per minute per week. Hypertension/Hyperlipidemia/Diabetes mellitus defined as medical record diagnosis of hypertension/hyperlipidemia/diabetes mellitus and/or current use of antihypertensive/lipid- or glucose lowering pharmacologic therapies. Obesity defined as body mass index ≥30.0 kg/m2. Smoking was defined as current cigarette smoker.
NL>CV, P.
NL>CV, P.
I>P.
CV>NL.
P>NL, I.
NL>CV, P, I.
P<NL.
NL<CV, P, I.
Assessment of prespecified physiologic predictors of peak VO2 listed in Table 7 at univariate analysis revealed significant associations with radiotherapy dose, cardiac, body composition, and ventilatory parameters and includes the variables retained in a multivariate model. The strongest correlation was seen between the DFRI and peak VO2 reflecting impaired myocardial relaxation with exercise (Figure 2). The radiotherapy, cardiac, pulmonary, and body composition parameters demonstrating a significant relation to peak VO2 (p <0.05) were entered into a stepwise multivariate regression model revealing that the DFRI and NTproBNP remained the only 2 independent predictors of peak VO2 in the entire cohort (model-adjusted R2 = 0.73, p <0.01; DFRI, = +0.813, p <0.01; NTproBNP, = −0.414, p = 0.04).
Table 7.
Multivariate analysis of predictors of peak oxygen consumption for the entire cohort
| Variable | R-value | Univariate p value | Multivariate p value |
|---|---|---|---|
| Radiotherapy parameters | |||
| Total prescribed dose (Gy) | +0.029 | 0.89 | |
| MCRD (Gy) | −0.445 | 0.02 | 0.31 |
| MLRD (Gy) | +0.529 | 0.13 | |
| Cardiac parameters LVEF (%) | +0.026 | 0.90 | |
| Rest e’ | +0.514 | 0.01 | 0.24 |
| Rest E/e’ | −0.613 | <0.01 | 0.80 |
| Exercise e’ | +0.613 | 0.03 | 0.86 |
| Exercise E/e’ | −0.406 | 0.19 | |
| Δ E/e’exercise | −0.245 | 0.44 | |
| e’/DT ratio | +0.422 | 0.04 | 0.05 |
| DFRI | +0.629 | 0.02 | <0.01 |
| Exercise VTICO | +0.210 | 0.51 | |
| Δ VTICO exercise | +0.773 | <0.01 | 0.21 |
| NTproBNP | −0.588 | <0.01 | 0.04 |
| hs-cTnI | −0.552 | <0.01 | 0.76 |
| hs-cTnT | −0.541 | <0.01 | 0.26 |
| Galectin-3 | −0.299 | 0.15 | |
| hsCRP | −0.333 | 0.11 | |
| sST2 | −0.200 | 0.35 | |
| Body composition parameters | |||
| Weight (kg) | −0.197 | 0.35 | |
| BMI | −0.097 | 0.65 | |
| Waist circumference | −0.371 | 0.08 | |
| Waist-hip ratio | −0.444 | 0.03 | 0.57 |
| Fat mass% | −0.062 | 0.77 | |
| Fat-free mass% | +0.062 | 0.77 | |
| Ventilatory parameters | |||
| FVC | +0.538 | <0.01 | 0.83 |
| FEV1 | +0.760 | <0.01 | 0.37 |
| FEV1/FVC | +0.594 | <0.01 | 0.36 |
| Direct MVV | +0.723 | <0.01 | 0.23 |
| Δ SpO2 exercise | −0.490 | 0.02 | 0.65 |
Bold values indicate significance at a p-value <0.05.
Gy = Gray; MCRD = mean cardiac radiation dose; MLRD = mean lung radiation dose; LVEF = left-ventricular ejection fraction; e’ = early diastolic mitral annular velocity; E/e’ = ratio of early transmitral velocity to early diastolic mitral annular velocity; D = delta; e’/DT = ratio of early diastolic mitral annular velocity to deceleration time; DFRI = diastolic functional reserve index; VTICO = left-ventricular outflow tract velocity time integral cardiac output; NTproBNP = N-terminal pro-brain natriuretic peptide; hs-cTnI = high-sensitivity cardiac troponin I; hs-cTnT = high-sensitivity cardiac troponin T; hsCRP = high-sensitivity C-reactive protein; sST2 = soluble suppression of tumorigenicity-2; kg = kilograms; BMI = body mass index; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1-second; MVV = maximal voluntary ventilation; SpO2 = oxygen saturation.
Figure 2.
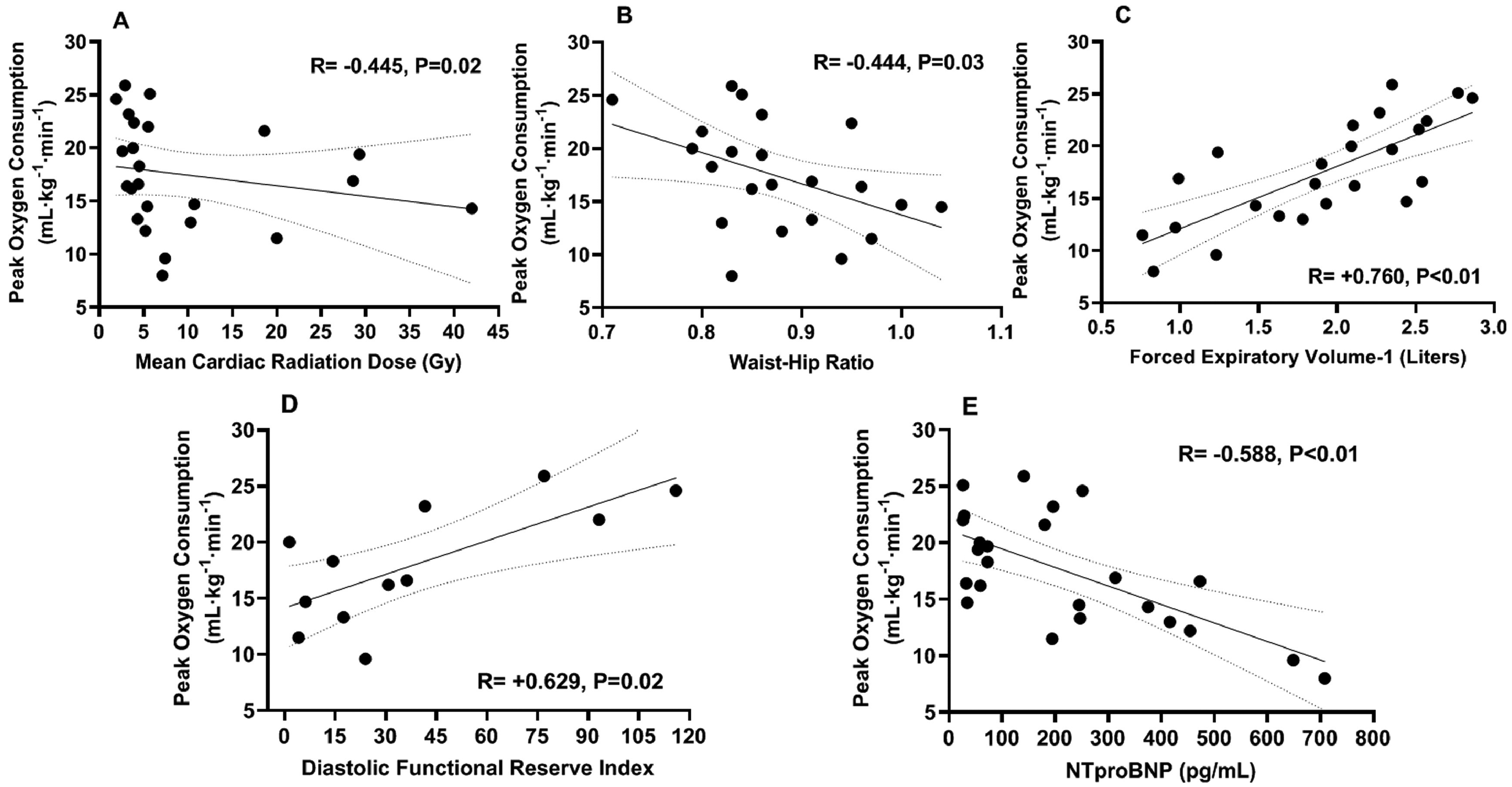
Cardiovascular, pulmonary, and body composition predictors of peak oxygen consumption. Peak oxygen consumption (VO2) was inversely associated with the mean cardiac radiation dose (Panel A). Peak VO2 was independently associated with Doppler stress echo-derived diastolic functional reserve index and NTproBNP levels (Panels D, E). The anthropometric waist-hip ratio (Panel B) and pulmonary forced expiratory volume-1 second (Panel C) were inversely associated with peak VO2. Abbreviations: Gy = Gray units; mL·kg−1·min−1 = milliliters per kilogram per minute; NTproBNP = N-terminal pro-brain natriuretic peptide; pg/mL = picograms per milliliter.
The DFRI and NTproBNP were then entered into a general linear model with the addition of cancer-type (breast vs lung) as a categorical predictor identified in the clinical characteristics associated with differences in peak VO2. This resulted in the loss of NTproBNP as an independent significant predictor, whereas the DFRI remained strongly associated with peak VO2 (R2 = 0.734, p = 0.02).
Dividing the cohort by primary exercise limitation (cardiac vs pulmonary vs indeterminate) and examining the predictors of peak VO2 in those with a cardiac limitation to exercise revealed the resting early transmitral flow to early diastolic mitral annular velocity ratio and the exercise early diastolic mitral annular velocity were retained as independent predictors of peak VO2 at multivariate analysis (model-adjusted R2 = 0.79, p <0.01).
Discussion
In this cohort of patients without known pre-existing cardiovascular disease who had received a cardiotoxic dose of thoracic radiotherapy, most patients had impaired CRF as indicated by a reduction in peak VO2 (median reduction of 38% below normative values). Moreover, 24% demonstrated an abnormal cardiovascular limitation to exercise, and the Doppler-stress echo-derived DFRI and the cardiac-biomarker NTproBNP were indeed strong, independent predictors of peak VO2.
These results have multiple implications. First, they confirm that impairment in CRF is common in cancer survivors. Second, it indicates that assessment of CRF is sensitive to detect latent cardiovascular abnormalities in this cohort without a history of cardiovascular disease. Third, it confirms a dose-dependent relation between impaired CRF and radiotherapy heart dose.
The finding of reduced CRF in cancer survivors following anticancer therapies has been previously reported24 although relations between radiotherapy regimen and peak VO2 have not been reported. A review by Peel et al involving a total of 1,856 female breast cancer subjects (chemotherapy: n = 78%, radiotherapy: n = 56%) found peak VO2 in the postadjuvant setting was 25% lower compared with healthy, sedentary values.24 The novelty of the present study lies in its characterization of the exercise response in cancer survivors with significant radiotherapy heart dose and the implicated mechanisms of impaired CRF.
The DFRI (the Doppler-echo product of resting early diastolic mitral annular velocity multiplied by the delta early diastolic mitral annular velocity during exercise) has previously been shown to predict exercise capacity in individuals with exertional diastolic dysfunction.25,26 The utility of the DFRI is its ability to identify diastolic abnormalities not apparent at rest. The early mitral annulus diastolic velocity, a surrogate of myocardial relaxation, demonstrates a strong inverse correlation with the isovolumetric time constant (tau) a reference marker of left-ventricular relaxation.27 The finding of reduced CRF and its strong association with DFRI in the present study indicates impaired relaxation is driving the exercise intolerance.
In a population-based case-control study of incident heart failure in female breast cancer patients (MCRD = 2.5 Gray, mean time postradiotherapy = 5.8 years), a dose-response relation between MCRD and the incidence of heart failure was present.3 The odds ratio (95% confidence interval) for heart failure per log MCRD was 9.1 (3.4 to 24.4) for any heart failure and 16.9 (3.9 to 73.7) for heart failure with preserved ejection fraction. In an elegant animal model, Saiki et al demonstrated reduced exercise capacity, increased left-ventricular diastolic stiffness, impaired myocardial relaxation, elevated filling-pressures, but similar left-ventricular ejection fraction compared with controls in rats following cardiac irradiation.28 Post hoc analysis showed evidence of a significant inverse linear trend between exercise capacity and radiation dose suggesting a dose-response relation. In another animal study by Mezzaroma et al, contractile reserve measured by isoproterenol challenge decreased in a dose-dependent manner in mice exposed to different radiotherapy doses.29 These studies provide mechanistic insight into the pathological link between heart failure, diastolic dysfunction, radiotherapy exposure, and resultant impairment of cardiac reserve.
The primary limitations of this study are the small number of cases and its cross-sectional nature rather than longitudinal assessment of the disease. Therefore, despite the multiple correlations between cardiac variables, CRF, and radiotherapy dose a cause-effect link cannot be proven, and these associations may reflect biases between primary diagnosis, treatment modalities, and concomitant cardiovascular risk factors. Moreover, there was significant heterogeneity in cancer-type and use of chemotherapy systemic agents investigated with this study. Nevertheless, the comprehensive characterization of the patients using cardiac and pulmonary-targeted diagnostic tests, and the results of a unifying significant cardiac radiation dose threshold, but varying dose amounts based upon cancer type and guideline-directed treatments (i.e., lower in breast cancer vs higher in lung cancer) may have allowed detection of the observed dose-response relation. From a technical standpoint, an additional limitation when ascertaining organ-system limitation to exercise is that no specific procedures were performed that directly measured peripheral vascular function or skeletal muscle characteristics both of which are known to contribute to exercise capacity.30
In conclusion, impairment in CRF shows a dose-dependent relation with the radiation dose to the heart, and is primarily related to impaired diastolic reserve. This study warrants further investigation into radiation-induced exercise intolerance and the efficacy of interventions to improve CRF in this population.
Supplementary Material
Acknowledgment
The authors would like to thank all the patients and the families in the Virginia Commonwealth University Massey Cancer Center who participated in the screening and testing in this study. The authors would also like to thank the staff of the Virginia Commonwealth University Clinical Research Unit and the Massey Cancer for their support during the conduct of the study.
Funding information. This research was supported through a Virginia Commonwealth University Massey Cancer Center Pilot Project grant #P30CA016059K and Virginia Commonwealth University’s grant #1UL1TR002649 from the National Center for Advancing Translational Science.
Footnotes
Disclosures
The authors have nothing to disclose and no conflicts of interest exist for the listed authors.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2019.12.019.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Rygiel K Cardiotoxic effects of radiotherapy and strategies to reduce them in patients with breast cancer: an overview. J Cancer Res Ther 2017;13:186–192. [DOI] [PubMed] [Google Scholar]
- 3.Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, Ruddy KJ, Yan E, Redfield MM. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation 2017;135:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenneman CG, Sawyer DB. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res 2016;118:1008–1020. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs LD, Salgo IS, Goonewardena S, Weinert L, Coon P, Bardo D, Gerard O, Allain P, Zamorano JL, Isla LP de, Mor-Avi V, Lang RM. Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur Heart J 2006;27: 460–468. [DOI] [PubMed] [Google Scholar]
- 6.Kaminsky LA, Arena R, Ellingsen O, Harber MP, Myers J, Ozemek C, Ross R. Cardiorespiratory fitness and cardiovascular disease - The past, present, and future. Prog Cardiovasc Dis 2019;62:86–93. [DOI] [PubMed] [Google Scholar]
- 7.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J 2004;147:354–360. [DOI] [PubMed] [Google Scholar]
- 8.Peel JB, Sui X, Adams SA, Hebert JR, Hardin JW, Blair SN. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med Sci Sports Exerc 2009;41:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res 2019;124: 799–815. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA, American Heart Association Exercise Cardiac Rehabilitation, of the Council on Clinical Cardiology Council on Nutrition PA, Metabolism C on C, Stroke Nursing, on Epidemiology C, Prevention. Exercise standards for testing and training: a scientific statement from the American heart association. Circulation 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 11.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. 4th ed. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 12.Oliveira RB, Myers J, Araujo CG, Arena R, Mandic S, Bensimhon D, Abella J, Chase P, Guazzi M, Brubaker P, Moore B, Kitzman D, Peberdy MA. Does peak oxygen pulse complement peak oxygen uptake in risk stratifying patients with heart failure? Am J Cardiol 2009;104:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun XG, Hansen JE, Stringer WW. Oxygen uptake efficiency plateau: physiology and reference values. Eur J Appl Physiol 2012;112: 919–928. [DOI] [PubMed] [Google Scholar]
- 14.Barron A, Francis DP, Mayet J, Ewert R, Obst A, Mason M, Elkin S, Hughes AD, Wensel R. Oxygen uptake efficiency slope and breathing reserve, not anaerobic threshold, discriminate between patients with cardiovascular disease over chronic obstructive pulmonary disease. JACCHeart Fail 2016;4:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, MacKo R, Mancini D, Milani RV. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American heart association. Circulation 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AT. Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 17.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J, EACPR, AHA. EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J 2012;33:2917–2927. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 20.Haites NE, McLennan FM, Mowat DH, Rawles JM. Assessment of cardiac output by the Doppler ultrasound technique alone. Br Heart J 1985;53:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 2000;102:1788–1794. [DOI] [PubMed] [Google Scholar]
- 22.Gibby C, Wiktor DM, Burgess M, Kusunose K, Marwick TH. Quantitation of the diastolic stress test: filling pressure vs. diastolic reserve. Eur Heart J Cardiovasc Imaging 2013;14:223–227. [DOI] [PubMed] [Google Scholar]
- 23.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 24.Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc 2014;3:e000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha JW, Choi D, Park S, Choi EY, Shim CY, Kim JM, Ahn JA, Lee SW, Oh JK, Chung N. Left ventricular diastolic functional reserve during exercise in patients with impaired myocardial relaxation at rest. Heart 2009;95:399–404. [DOI] [PubMed] [Google Scholar]
- 26.Trankle C, Canada JM, Buckley L, Carbone S, Dixon D, Arena R, Van Tassell B, Abbate A. Impaired myocardial relaxation with exercise determines peak aerobic exercise capacity in heart failure with preserved ejection fraction. ESC Hear Fail 2017;4:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474–480. [DOI] [PubMed] [Google Scholar]
- 28.Saiki H, Moulay G, Guenzel AJ, Liu W, Decklever TD, Classic KL, Pham L, Chen HH, Burnett JC, Russell SJ, Redfield MM. Experimental cardiac radiation exposure induces ventricular diastolic dysfunction with preserved ejection fraction. Am J Physiol Hear Circ Physiol 2017;313:H392–H407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mezzaroma E, Mikkelsen RB, Toldo S, Mauro AG, Sharma K, Marchetti C, Alam A, Van Tassell B, Gewirtz DA, Abbate A. Role of interleukin-1 in radiation-induced cardiomyopathy. Mol Med 2015;21: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buono MG Del, Arena R, Borlaug BA, Carbone S, Canada JM, Kirk-man DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, Abbate A. Exercise intolerance in patients with heart failure. J Am Coll Cardiol 2019;73:2209–2225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


