Abstract
Background
Previous research regarding long-term glucose variability over several years which is an emerging indicator of glycemic control in diabetes showed several limitations. We investigated whether variability in long-term fasting plasma glucose (FG) can predict the development of stroke, myocardial infarction (MI), and all-cause mortality in patients with diabetes.
Methods
This is a retrospective cohort study using the data provided by the Korean National Health Insurance Corporation. A total of 624,237 Koreans ≥ 20 years old with diabetes who had undergone health examinations at least twice from 2005 to 2008 and simultaneously more than once from 2009 to 2010 (baseline) without previous histories of stroke or MI. As a parameter of variability of FG, variability independent of mean (VIM) was calculated using FG levels measured at least three times during the 5 years until the baseline. Study endpoints were incident stroke, MI, and all-cause mortality through December 31, 2017.
Results
During follow-up, 25,038 cases of stroke, 15,832 cases of MI, and 44,716 deaths were identified. As the quartile of FG VIM increased, the risk of clinical outcomes serially increased after adjustment for confounding factors including duration and medications of diabetes and the mean FG. Adjusted hazard ratios (95% confidence intervals) of FG VIM quartile 4 compared with quartile 1 were 1.20 (1.16–1.24), 1.20 (1.15–1.25), and 1.32 (1.29–1.36) for stroke, MI and all-cause mortality, respectively. The impact of FG variability was higher in the elderly and those with a longer duration of diabetes and lower FG levels.
Conclusions
In diabetes, long-term glucose variability showed a dose–response relationship with the risk of stroke, MI, and all-cause mortality in this nationwide observational study.
Keywords: Diabetes mellitus Glucose variability, Cardiovascular disease, Stroke, All-cause mortality, The Korean National Health Insurance Corporation
Background
For individuals with diabetes, maintaining optimal blood glucose level is required to prevent complications and death [1]. However, as incident cardiovascular disease (CVD) and deaths associated with diabetes cannot be fully explained by increased HbA1c itself [2–4], more attention has been focused on non-traditional risk factors such as glucose variability (GV). Patients with diabetes with similar HbA1c values can have different daily glycemic profiles with variations in the duration and frequency of glycemic excursions [5]. Short-term GV usually means same-day or between-day glycemic oscillations and might be influenced by the individuals’ diet, exercise, and treatment modality. Long-term GV represents the visit-to-visit variability in fasting glucose (FG) estimated over months to years; compliance with medication; and deterioration of insulin secretion and resistance, which can be important issues [6].
During the past decade, detrimental effects of GV on patients with diabetes have been proposed for various medical conditions [7–13]. GV could not only predict diabetic vascular complications [7, 14, 15], heart failure [9], and postoperative complications of aortic valve implantation [11], but also indicate poor prognosis for in-patients with acute lung diseases [8] and acute coronary syndrome [13]. Moreover, because GV could modify the correlation between time in range and estimated HbA1c, GV consideration was recommended when setting individualized goals for glycemic control [10].
Although there have been a few attempts to verify if GV can be a contributor to CVD or mortality [6, 7, 15–21], there is no consensus for patients with diabetes. Previous studies have yielded partial significance according to age, study group, or status of glycemic control [6, 7, 15–21]. In addition, they have several limitations such as small sample size and short-term follow-up periods.
In the present study, we used a nationwide population-based database in Korea to investigate whether long-term variability in FG can predict the risk of stroke, myocardial infarction (MI), and all-cause mortality during a median 8 years of follow-up, to specify the population prone to the risk of higher GV, and to compare it with single measurements of FG.
Methods
Study design and subjects
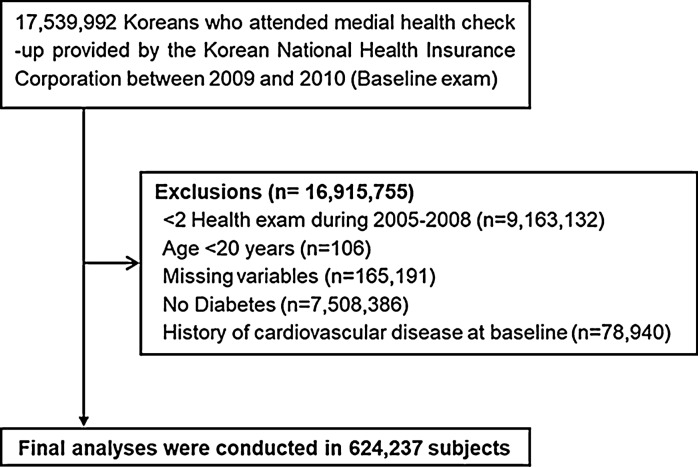
As shown in Fig. 1, we analyzed the data of patients with diabetes who had undergone health examinations provided by the National Health Insurance Corporation (NHIC) at least twice from 2005 to 2008 and simultaneously more than once between January 1, 2009, and December 31, 2010 (baseline exam). Among them, we excluded individuals aged < 20 years and those with histories of stroke or MI, along with those missing data in the inclusion criteria. After those exclusions, 624,237 individuals were included in this study.
Fig. 1.
Selection of study subjects
The NHIC is the single health insurance system operated by the Korean government and covers about 97% of the Korean population. It has an eligibility profile, health examination database (general health examinations and questionnaires on lifestyle), medical treatment information identified by the medical bills submitted by healthcare providers, and a medical care institution database [22, 23]. Enrollees are advised to undergo a standardized medical examination annually or biannually. The entire database of the NHIC is now open to all researchers.
The presence of diabetes was defined as at least one claim per year for the prescription of anti-diabetic medications under the International Classification of Diseases, Tenth Revision (ICD-10) codes E10–14 or a fasting plasma glucose level ≥ 7 mm ol/L. Type 1 diabetes (T1DM) was defined as ICD-10 code E10 with at least one prescription history of insulin. The remained subjects were referred to as type 2 diabetes (T2DM).
This study was approved by the official review committee in the NHIC and the Institutional Review Board of the Korea University Ansan Hospital (Institutional Review Board number 2019AS0158) and was carried out in accordance with the Helsinki Declaration of 1975.
Definition of GV
The variability independent of the mean (VIM) of FG was used as a primary variability parameter calculated from FG levels measured at least three times during the 5 years prior to the baseline (Fig. 2). The FG level at baseline was included in calculation of FG variability. Additionally, standard deviation (SD), coefficient of variation (CV, SD/mean), and average real variability (ARV) of FG during the same period were calculated using the following equation:
where β is derived by nonlinear regression analysis, based on the natural logarithm of SD over the natural logarithm of the mean [24].
where k ranges from 1 to n-1, and n is the number of FG measurements.
Fig. 2.
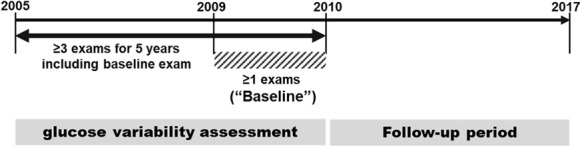
Assessment of glucose variability and follow-up period
Study outcomes
Incidences of stroke, MI, or death until December 31, 2017, were the endpoints of this study. Stroke was defined as the recording of ICD-10 codes I63 or I64 during admission with simultaneous claims for brain CT or MRI. MI was diagnosed by the recording of ICD-10 codes I21 or I22 with hospitalization. Deceased cases were identified based on nationwide death certificate data from the Korea National Statistical Office regardless of a previous diagnosis of stroke or MI. The time interval between the baseline examination and the date of study outcome or December 31, 2017 is defined as the follow-up period.
Anthropometric and laboratory measurements
We used data from questionnaires regarding demographic characteristics, lifestyle, medical history, and medications during the medical examination. Alcohol consumption habit was divided into near abstinence, moderate (< 30 g/day), or severe (≥ 30 g/day). Smoking history was categorized as never, ex-, and current smokers. Doing regular exercise was defined > 20 min of vigorous-intensity or > 30 min of moderate-intensity exercise at least once per week [25]. Body mass index (BMI) was estimated by weight in kilograms divided by the square of height in meters. Waist circumference was checked at the middle point between the iliac crest and the rib cage.
The presence of hypertension was defined as systolic blood pressure (BP) ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, or the presence of at least one prescription of anti-hypertensive medications under ICD-10 codes I10–I15 per year. The presence of malignancy was determined by registration in the Korea Central Cancer Registry under the International Classification of Diseases, ICD-10 C00-C96 before baseline examination. Low-income status was defined by the lowest 20%.
Venous blood samples were drawn in the morning after an overnight fast of at least 8 hours to measure the levels of plasma glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol (LDL-C), and creatinine.
Dyslipidemia was defined as total cholesterol level ≥ 6.21 mmol/L or the presence of at least one claim per year for the prescription of anti-hyperlipidemic drugs under ICD-10 code E78. The estimated glomerular filtration rate (eGFR) was estimated by the Modification of Diet in Renal Disease formula [26], and eGFR < 60 mL/min/1.73 m2 was classified as chronic kidney disease (CKD) [27]. The number of oral anti-diabetic medication among metformin, sulfonylurea, meglitinide, thiazolidinedione, inhibitors of dipeptidyl peptidase 4 (DPP-4 inhibitors), and acarbose taken in the 12 months prior to baseline was identified. The history of heart disease was identified by self-report.
Quality control of the laboratory tests was conducted in accordance with the Korean Association of Laboratory Quality Control.
Statistical analysis
Data are presented as mean ± SD, geometric mean (95% confidence intervals [CIs]), or number (%). The baseline characteristics were compared using Chi square tests for categorical variables and analysis of variance for continuous variables after dividing the subjects according to the FG VIM quartile. Triglyceride levels were log-transformed for analysis.
To assess the risks of stroke, MI, and all-cause mortality, we conducted multivariate-adjusted Cox proportional hazards analyses according to FG VIM quartiles and deciles, using quartile 1 or decile 1 as the reference group.
We adjusted for confounders at baseline using two models. Model 1 was adjusted for age, sex, BMI, alcohol drinking, smoking, regular exercise, presence of hypertension and dyslipidemia, CKD, and low-income status. Model 2 is the same as model 1, plus further adjustment for duration of diabetes over 5 years, the number of classes of oral anti-diabetic medication, insulin prescription history taken in the 12 months prior to baseline, and mean FG during the 5 years preceding the baseline exam.
We conducted several subgroup analyses to evaluate the effects of age; sex; BMI; smoking; income status; presence of hypertension, dyslipidemia, and malignancy; duration of diabetes; baseline FG level; subtype of diabetes; and anti-diabetic medication. The hazard ratios (HRs) and 95% CIs for stroke, MI, and all-cause death in FG VIM quartile 4 versus quartiles 1–3, with adjustments for confounders, were estimated.
To compare with the predictive value of a single FG level, we performed the above-mentioned Cox analysis according to baseline FG level, with FG levels of 100-119 mg/dL being the reference group. The mean of FG was excluded as a confounder in this analysis. Additionally, we repeated this analysis after stratifying the subjects according to prescription history of anti-diabetic medication.
We checked a variable inflation factor for all covariates of less than 2.0 and found no relevant multicollinearity in covariates. SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. A p value of < 0.05 was considered to be statistically significant.
Results
FG VIM quartile 4 had more males and current smokers and showed higher FG and triglyceride levels compared with quartile 1 (Table 1). On the other hand, this group was younger, had a lower BP and LDL-C level, and had lower proportions of hypertension, dyslipidemia, and heart disease versus quartile 1. About 46% of the highest VIM quartile was not treated with any anti-hyperglycemic agents. However, a higher proportion of them was treated with insulin for the preceding 12 months than other tertiles.
Table 1.
Baseline characteristics of the study subjects according to quartiles of fasting glucose variability
| Characteristics | VIM Q1 (n = 156061) | VIM Q2 (n = 156058) | VIM Q3 (n = 156060) | VIM Q4 (n = 156058) | P value |
|---|---|---|---|---|---|
| Age (years) | 58.6 ± 10.6 | 57.4 ± 11.0 | 56.5 ± 11.7 | 54.7 ± 13.1 | < 0.001 |
| Sex, male (%) | 98563 (63.2) | 101947 (65.3) | 103675 (66.4) | 106869 (68.5) | < 0.001 |
| BMI (kg/m2) | 24.9 ± 3.0 | 25.0 ± 3.1 | 25.1 ± 3.2 | 24.9 ± 3.4 | < 0.001 |
| WC (cm) | 85.2 ± 8.0 | 85.5 ± 8.0 | 85.6 ± 8.2 | 85.1 ± 8.6 | < 0.001 |
| Systolic BP (mmHg) | 128.6 ± 15.2 | 128.8 ± 15.0 | 128.6 ± 15.0 | 127.8 ± 15.0 | < 0.001 |
| Fasting glucose (mg/dL) | 143.8 ± 40.5 | 142.0 ± 38.0 | 142.4 ± 41.1 | 146.2 ± 50.7 | < 0.001 |
| Triglyceride (mg/dL) | 141.9 (141.5–142.3) | 146.7 (146.3–147.1) | 149.7 (149.3–150.1) | 148.8 (148.3–149.2) | < 0.001 |
| HDL-C (mg/dL) | 52.0 ± 21.8 | 51.8 ± 21.2 | 51.6 ± 21.1 | 51.7 ± 20.9 | < 0.001 |
| LDL-C (mg/dL) | 111.6 ± 45.0 | 111.9 ± 44.4 | 111.4 ± 44.7 | 110.3 ± 46.5 | < 0.001 |
| GLU_SD (mg/dL) | 12.4 ± 9.8 | 22.7 ± 14.1 | 32.3 ± 18.7 | 47.1 ± 25.3 | < 0.001 |
| GLU_CV (%) | 8.1 ± 4.0 | 15.5 ± 5.0 | 22.9 ± 6.9 | 35.5 ± 11.9 | < 0.001 |
| GLU_VIM (%) | 4.1 ± 1.8 | 9.6 ± 1.3 | 14.6 ± 1.7 | 24.3 ± 5.7 | < 0.001 |
| GLU_ARV (mg/dL) | 15.2 ± 12.9 | 27.0 ± 18. 8 | 37.8 ± 25.2 | 52.4 ± 33.7 | < 0.001 |
| Current smoker (%) | 33185 (21.3) | 37301 (23.9) | 42135 (27) | 48736 (31.2) | < 0.001 |
| Heavy drinking (%) | 14997 (9.6) | 15563 (10.0) | 15755 (10.1) | 14992 (9.6) | < 0.001 |
| Regular exercise (%) | 40718 (26.0) | 38546 (24.7) | 36142 (23.2) | 32955 (21.1) | < 0.001 |
| Comorbidities | |||||
| Hypertension (%) | 89659 (57.5) | 87808 (56.3) | 84796 (54.3) | 78148 (50.1) | < 0.001 |
| Dyslipidemia (%) | 60077 (38.5) | 58915 (37.8) | 56500 (36.2) | 50461 (32.3) | < 0.001 |
| CKD (%) | 15740 (10.1) | 15962 (10.2) | 16598 (10.7) | 17320 (11.1) | < 0.001 |
| Heart disease (%) | 6265 (4.6) | 5786 (4.3) | 5189 (4.0) | 4564 (3.7) | < 0.001 |
| Any malignancy (%) | 4125 (2.6) | 3930 (2.5) | 3772 (2.4) | 3892 (2.5) | 0.001 |
| Income (lower 20%, %) | 34507 (22.1) | 35412 (22.7) | 37482 (24.0) | 39595 (25.4) | < 0.001 |
| Antidiabetic medication | |||||
| Metformin | 84747 (54.3) | 77947 (50.0) | 72119 (46.2) | 62185 (39.9) | < 0.001 |
| Sulfonylurea | 81607 (52.3) | 77767 (49.8) | 74710 (47.9) | 66155 (42.4) | < 0.001 |
| Meglitinide | 4246 (2.7) | 4050 (2.6) | 3850 (2.5) | 3785 (2.4) | < 0.001 |
| Thiazolidinedione | 14054 (9.0) | 13354 (8.6) | 12355 (7.9) | 10933 (7.0) | <0.001 |
| DPP-4 inhibitor | 13802 (8.8) | 12730 (8.2) | 11766 (7.5) | 9535 (6.1) | < 0.001 |
| a-Glucosidase inhibitor | 21803 (14.0) | 20564 (13.2) | 20055 (12.9) | 18458 (11.8) | < 0.001 |
| Insulin | 10064 (6.5) | 9936 (6.4) | 10655 (6.8) | 13320 (8.5) | < 0.001 |
| Number of oral anti-diabetic medications | < 0.001 | ||||
| 0 | 42298 (27.1) | 51240 (32.8) | 58967 (37.8) | 72375 (46.4) | |
| 1 | 38976 (25.0) | 33797 (21.7) | 29285 (18.8) | 23217 (14.9) | |
| 2 | 48766 (31.3) | 45997 (29.5) | 43216 (27.7) | 38441 (24.6) | |
| ≥ 3 | 26021 (16.7) | 25024 (16.0) | 24592 (15.8) | 22025 (14.1) | |
| Duration of diabetes ≥5 years (%) | 62489 (40.0) | 55604 (35.6) | 50193 (32.2) | 43641 (28.0) | < 0.001 |
| Type 1 diabetes (%) | 3104 (2.0) | 3214 (2.1) | 3745 (2.4) | 5548 (3.6) | < 0.001 |
| Number of exams | < 0.001 | ||||
| 3 | 126421 (81.0) | 113451 (72.7) | 108293 (69.4) | 104468 (66.9) | |
| 4 | 14692 (9.4) | 19227 (12.3) | 21858 (14.0) | 24324 (15.6) | |
| 5 | 14948 (9.6) | 23380 (15.0) | 25909 (16.6) | 27266 (17.5) | |
| Time interval between adjacent exams (years) | 1.8 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | <0.001 |
Q1:0–7.4; Q2:7.4–11.9; Q3:11.9–17.8; Q4:17.8–87.7. Data are presented as mean ± standard deviation, geometric mean (95% confidence interval), or number (%). One-way analysis of variance and Chi squared tests were used to compare the characteristics of the study subjects at baseline. Post-hoc multiple comparison analysis was performed with Bonferroni correction, and triglyceride levels were log-transformed for analysis. ARV, average real variability; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; CV, coefficient of variation; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; SD, standard deviation; VIM, variability independent of mean; WC, waist circumference
During the median (interquartile range) follow-up period of 8.0 (7.3–8.4) years, 25,038 cases of stroke, 15,832 cases of MI, and 44,716 cases of death were identified. As shown in Table 2, the HRs for stroke, MI, and all-cause mortality serially increased as the FG VIM quartile increased. Subjects in FG VIM quartile 4 had a 20% higher risk for stroke and MI, and a 32% higher risk for all-cause mortality, versus those in quartile 1, even after adjustment for several risk factors and mean FG. We also tested the following GV calculation methods: SD, CV, and ARV. A similar relationship was obtained in FG SD, CV, and ARV quartiles instead of VIM (Additional file 1: Table S1).
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of stroke, myocardial infarction, and all-cause mortality by quartile of fasting glucose variability
| Events (n) | Follow-up duration (person-years) | Incidence rate (per 1000 person-years) | Age- and sex- Adjusted HR (95% CI) | Multivariate-adjusted HR (95% CI) | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| Stroke | ||||||
| VIM Q1 (n = 156061) | 6350 | 1185704.0 | 5.36 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| VIM Q2 (n = 156058) | 6162 | 1191326.6 | 5.17 | 1.03 (0.99–1.07) | 1.03 (0.99–1.06) | 1.06 (1.02–1.09) |
| VIM Q3 (n = 156060) | 6214 | 1193013.0 | 5.21 | 1.08 (1.04–1.12) | 1.05 (1.02–1.09) | 1.10 (1.06–1.14) |
| VIM Q4 (n = 156058) | 6312 | 1191670.8 | 5.30 | 1.17 (1.13–1.21) | 1.10 (1.07–1.14) | 1.20 (1.16–1.24) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||
| Myocardial infarction | ||||||
| VIM Q1 (n = 156061) | 3859 | 1194006.5 | 3.23 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| VIM Q2 (n = 156058) | 3899 | 1199121.6 | 3.25 | 1.06 (1.01–1.11) | 1.05 (1.00–1.10) | 1.07 (1.02–1.12) |
| VIM Q3 (n = 156060) | 3978 | 1200620.1 | 3.31 | 1.11 (1.07–1.16) | 1.09 (1.04–1.13) | 1.12 (1.07–1.17) |
| VIM Q4 (n = 156058) | 4096 | 1199009.4 | 3.42 | 1.21 (1.16–1.27) | 1.14 (1.09–1.20) | 1.20 (1.15–1.25) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||
| All-cause mortality | ||||||
| VIM Q1 (n = 156061) | 10506 | 1205853.5 | 8.71 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| VIM Q2 (n = 156058) | 10608 | 1211059.8 | 8.76 | 1.09 (1.06–1.119) | 1.08 (1.05–1.11) | 1.10 (1.07–1.13) |
| VIM Q3 (n = 156060) | 11181 | 1212864.1 | 9.22 | 1.19 (1.16–1.22) | 1.15 (1.12–1.18) | 1.17 (1.14–1.20) |
| VIM Q4 (n = 156058) | 12421 | 1211473.9 | 10.25 | 1.40 (1.36–1.44) | 1.29 (1.26–1.33) | 1.32 (1.29–1.36) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||
Q1:0–7.4; Q2:7.4–11.9; Q3:11.9–17.8; Q4:17.8–87.7. Model 1 is adjusted for age, sex, body mass index, alcohol drinking, smoking, regular exercise, presence of hypertension, dyslipidemia, chronic kidney disease, and lower 20% income. Model 2 is the same as model 1, plus further adjustment for duration of diabetes over 5 years, the number of classes of oral anti-diabetic medication taken in the 12 months prior to baseline, presence of prescription history of insulin, and mean of fasting glucose. VIM, variability independent of mean
When we divided individuals into FG VIM deciles, the risks of stroke, MI, and all-cause death showed a positive dose–response association with the FG VIM decile (Fig. 3 and Additional file 1: Table S2).
Fig. 3.
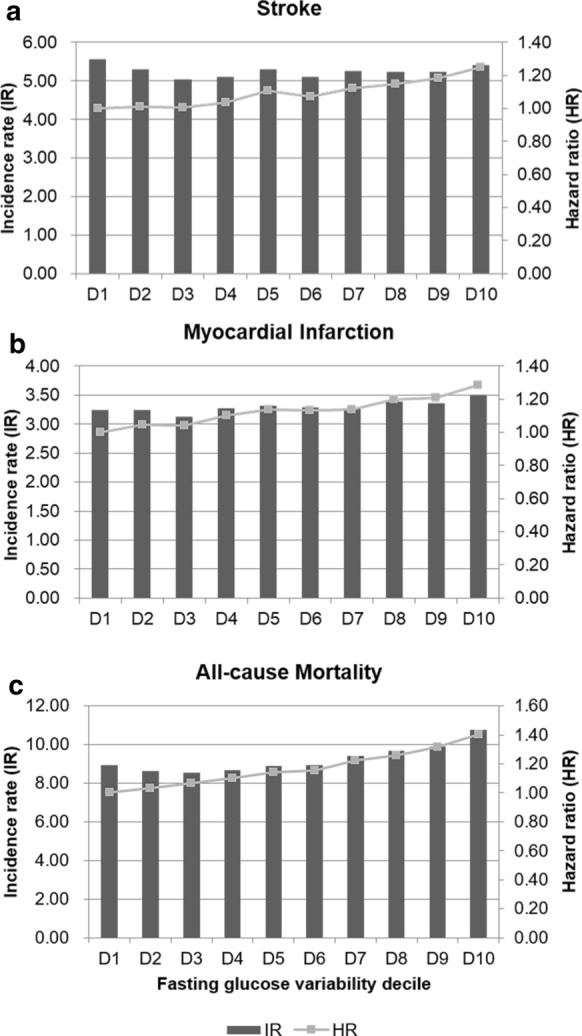
Hazard ratios (HRs) and incidence rates of (a) stroke, (b) myocardial infarction, and (c) all-cause mortality by deciles of fasting glucose variability, assessed by variability independent of mean
In the subgroup analyses, FG VIM quartile 4 showed a consistently increased risk for all-cause mortality (Tables 3 and 4) except in young individuals aged 20-39. A similar finding was observed for stroke and MI, with exceptions in younger individuals and those with malignancy, shorter diabetes duration, baseline FG ≥ 126 mg/dL, T1DM, and treatment with thiazolidinedione and insulin.
Table 3.
Subgroup analysis according to clinically relevant factors in quartile 4 versus fasting glucose variability of quartiles 1–3
| Stroke | Myocardial infarction | All-cause mortality | ||||
|---|---|---|---|---|---|---|
| IR per 1000 | HR (95% CI) | IR per 1000 | HR (95% CI) | IR per 1000 | HR (95% CI) | |
| Age (years) | ||||||
| 20–39 (n = 51687) | 0.56 | 0.78 (0.59–1.03) | 0.93 | 0.89 (0.72–1.09) | 1.14 | 1.01 (0.84–1.22) |
| 40–64 (n = 413136) | 3.43 | 1.04 (0.99–1.09) | 2.50 | 1.07 (1.01–1.12) | 4.82 | 1.17 (1.13–1.21) |
| ≥ 65 (n = 159414) | 12.08 | 1.13 (1.09–1.18) | 6.35 | 1.12 (1.07–1.18) | 24.15 | 1.18 (1.15–1.21) |
| P for interaction | 0.001 | 0.056 | 0.102 | |||
| Sex | ||||||
| Male (n = 411054) | 5.07 | 1.05 (1.01–1.09) | 3.29 | 1.06 (1.01–1.11) | 10.15 | 1.17 (1.14–1.20) |
| Female (n = 213183) | 5.62 | 1.13 (1.07–1.18) | 3.33 | 1.13 (1.07–1.21) | 7.49 | 1.19 (1.14–1.24) |
| P for interaction | 0.028 | 0.071 | 0.387 | |||
| BMI | ||||||
| < 25 kg/m2 (n = 331714) | 5.66 | 1.10 (1.06–1.14) | 3.44 | 1.08 (1.03–1.13) | 11.31 | 1.19 (1.16–1.22) |
| ≥ 25 kg/m2 (n = 292523) | 4.81 | 1.05(1.01–1.10) | 3.15 | 1.10 (1.04–1.16) | 6.91 | 1.14 (1.10–1.18) |
| P for interaction | 0.117 | 0.477 | 0.020 | |||
| Current smoking | ||||||
| No (n = 462880) | 5.33 | 1.13 (1.09–1.17) | 3.23 | 1.12 (1.07–1.17) | 9.11 | 1.19 (1.16–1.22) |
| Yes (n = 161357) | 5.06 | 0.94 (0.89–0.99) | 3.53 | 1.01 (0.95–1.08) | 9.59 | 1.15 (1.10–1.19) |
| P for interaction | <0.001 | 0.026 | 0.089 | |||
| Hypertension | ||||||
| No (n = 283826) | 3.35 | 1.02 (0.97–1.08) | 2.38 | 1.01 (0.95–1.08) | 6.50 | 1.17 (1.13–1.21) |
| Yes (n = 340411) | 6.89 | 1.11 (1.07–1.14) | 4.09 | 1.13 (1.09–1.19) | 11.55 | 1.19 (1.16–1.22) |
| P for interaction | 0.011 | 0.001 | 0.845 | |||
| Dyslipidemia | ||||||
| No (n = 398284) | 5.24 | 1.05 (1.01–1.09) | 3.09 | 1.09 (1.04–1.14) | 9.99 | 1.17 (1.15–1.20) |
| Yes (n = 225953) | 5.29 | 1.13 (1.07–1.18) | 3.68 | 1.10 (1.04–1.16) | 7.93 | 1.19 (1.15–1.24) |
| P for interaction | 0.007 | 0.397 | 0.476 | |||
| Malignancy | ||||||
| No (n = 608518) | 5.25 | 1.15 (1.11–1.18) | 3.39 | 1.13 (1.09–1.18) | 9.75 | 1.24 (1.21–1.27) |
| Yes (n = 15719) | 7.22 | 1.03 (0.88–1.22) | 4.68 | 1.11 (0.90–1.36) | 32.14 | 1.18 (1.09–1.28) |
| P for interaction | 0.213 | 0.682 | 0.702 | |||
| Income lower 20% | ||||||
| No (n = 477241) | 5.08 | 1.08 (1.05–1.12) | 3.22 | 1.10 (1.05–1.14) | 8.82 | 1.21 (1.18–1.24) |
| Yes (n = 146996) | 5.83 | 1.07 (1.01–1.13) | 3.57 | 1.07 (1.00–1.15) | 10.57 | 1.12 (1.08–1.17) |
| P for interaction | 0.759 | 0.618 | 0.001 | |||
Adjusted for age, sex, body mass index, alcohol drinking, smoking, regular exercise, presence of hypertension, dyslipidemia, chronic kidney disease, and lower 20% income, duration of diabetes over 5 years, the number of classes of oral anti-diabetic medication taken in the 12 months prior to baseline, presence of prescription history of insulin, and mean of fasting glucose
Table 4.
Subgroup analysis according to the characteristics of diabetes in quartile 4 versus fasting glucose variability of quartiles 1–3
| Stroke | Myocardial infarction | All-cause mortality | ||||
|---|---|---|---|---|---|---|
| IR per 1000 | HR (95% CI) | IR per 1000 | HR (95% CI) | IR per 1000 | HR (95% CI) | |
| Duration of diabetesa | ||||||
| < 5 years (n = 412310) | 4.02 | 1.01 (0.97–1.06) | 2.63 | 1.03 (0.98–1.08) | 7.41 | 1.16 (1.12–1.19) |
| ≥ 5 years (n = 211927) | 7.74 | 1.17 (1.12–1.22) | 4.66 | 1.17 (1.11–1.24) | 12.87 | 1.22 (1.18–1.26) |
| P for interaction | < 0.001 | 0.001 | 0.011 | |||
| Baseline fasting glucosea | ||||||
| < 126 mg/dL (n = 188779) | 6.30 | 1.21 (1.15–1.27) | 3.94 | 1.16 (1.09–1.23) | 11.64 | 1.17 (1.13–1.21) |
| ≥ 126 mg/dL (n = 435458) | 4.81 | 0.95 (0.92–0.99) | 3.03 | 1.01 (0.96–1.06) | 8.20 | 1.11 (1.08–1.14) |
| P for interaction | < 0.001 | < 0.001 | < 0.001 | |||
| Subtype of diabetesa | ||||||
| T2DM (n = 608626) | 5.02 | 1.14 (1.11–1.17) | 3.26 | 1.14 (1.10–1.19) | 9.70 | 1.22(1.19–1.25) |
| T1DM (n = 15611) | 13.49 | 1.11 (0.99–1.24) | 8.01 | 0.96 (0.84–1.11) | 26.23 | 1.25(1.15–1.35) |
| P for interaction | 0.267 | 0.065 | 0.186 | |||
| Metforminb | ||||||
| No (n = 327239) | 4.05 | 1.17 (1.12–1.22) | 2.48 | 1.18 (1.12–1.25) | 8.59 | 1.30 (1.26–1.34) |
| Yes (n = 296998) | 7.22 | 1.18 (1.13–1.22) | 4.72 | 1.16 (1.11–1.22) | 12.80 | 1.25 (1.22–1.29) |
| P for interaction | 0.622 | 0.976 | 0.150 | |||
| Sulfonylureab | ||||||
| No (n = 323998) | 3.56 | 1.19 (1.13–1.24) | 2.48 | 1.18 (1.12–1.25) | 7.42 | 1.30 (1.26–1.34) |
| Yes (n = 300239) | 7.72 | 1.17 (1.13–1.21) | 4.72 | 1.16 (1.11–1.22) | 14.16 | 1.26 (1.23–1.30) |
| P for interaction | 0.858 | 0.976 | 0.380 | |||
| Meglitinideb | ||||||
| No (n = 608306) | 5.19 | 1.17 (1.13–1.20) | 3.34 | 1.16 (1.12–1.20) | 10.02 | 1.27 (1.25–1.30) |
| Yes (n = 15931) | 10.02 | 1.28 (1.11–1.48) | 6.51 | 1.37(1.15–1.64) | 20.14 | 1.37 (1.24–1.52) |
| P for interaction | 0.139 | 0.056 | 0.080 | |||
| Thiazolidinedioneb | ||||||
| No (n = 573541) | 5.21 | 1.17 (1.14–1.21) | 3.37 | 1.17 (1.13–1.22) | 10.17 | 1.28 (1.25–1.31) |
| Yes (n = 50696) | 6.44 | 1.17 (1.06–1.29) | 3.97 | 1.13 (0.99–1.28) | 11.37 | 1.25 (1.16–1.34) |
| P for interaction | 0.969 | 0.561 | 0.526 | |||
| DPP-4 inhibitorb | ||||||
| No (n = 576404) | 5.25 | 1.17 (1.13–1.20) | 3.37 | 1.17 (1.13–1.21) | 10.23 | 1.27 (1.25–1.30) |
| Yes (n = 47833) | 6.06 | 1.21 (1.09–1.35) | 4.06 | 1.18 (1.03–1.35) | 10.63 | 1.35 (1.24–1.46) |
| P for interaction | 0.287 | 0.835 | 0.079 | |||
| a-Glucosidase inhibitorb | ||||||
| No (n = 543357) | 4.70 | 1.15 (1.12–1.19) | 3.09 | 1.15 (1.10–1.20) | 9.29 | 1.28 (1.25–1.31) |
| Yes (n = 80880) | 9.92 | 1.23 (1.16–1.32) | 5.94 | 1.24 (1.14–1.34) | 17.62 | 1.25 (1.19–1.31) |
| P for interaction | 0.053 | 0.157 | 0.519 | |||
| Insulinb | ||||||
| No (n = 580262) | 4.75 | 1.15 (1.11–1.18) | 3.05 | 1.14 (1.10–1.19) | 9.02 | 1.24 (1.22–1.27) |
| Yes (n = 43975) | 11.68 | 1.11 (1.03–1.19) | 7.68 | 1.08 (0.99–1.18) | 24.31 | 1.19 (1.13–1.25) |
| P for interaction | 0.874 | 0.568 | 0.492 | |||
aAdjusted for age, sex, body mass index, alcohol drinking, smoking, regular exercise, presence of hypertension, dyslipidemia, chronic kidney disease, and lower 20% income, duration of diabetes over 5 years, the number of classes of oral anti-diabetic medication taken in the 12 months prior to baseline, presence of prescription history of insulin, and mean of fasting glucose
bAdjusted for age, sex, body mass index, alcohol drinking, smoking, regular exercise, presence of hypertension, dyslipidemia, chronic kidney disease, and lower 20% income, duration of diabetes over 5 years, and mean of fasting glucose
On the other hand, the relationship between FG status and the risk of clinical outcomes showed a U-shaped association (Fig. 4 and Additional file 1: Table S3). Compared to individuals whose FG levels were in the range 100-119 mg/dL, those with FG < 100 mg/dL or ≥ 160 mg/dL had significantly higher HRs. This relationship was consistent after dividing the subjects by prescription of anti-diabetic medications (Additional file 1: Table S4).
Fig. 4.
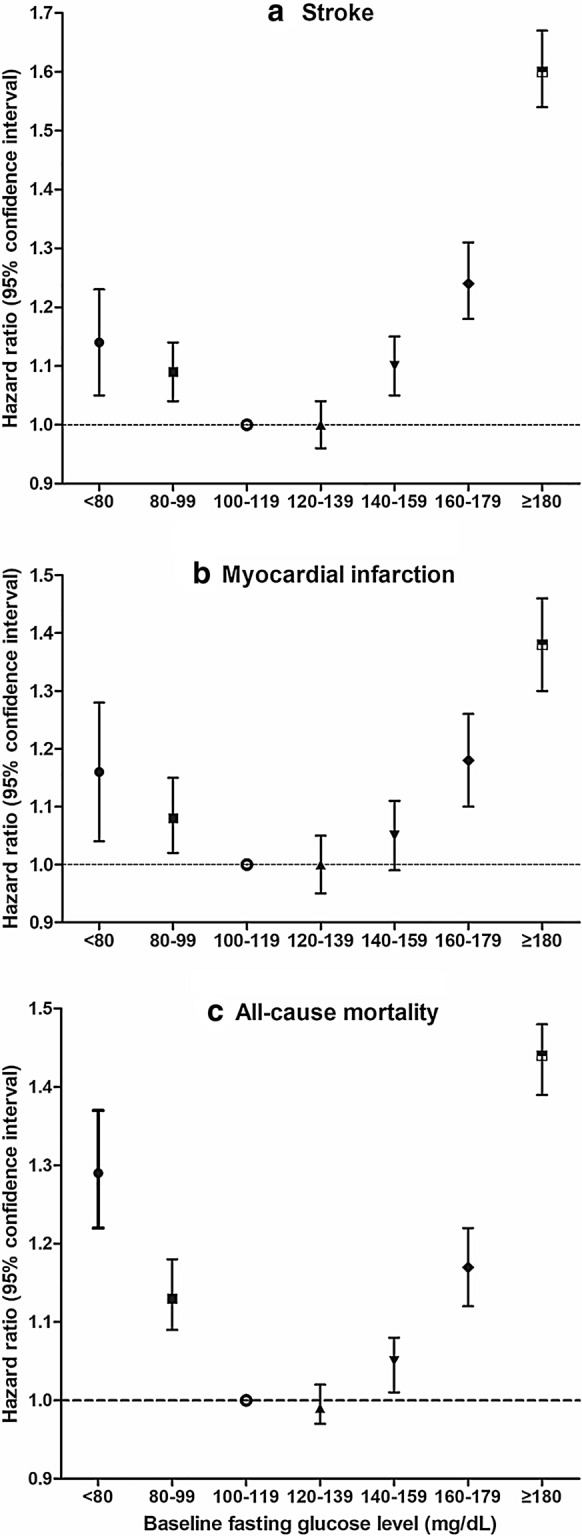
Hazard ratios (HRs) of (a) stroke, (b) myocardial infarction, and (c) all-cause mortality according to baseline fasting glucose level
Discussion
This nationwide population-based study in diabetes revealed that long-term GV for 5 years predicted future development of stroke, MI, and all-cause death, independent of anti-diabetic medication, metabolic risk factors, and mean fasting glucose. This association showed a linear fashion, compared to the U-shaped association between FG and outcomes. In the subgroup analysis, the impact of GV was higher in the elderly and those with a longer duration of diabetes and lower FG levels.
Long-term glucose variability and clinical outcomes
Several medium-sized cohort studies have reported the impact of long-term GV [6, 15, 17–21]. Variability in FG is an independent predictor of all-cause mortality, and the highest tertile group in 1400 type 2 diabetes patients included in the VERONA study had a 67% higher risk [17, 21]. In the sub-analysis of the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial, variability in FG was associated with an increased risk of death from cardiovascular causes, nonfatal MI, or nonfatal stroke [15]. Furthermore, GV might reflect better the impact of glycemic control on diabetic complications than HbA1c, or at least, GV can complement the power of HbA1c in risk prediction [7, 14, 15]. In the ADVANCE trial, HRs (95% CIs) estimated for each 10-percentile point increase in SD of FG and HbA1c were 1.12 (1.08–1.16) and 1.05 (1.01–1.09), respectively [15].
However, these studies have limitations such as short follow-up period [15] and a relatively small population size, and the impact of GV was only shown in some subgroups, but not the entire population [18]. In the present study, we computed long-term GV over 5 years and assessed risk for a median of 8 years of follow-up. In addition, this dataset includes almost all Korean diabetic patients. In this respect, the worst outcome in the highest GV quartile even after adjustment for mean FG provides a clear indication that GV can be considered as a new risk factor for stroke, MI, and all-cause mortality.
Interpretation of the impact of glucose variability
A few mechanisms might be involved in the relationship between FG variability and clinical outcomes. Transient high glucose spikes have been shown to impair endothelial function [28, 29], increase oxidative stress more than sustained chronic hyperglycemia [30, 31], and induce β-cell dysfunction [12]. Furthermore, higher GV was strongly associated with increased lipid level and decreased fibrous content with a larger plaque burden [32].
In subgroup analysis of this study, the impact of GV was greater in the elderly and those with a longer duration of diabetes and FG level < 126 mg/dL. These findings were partially in accordance with previous publications [18, 20]. In the Verona Diabetes Study, the positive association between GV and mortality risk was confined to patients older than 65 years [18]. The elderly are known to be more susceptible to oxidative stress than younger individuals because their defense mechanisms are less efficient [19]. The limited significance seen in current smokers could be explained by the higher proportion of young subjects in the current smoker group. In the same notion, patients with a longer diabetes duration and lower FG might reflect a population that is more vulnerable to GV. It is interesting that contrary to other studies [15, 19, 20], GV had a greater impact among lower FG patients than in higher FG patients, which may be due to an ethnic difference, but the reason is difficult to ascertain from this study. As we also showed that lower FG level was significantly associated with more CVD and death events, the higher GV in the lower FG group augmented the risk for future CVD and death, indicating the target population for reducing these outcomes.
In addition to this pathophysiologic interpretation, elevated GV might be an indicator of irregular compliance to therapy, comorbidity, poor health, or diabetic complications resulting in the increase of mortality [17]. However, contrary to other studies [14, 17, 20], our study population with higher GV had a shorter duration of diabetes, had fewer comorbidities, and about half of them were not treated with anti-diabetic medication. Although there was a greater proportion of insulin users, the contribution of insulin users to the outcome was small since less than 10% of the study population was treated with insulin. We speculate that, compared to other hospital-based studies, this study included more low-risk diabetic patients since diagnosis of diabetes was based on not only health insurance claims data, but also general health screening exams, which resulted in detection of undiagnosed mild diabetic cases. Therefore, we can evaluate the impact of GV itself, rather than combined poor health status and comorbidities as in other studies.
In subgroup analysis according to anti-diabetic medications, interaction analysis did not find any significance, contrary to previous studies which showed sulfonylurea increases glucose fluctuation and risk of hypoglycemia [33], and DPP-4 inhibitors and the novel insulin analogue degludec reduced GV [34–36]. Given that actual drug exposure could not be determined because of the retrospective study design, a future well-designed, randomized controlled trial is expected to resolve this concern.
Assessment of glycemic variability
There are no standard indices for quantification of long-term glycemic variability [37], so each value has distinct characteristics. The SD reflects dispersion of measurements around the mean and is sensitive to low sampling frequency, while CV is a standardized variation providing direct comparison among study groups. The ARV index, which averages the absolute differences between successive measurements, might be a reliable index for time series variability [38, 39]. However, SD, CV, and ASV are partially dependent on the mean and its changes over time, and this may not be resolved even if adjusted for mean value [40]. On the other hand, VIM is a measure of variability designed not to correlate with mean level [24, 41] but is sample-specific [42, 43]. Therefore, we used VIM as the main measurement of FG variability, and similar results to other estimates of glycemic variation support the robustness of our study (Additional file 1: Table S1).
Limitations of this study
This is the first large-scale epidemiologic study demonstrating the impact of long-term variability in FG on CVD outcomes separately and all-cause death with a long-term follow-up of 8.0 (7.3–8.4) years. However, we are aware of several limitations of this study. First, given that HbA1c or postprandial glucose level was not measured in this study, incident diabetes might be underestimated. To enhance the accuracy of diabetes diagnosis, we combined ICD-10 codes and patient prescription histories in addition to fasting glucose level. Second, possible selection bias due to extracting patients based on the number of health check-ups is one of the limitations. To overcome it, we conducted various subgroup analyses. Finally, classification of T1DM based on the presence of ICD-10 E10 with at least one prescription of insulin might not be accurate. Because ICD-10 code is based on claims data, but autoantibody, insulin, or c-peptide level was not measured, the prevalence of T1DM could be overestimated. However, we performed subgroup analysis after stratifying the subjects according to subtype of diabetes. There was a significance in all-cause mortality in T1DM. The absence of significance in stroke and MI was thought to be related to the small number of population.
Conclusion
This nationwide population-based study with long-term follow-up showed that GV had a dose–response relationship with the risk of stroke, MI, and all-cause mortality in diabetes, especially in the elderly and those with a longer duration of diabetes and lower FG levels. This important health issue will play a role in reducing future development of CVD and death attributed to the increasing population of diabetes.
Supplementary information
Additional file 1: Table S1. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of stroke, myocardial infarction, and all-cause mortality by quartiles of fasting glucose variability, assessed by standard deviation, coefficient of variation, and average real variability. Table S2. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of stroke, myocardial infarction, and all-cause mortality by deciles of fasting glucose variability. Table S3. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of stroke, myocardial infarction, and all-cause mortality according to baseline fasting glucose level. Table S4. Hazard ratios (HRs) and 95% confidence intervals (CIs) according to baseline fasting glucose level and antidiabetic medication (ADM).
Acknowledgements
Not applicable.
Abbreviations
- ARV
Average real variability
- BMI
Body mass index
- BP
Blood pressure
- CI
Confidence interval
- CKD
Chronic kidney disease
- CV
Coefficient of variation
- CVD
Cardiovascular disease
- DPP-4 inhibitors
Inhibitors of dipeptidyl peptidase 4
- eGFR
Estimated glomerular filtration rate
- FG
Fasting glucose
- GV
Glucose variability
- HR
Hazard ratio
- ICD-10
International Classification of Diseases, tenth revision
- LDL-C
low-density lipoprotein cholesterol
- MI
Myocardial infarction
- NHIC
National Health Insurance System
- SD
Standard deviation
- T1DM
Type 1 diabetes
- T2DM
Type 2 diabetes
- VIM
Variability independent of the mean
Authors’ contributions
Conception and design: DYL, KH and NHK (Nan Hee Kim); Analysis and interpretation of the data: DYL, SP, JAS, NHK (Nam Hoon Kim), HJY and NHK (Nan Hee Kim); Drafting of the article: DYL and JHY; Critical revision for important intellectual content: SGK, KMC and SHB; Final approval of the article: YGP and NHK (Nan Hee Kim); Statistical expertise, Collection and assembly of data: KH. SP and YGP; Obtaining of funding: DYL and NHK (Nan Hee Kim); Administrative, technical, or logistic support: YGP. All authors read and approved the final manuscript.
Funding
This study was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (NRF-2019M3E5D3073102 and NRF-2019R1H1A2039682) and supported by Korea University Ansan Hospital Grant (O1903661). However, funders did not participate in the study’s design or reporting.
Availability of data and materials
The data that support the findings of this study are available from the National Health Insurance Corporation but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the National Health Insurance Corporation.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Da Young Lee and Kyungdo Han contributed equally to this article
Contributor Information
Yong Gyu Park, Email: ygpark@catholic.ac.kr.
Nan Hee Kim, Email: nhkendo@gmail.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-020-01134-0.
References
- 1.6 Glycemic Targets: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S55-S64. [DOI] [PubMed]
- 2.Wan EYF, Yu EYT, Fung CSC, Chin WY, Fong DYT, Chan AKC, et al. Relation between HbA1c and incident cardiovascular disease over a period of 6 years in the Hong Kong population. Diabetes Metab. 2018;44:415–423. doi: 10.1016/j.diabet.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Arem H, Moore SC, Patel A, Hartge P, de Gonzalez BA, Visvanathan K, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175:959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Škrha J, Šoupal J, Škrha J, Jr, Prázný M. Glucose variability, HbA1c and microvascular complications. Rev Endocrine Metab Disorders. 2016;17:103–110. doi: 10.1007/s11154-016-9347-2. [DOI] [PubMed] [Google Scholar]
- 5.Siegelaar SE, Holleman F, Hoekstra JBL, DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31:171–182. doi: 10.1210/er.2009-0021. [DOI] [PubMed] [Google Scholar]
- 6.Sun B, He F, Gao Y, Zhou J, Sun L, Liu R, et al. Prognostic impact of visit-to-visit glycemic variability on the risks of major adverse cardiovascular outcomes and hypoglycemia in patients with different glycemic control and type 2 diabetes. Endocrine. 2019;64:536–543. doi: 10.1007/s12020-019-01893-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhou JJ, Schwenke DC, Bahn G, Reaven P. Glycemic variation and cardiovascular risk in the Veterans Affairs Diabetes trial. Diabetes Care. 2018;41:2187–2194. doi: 10.2337/dc18-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira L, Moniz AC, Carneiro AS, Miranda AS, Fangueiro C, Fernandes D, et al. The impact of glycemic variability on length of stay and mortality in diabetic patients admitted with community-acquired pneumonia or chronic obstructive pulmonary disease. Diabetes Metab Syndr. 2019;13:149–153. doi: 10.1016/j.dsx.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Yokota S, Tanaka H, Mochizuki Y, Soga F, Yamashita K, Tanaka Y, et al. Association of glycemic variability with left ventricular diastolic function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2019;18:166. doi: 10.1186/s12933-019-0971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Ma X, Zhang L, Mo Y, Lu W, Zhu W, et al. Glycemic variability modifies the relationship between time in range and hemoglobin A1c estimated from continuous glucose monitoring: a preliminary study. Diabetes Res Clin Pract. 2020;161:108032. doi: 10.1016/j.diabres.2020.108032. [DOI] [PubMed] [Google Scholar]
- 11.Besch G, Pili-Floury S, Morel C, Gilard M, Flicoteaux G, du Mont SL, et al. Impact of post-procedural glycemic variability on cardiovascular morbidity and mortality after transcatheter aortic valve implantation: a post hoc cohort analysis. Cardiovasc Diabetol. 2019;18:27. doi: 10.1186/s12933-019-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Dai J, Han X, Zhao Y, Zhang H, Liu X, et al. Glycemic variability indices determined by self-monitoring of blood glucose are associated with β-cell function in Chinese patients with type 2 diabetes. Diabetes Res Clin Pract. 2020;164:108152. doi: 10.1016/j.diabres.2020.108152. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi H, Iwahashi N, Kirigaya J, Kataoka S, Minamimoto Y, Gohbara M, et al. Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc Diabetol. 2018;17:116. doi: 10.1186/s12933-018-0761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol. 2018;17:33. doi: 10.1186/s12933-018-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37:2359–2365. doi: 10.2337/dc14-0199. [DOI] [PubMed] [Google Scholar]
- 16.Tang X, Li S, Wang Y, Wang M, Yin Q, Mu P, et al. Glycemic variability evaluated by continuous glucose monitoring system is associated with the 10-y cardiovascular risk of diabetic patients with well-controlled HbA1c. Clin Chim Acta. 2016;461:146–150. doi: 10.1016/j.cca.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Muggeo M, Zoppini G, Bonora E, Brun E, Bonadonna RC, Moghetti P, et al. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000;23:45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Zoppini G, Verlato G, Targher G, Bonora E, Trombetta M, Muggeo M. Variability of body weight, pulse pressure and glycaemia strongly predict total mortality in elderly type 2 diabetic patients. The Verona Diabetes Study. Diabetes Metab Res Rev. 2008;24:624–628. doi: 10.1002/dmrr.897. [DOI] [PubMed] [Google Scholar]
- 19.Rizvi SI, Maurya PK. Markers of oxidative stress in erythrocytes during aging in humans. Ann N Y Acad Sci. 2007;1100:373–382. doi: 10.1196/annals.1395.041. [DOI] [PubMed] [Google Scholar]
- 20.Xu D, Fang H, Xu W, Yan Y, Liu Y, Yao B. Fasting plasma glucose variability and all-cause mortality among type 2 diabetes patients: a dynamic cohort study in Shanghai, China. Sci Rep. 2016;6:39633. doi: 10.1038/srep39633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muggeo M, Verlato G, Bonora E, Zoppini G, Corbellini M, de Marco R. Long-term instability of fasting plasma glucose, a novel predictor of cardiovascular mortality in elderly patients with non-insulin-dependent diabetes mellitus: the Verona Diabetes Study. Circulation. 1997;96:1750–1754. doi: 10.1161/01.cir.96.6.1750. [DOI] [PubMed] [Google Scholar]
- 22.Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YH, Han K, Ko SH, Ko KS, Lee KU. Data analytic process of a nationwide population-based study using National Health Information Database established by National Health Insurance Service. Diabetes Metab J. 2016;40:79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 25.Oh JY, Yang YJ, Kim BS, Kang JH. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J Korean Acad Fam Med. 2007;28:532–541. [Google Scholar]
- 26.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 27.Group KDIGOCW KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease: Chapter 1: definition and classification of CKD. Kidney Int Suppl. 2013;2013(3):19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XG, Zhang YQ, Zhao DK, Wu JX, Zhao J, Jiao XM, et al. Relationship between blood glucose fluctuation and macrovascular endothelial dysfunction in type 2 diabetic patients with coronary heart disease. Eur Rev Med Pharm Sci. 2014;18:3593–3600. [PubMed] [Google Scholar]
- 29.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 30.Salisbury D, Bronas U. Reactive oxygen and nitrogen species: impact on endothelial dysfunction. Nurs Res. 2015;64:53–66. doi: 10.1097/NNR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 31.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada K, Hibi K, Gohbara M, Kataoka S, Takano K, Akiyama E, et al. Association between blood glucose variability and coronary plaque instability in patients with acute coronary syndromes. Cardiovasc Diabetol. 2015;14:111. doi: 10.1186/s12933-015-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamazaki M, Hasegawa G, Majima S, Mitsuhashi K, Fukuda T, Iwase H, et al. Effect of repaglinide versus glimepiride on daily blood glucose variability and changes in blood inflammatory and oxidative stress markers. Diabetol Metab Syndr. 2014;6:54. doi: 10.1186/1758-5996-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh S, Kim H, Dang-Vu TT, Joo E, Shin C. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep. 2016;39:161–171. doi: 10.5665/sleep.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs. Diabetes Care. 2015;38:716–722. doi: 10.2337/dc15-0168. [DOI] [PubMed] [Google Scholar]
- 36.Vora J, Cariou B, Evans M, Gross JL, Harris S, Landstedt-Hallin L, et al. Clinical use of insulin degludec. Diabetes Res Clin Pract. 2015;109:19–31. doi: 10.1016/j.diabres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Inzucchi SE, Umpierrez G, DiGenio A, Zhou R, Kovatchev B. How well do glucose variability measures predict patient glycaemic outcomes during treatment intensification in type 2 diabetes? Diabetes Res Clin Pract. 2015;108:179–186. doi: 10.1016/j.diabres.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, et al. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. doi: 10.1161/HYPERTENSIONAHA.109.140798. [DOI] [PubMed] [Google Scholar]
- 39.Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–511. doi: 10.1097/01.hjh.0000160205.81652.5a. [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Choi EK, Han KD, Lee E, Moon I, Lee SR, et al. Bodyweight fluctuation is associated with increased risk of incident atrial fibrillation. Heart Rhythm. 2020;17:365–371. doi: 10.1016/j.hrthm.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 41.Yano Y. Visit-to-visit blood pressure variability-what is the current challenge? Am J Hypertens. 2017;30:112–114. doi: 10.1093/ajh/hpw124. [DOI] [PubMed] [Google Scholar]
- 42.Bangalore S, Breazna A, DeMicco DA, Wun CC, Messerli FH. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol. 2015;65:1539–1548. doi: 10.1016/j.jacc.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Dolan E, O’Brien E. Blood pressure variability: clarity for clinical practice. Hypertension. 2010;56:179–181. doi: 10.1161/HYPERTENSIONAHA.110.154708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of stroke, myocardial infarction, and all-cause mortality by quartiles of fasting glucose variability, assessed by standard deviation, coefficient of variation, and average real variability. Table S2. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of stroke, myocardial infarction, and all-cause mortality by deciles of fasting glucose variability. Table S3. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of stroke, myocardial infarction, and all-cause mortality according to baseline fasting glucose level. Table S4. Hazard ratios (HRs) and 95% confidence intervals (CIs) according to baseline fasting glucose level and antidiabetic medication (ADM).
Data Availability Statement
The data that support the findings of this study are available from the National Health Insurance Corporation but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the National Health Insurance Corporation.