Abstract
Objectives
This scoping review sought to summarize available data on the prevalence, associated factors, etiology, comorbidities, treatment, cost and mortality of chronic kidney disease (CKD) in Cameroon.
Methods
We searched PubMed, Scopus and African Journals Online from database inception to 31 March, 2020 to identify all studies published on the prevalence, associated factors, etiology, comorbidities, treatment, cost and mortality of CKD in Cameroon.
Results
Thirty studies were included. The prevalence of CKD varied from 3 to 14.1 and 10.0%–14.2% in rural and urban areas, respectively. The prevalence of CKD in patients with hypertension, diabetes mellitus, and human immunodeficiency virus was 12.4–50.0, 18.5%, and 3.0–47.2%, respectively. Hypertension (22.3–59.1%), chronic glomerulonephritis (15.8–56.2%), and diabetes mellitus (15.8–56.2%) were the most common causes of CKD. The cause was unknown in 13.5–17.0% of the cases. Advanced age, hypertension, diabetes mellitus, and obesity were frequent associated factors. Hemodialysis was the main treatment modality in patients with End Stage Renal Disease (ESRD). The monthly cost of management of non-dialyzed CKD was 163 US dollars. The one-year mortality rate of ESRD was 26.8–38.6%.
Conclusion
Chronic kidney disease affects about one in 10 adults in the general population in Cameroon. Patients with hypertension, diabetes mellitus, and human immunodeficiency virus bear the greatest burden of CKD in Cameroon. Advanced age, hypertension, diabetes mellitus, and obesity are major factors associated with CKD. Chronic kidney disease in Cameroon is associated with high morbidity and mortality and huge economic cost on the patient.
Keywords: Chronic kidney disease, End stage renal disease, Cameroon
Background
Chronic Kidney Disease (CKD) is an abnormality in kidney structure or function assessed using a matrix of variables including glomerular filtration rate (GFR), thresholds of albuminuria and duration of injury [1]. The global prevalence of CKD in 2015 was estimated at 13.4% [2], with a prevalence as high as 36.1% amongst high-risk populations [3]. Chronic kidney disease poses a serious threat to global health due to its high morbidity and mortality rate [4]. According to the 2015 Global Burden of Disease Study, CKD was the 12th common cause of mortality, accounting for about 1.1 million deaths worldwide [5]. Mortality due to CKD increased by 31.7% over the past decade to represent one of the rapidly rising causes of death worldwide [5]. Chronic kidney disease is the 17th leading cause of global disability-adjusted life years (DALYs) lost to disease [5].
Chronic kidney disease disproportionately affects low-income and middle-income countries (LMICs) with a prevalence that is 15% higher than that in high-income countries [3]. In addition to poorly controlled diabetes mellitus and hypertension, infection, and herbal and environmental toxins play an essential role in the epidemiology of CKD in these settings [6]. Chronic kidney disease is both a cause and consequence of non-communicable diseases (NCDs) [7, 8]. The burden of CKD in LMICs is worsened by limited accessibility to and affordability of renal replacement therapy (RRT) [9]. The number of people requiring RRT worldwide is projected to increase from 3.3 million to 5.4 million people by 2030 with most of this increase in developing countries [10].
High-risk groups for CKD include persons living with hypertension, diabetes mellitus, overweight, obesity [11, 12] and human immune deficiency virus (HIV) [13] as well as the elderly. A meta-analysis conducted in 2018 estimated the pooled prevalence of CKD stages 1–5 and 3–5 in the general African population at 15.8 and 4.6%, respectively [13]. Among high-risk populations, the prevalence of CKD stage 1–5 and 3–5 were 32.3 and 13.3%, respectively [13]. Moreover, the prevalence of CKD was about four times higher in Sub-Sahara Africa compared to North Africa. A large-scale population-based study of about 8000 participants aged 40–60 years from six communities in sub-Saharan Africa revealed an age-standardized prevalence of CKD of 2.4% [14]. By 2030, it is estimated that over 70% of people with end-stage kidney disease will be living in developing countries like countries in sub-Saharan Africa [15] due to the rising prevalence of diabetes mellitus, hypertension, obesity, and HIV in these sub-Saharan countries [16].
The prevalence of CKD in adult Cameroonians varied between 11 and 14.2% [11, 17]. The prevalence of hypertension (31%) [18], diabetes mellitus (6%) [19], and obesity (15%) [20] are high with a prevalence of HIV of 4% [21]. Dialysis was introduced in Cameroon in the early 1980s, and included both peritoneal and hemodialysis [22]. However, hemodialysis has been the only available modality of RRT for over two decades now [22].
This review sought to assess the burden of CKD in Cameroon. Specifically, we summarized data on the prevalence, incidence, risk factors, treatment, cost of treatment, and outcome of patients with CKD in Cameroon. Furthermore, we aimed to describe the economic burden and comorbidities of patients with CKD, and to identify research gaps.
Methods
This scoping review was conducted according to the approach proposed by Arsksey and O’Malley [23].
Literature search
PubMed, Scopus and African Journals Online were searched without language restriction to retrieve all publications on the prevalence, the incidence, comorbidity, risk factors, treatment, economic burden and outcome (length of hospital stay and mortality rate) of CKD in Cameroon from database inception to March 31, 2020. Table 1 depicts the search strategy for PubMed which was adapted to suit other databases. The reference list of the selected articles was searched to identify articles which might have been missed during the search.
Table 1.
Search strategy
| Search | Search terms |
|---|---|
| #1 | “Kidney disease*” OR “kidney failure” OR “Renal disease” OR “Renal insufficiency” OR “Chronic kidney” OR “Chronic renal” OR “CKD” OR “CKF” OR “CRD” OR “end-stage renal” OR “end-stage kidney” OR “endstage renal” OR “endstage kidney” OR “uremia” OR “uraemia” OR “dialysis” OR “hemofiltration” OR “haemofiltration” OR “hemodiafiltration” OR “haemodiafiltration” OR “hemodialysis” OR “haemodialysis” OR “renal dialysis” |
| #2 | Cameroon |
| #3 | #1 AND #2 |
| #4 | Publication date limits: from database 1 January 1967 to 31 May 2019 |
Selection of studies for the review
Cross-sectional, cohort, case-control studies and systematic reviews that reported relevant data on CKD in Cameroon were considered for inclusion. For this review, CKD was defined as estimated glomerular filtration rate < 60 mL/min/1.73m2 using either the Modification of Diet in Renal Disease (MDRD) study equation, the Cockcroft-Gault (CG) formula, or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations or proteinuria ≥1+ (or albuminuria ≥300 mg/g) or patients with known CKD on RRT [17]. Letters, commentaries, case reports, and case series with less than 30 participants were excluded. For duplicate publications, we considered the most comprehensive or recent report with the largest sample size.
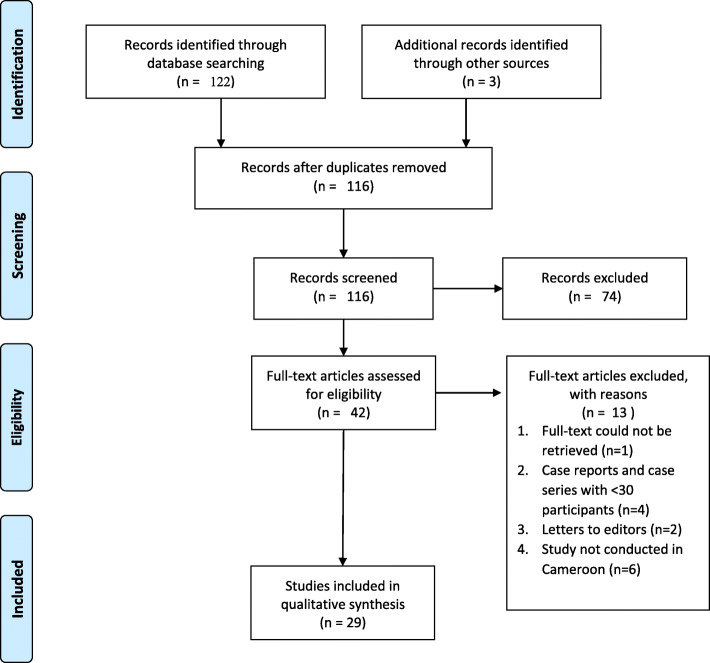
Two authors independently screened abstracts and citations retrieved from the online search and assessed the full texts of the relevant citations for inclusion in the review, Fig. 1. Disagreements during the study selection process were resolved through consensus, or arbitration by a third review author, in case a consensus could not be reached.
Fig. 1.
Flow diagram for study screening, selection and inclusion
Data charting
Relevant data were extracted with the aid of pre-structured abstraction sheets. We abstracted the following information from eligible articles: the surname of the first author of the article, publication year, study design and population studied, study setting (community-based or hospital-based), study area (rural or urban), percentage of males included in the study, mean or median age of the participants, sample size, measure used to assess kidney damage or function and if these measures were reassessed after 3 months of measurement, prevalence, comorbidity, treatment rate, median duration of hospital stay and mortality rate of CKD.
Results
In total, 122 records were retrieved from bibliographic searches. After screening titles and abstracts, 42 full-text papers were assessed for eligibility and 29 studies were retained for this review [11, 17, 24–49].
Prevalence of CKD in Cameroon
Table 2 summarizes the studies that reported on the prevalence of CKD in Cameroon. The prevalence of CKD was reported in 11 studies in Cameroon [11, 17, 24–32]. All studies were cross-sectional studies, 4 (36.4%) were community-based, and 2 (18.2%) were conducted in rural areas. The average age of the participants ranged from 35 to 61 years.
Table 2.
Prevalence of CKD in Cameroon
| First Author | Year of publication | Study Design | Study Setting | Study area | Disease specific population | Mean Age (in years) | Male (%) | Sample Size | Measure of Kidney damage or Function | Prevalence of CKD |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaze [24] | 2013 | Cross-sectional | Hospital-based | Urban | HAART-naïve PLWHA | 35.0 | 32.0% | 104 | eGFR < 60 based on MDRD and CG or at least 1+ proteinuria | 3% |
| Kaze [17] | 2015 | Cross-sectional | Community-based | Urban | General adult population | 36.5 | 48.7% | 119 | eGFR < 60 based on MDRD, CG and CKD-EPI or albuminuria ≥30 mg/g | 10.9% |
| Kaze [17] | 2015 | Cross-sectional | Community-based | Rural | General adult population | 51 | 39.7 | 320 | eGFR < 60 based on MDRD, CG and CKD-EPI or albuminuria ≥30 mg/g | 14.1% |
| Kaze [11] | 2015 | Cross-sectional | Community-based | Urban | General adult population | 45.3 | 53.4% | 500 | eGFR < 60 based on MDRD, CG and CKD-EPI or albuminuria ≥30 mg/g | 10.0, 11.0 and 14.2% using CKD-EPI, MDRD and CG, respectively. |
| Feteh [25] | 2016 | Cross-sectional | Hospital-based | Urban | Patients with type 2 diabetes mellitus | 56.5 | 53.1% | 636 | eGFR < 60 based on MDRD | 18.5% |
| Kaze [30] | 2016 | Cross-sectional | Hospital-based | Urban | Hypertensive adult | 60.9 | 36.6% | 336 | eGFR < 60 based on MDRD, CG and CKD-EPI or albuminuria ≥30 mg/g | 49.7, 50.0 and 52.1% according to MDRD, CKD-EPI and CG equations respectively. |
| Kamdem [28] | 2017 | Cross-sectional | Hospital-based | Urban | newly diagnosed and untreated hypertensive patients | 51.0 | 49.1% | 839 | eGFR < 60 based on MDRD | 12.4% |
| Hamadou [27] | 2017 | Cross-sectional | Hospital-based | Urban | Hypertensive patients | 54.2 | 33% | 400 | eGFR < 60 based on CKD-EPI or proteinuria | 32.3% |
| Ekiti [26] | 2018 | Cross-sectional | Community-based | Rural | Sugarcane plantation workers | 39.0 | 75% | 204 | eGFR < 60 based on CKD-EPI or at least 1+ proteinuria | 3.4% |
| Halle [32] | 2018 | Cross-sectional | Hospital-based | Urban | PLWHA attending HIV day clinic | 37.1 | 26.7% | 709 | eGFR < 60 based on MDRD and CKD-EPI or at least 1+ proteinuria | 44% based on CKD-EPI and 47.2% based on MDRD |
| Kaze [31] | 2019 | Cross-sectional | Community-based | Urban | General adult population | 45.0 | 48.7% | 433 | eGFR < 60 based on CKD-EPI or albuminuria > 30 mg/g | 11.7% |
| Temgoua [29] | 2019 | Cross-sectional | Hospital-based | Urban | First-degree family relatives of HDP | 38.3 | 28.0% | 82 | eGFR < 60 based on MDRD or at least 1+ proteinuria or diagnosis by a Nephrologist | 15.9% |
NR Not Reported, NA Not Available, HIV Human immunodeficiency virus, AIDS Acquired immune deficiency syndrome, HAART Highly active antiretroviral therapy, PLWHA Persons living with HIV/AIDS, OR odds ratio, CI confidence interval, GFR Glomerular Filtration Rate, HDP Hemodialysis patients, MDRD Modification of Diet in Renal Disease, CG Cockcroft-Gault, CKD-EPI Chronic Kidney Disease Epidemiology
Overall, the prevalence of CKD in the general population ranged from 10.0–14.2%, [11, 17, 31]. The prevalence of CKD ranged from 3.4–14.1% and 10.0–14.2% in the general population in rural [17, 26] and urban areas [17, 31], respectively.
The prevalence of CKD among patients with hypertension ranged from 12.4–52.1% [27, 28, 30], Table 2. Thirty percent of hypertensive patients on treatment in a community-based study were diagnosed with CKD [27], and 12.4% in treatment naïve patients [28]. One study reported a prevalence of CKD of 18.5% among patients with type 2 diabetes mellitus [25]. Two studies evaluated the prevalence of CKD among persons living with HIV/AIDS (PLWHA). The prevalence of CKD in PLWHA ranged from 3.0–47.2% [24, 32].
The prevalence of CKD among sugarcane plantation workers was 3.4% [26]. The prevalence of CKD among first-degree family relatives of persons living with CKD on hemodialysis was 15.9% [29].
Factors associated with CKD
Table 3 depicts the factors associated with CKD. Advanced age [11, 26–28, 30, 32], female sex [27, 29], obesity/adiposity [17, 27, 30], hyperuricemia/gout [27, 30, 31], longer duration of HIV [32], CD4 count less than 200 cells/mL [32], hyperkalemia [28], dyslipidemia [28, 30], hypertension, diabetes mellitus [11, 17, 30], smoking [17, 30], consumption of alcohol [17, 30] and herbal medication [17], self-medication [30] were associated with increased odds of CKD.
Table 3.
Factors associated factors of chronic kidney disease in Cameroon
| First Author | Year of publication | Study Design | Study Setting | Disease specific population | Mean Age (in years) | Sample Size | Associated Factors (adjusted Odds Ratio; 95% Confidence Interval) |
|---|---|---|---|---|---|---|---|
| Kaze [17] | 2015 | Cross-sectional | Community-based | General adult population | 47.0 | 439 | History of hypertension (aOR: 3.95; 95% CI, 2.09–7.46), |
| History of diabetes mellitus (aOR: 6.64; 95% CI: 2.63–16.75) | |||||||
| Elevated systolic blood pressure (aOR: 1.01; 95% CI, 1.00–1.02) | |||||||
| Kaze [11] | 2015 | Cross-sectional | Community-based | General adult population | 45.3 | 500 | Advanced age (aOR: 1.09; 95% CI, 1.07–1.12), |
| Known hypertension (aOR: 2.40; 95% CI, 1.19–4.82) | |||||||
| Existing diabetes mellitus (aOR: 3.36; 95% CI, 1.02–11.07), | |||||||
| Overweight/obesity (aOR: 0.30; 95% CI, 0.17–0.54) | |||||||
| Kaze [30] | 2016 | Cross-sectional | Hospital-based | Hypertensive adult | 60.9 | 336 | Advanced age [aOR: 1.05; 95% CI, 1.02–1.07) |
| Raised systolic blood pressure (aOR: 1.01; 95% CI, 1.00–1.02) | |||||||
| Hamadou [27] | 2017 | Cross-sectional | Hospital-based | Hypertensive patients | 54.2 | 400 | Age > 50 years (aOR: 1.75; 95% CI: 1.06–2.89), |
| Females (aOR: 2.21; 95% CI: 1.29–3.78), obesity (aOR: 1.58; 95% CI: 1.36–1.95), hyperuricemia (aOR: 3.67; 95% CI: 1.78–7.58) | |||||||
| Kamdem [28] | 2017 | Cross-sectional | Hospital-based | newly diagnosed and untreated hypertensive patients | 51.0 | 839 | Age > 55 years (aOR: 5.29; 95% CI, 3.33–8.42), obesity (aOR: 0.15; 95% CI, 0.10–0.26), hyperkalemia (aOR: 1.33; 95% CI, 1.03–1.72) |
| Ekiti [26] | 2018 | Cross-sectional | Community-based | Sugarcane plantation workers | 39.0 | 204 | Age ≥ 40 years (aOR: 18.7; 95% CI: 1.5–236.4) |
| Halle [32] | 2018 | Cross-sectional | Hospital-based | PLWHA attending HIV day clinic | 37.1 | 709 | age > 35 years (aOR: 1.04; 95% CI: 1.02 to 1.06), longer duration of HIV (aOR: 2.60; 95% CI: 1.53 to 3.95), history of Hepatitis B (aOR: 3.04; 95% CI, 1.08 to 8.54), |
| CD4 count less than 200 cells/mL (aOR: 3.64; 95% CI, 2.55 to 5.21) | |||||||
| Kaze [31] | 2019 | Cross-sectional | Community-based | General adult population | 45.0 | 433 | Increased systolic blood pressure (aOR: 1.02; 95% CI, 1.00–1.04) per mmHg higher SBP), hyperglycemia (aOR: 4.73; 95% CI, 1.24–18.08) and hyperuricemia (aOR: 3.12; 95% CI, 1.58–6.16) |
HIV Human immunodeficiency virus, AIDS Acquired immune deficiency syndrome, HAART Highly active antiretroviral therapy, PLWHA Persons living with HIV/AIDS, aOR adjusted odds ratio, CI confidence interval, GFR Glomerular Filtration Rate, CKD-EPI Chronic Kidney Disease Epidemiology, SBP systolic blood pressure
Etiologies of chronic kidney disease in Cameroon
Eight studies reported on the etiologies of CKD in Cameroon, Table 4. Overall, hypertension (22.3–59.1%), chronic glomerulonephritis (15.8–56.2%), diabetes mellitus (7.3–24.0%) and HIV (6.6–11.5%) were the main etiological factors of CKD. The etiology of CKD was unknown in 13.5–17.0% of cases [35–42]. Halle et al 2016 reported hypertension (30.9%), glomerulonephritis (15.8%), diabetes mellitus (15.9%) and HIV (6.6%) as the major etiologies of CKD in a chart review of 863 medical records [37]. In a prospective study of 661 patients, the major etiologies of CKD were hypertension (28.3%), chronic glomerulonephritis (17.5%), diabetes mellitus (13.9%) and HIV (6.7%) [39].
Table 4.
Etiology of CKD in Cameroon
| First author | Year of publication | Study area | Study Design | Study setting | Study population | Mean age (in years) | Male (%) | Sample size | Etiologies |
|---|---|---|---|---|---|---|---|---|---|
| Halle [35] | 2014 | Urban | Cross-sectional | Hospital-based | Patients on maintenance hemodialysis | 49.4 | 66.4 | 113 | Hypertension (25.6%), Chronic glomerulonephritis (20.6%), diabetes mellitus (17.4%) |
| Kaze [36] | 2014 | Urban | Cross-sectional | Hospital-based | Patients on maintenance hemodialysis | 52.7 | 64.0 | 45 | Hypertension (29%), chronic glomerulonephritis (24%), Diabetes mellitus (24%) |
| Halle [37] | 2015 | Urban | Retrospective cohort | Hospital-based | Patients with ESRD | 47.4 | 66.0 | 863 | Hypertension (30.9%), glomerulonephritis (15.8%), diabetes mellitus (15.9%), HIV (6.6%), unknown (14.7%) |
| Kaze [38] | 2015 | Urban | Retrospective cohort | Hospital-based | Patients admitted in the nephrology unit | 44.8 | 60.0 | 225 | Chronic glomerulonephritis (25.9%), hypertension (22.3%), diabetes mellitus (20.1%) |
| Halle [39] | 2016 | Urban | Prospective cohort | Hospital-based | Patients on maintenance hemodialysis | 46.3 | 66.0 | 661 | Hypertension (28.3%), chronic glomerulonephritis (17.5%), diabetes mellitus (13.9%), hypertension and diabetes (7.3%), HIV (6.7%), unknown (16.9%) |
| Halle [40] | 2016 | Urban | Cross-sectional | Hospital-based | Maintenance hemodialysis | 51 | 66.0 | 97 | Hypertension (25.8%) Chronic glomerulonephritis (20.6%) Diabetes mellitus (17,5%) |
| Luma [41] | 2017 | Semi-urban | Cross-sectional | Hospital-based | Hemodialysis patients | 48 | 65.4 | 104 | Hypertension (40.4%), chronic glomerulonephritis (19.2%), HIVAN (11.5%), Diabetes mellitus (7.7%), obstructive nephropathy (2.9%), unknown (13.5%) |
| Moor [42] | 2017 | Urban | Cross-sectional | Hospital-based | Patients on maintenance hemodialysis | 55 | 75.0 | 44 | Hypertension (59.1%), Diabetes mellitus (11.4%) |
NR Not Reported, ESRD End stage renal disease, HIVAN HIV associated nephropathy
Major comorbidities in CKD patients in Cameroon
Thirteen studies discussed the comorbidities of CKD in Cameroon, Table 5. Ten or more of these studies reported hypertension (25.8–95.6%) and diabetes mellitus (11.40–41.54%)as major comorbidities associated with CKD patients. Also, viral infections such as HIV (4.4–13.5%), Hepatitis B (6.2–12.6%) and Hepatitis C (19.2–26.8%) infections were also important comorbidities associated with CKD. Furthermore, hyperuricemia, obesity, previous cardiovascular events, malnutrition, anemia, smoking, and alcohol use were major comorbidities.
Table 5.
Major comorbidities in Chronic Kidney Disease patients in Cameroon
| First author | Year of publication | Study area | Study population | Mean age (in years) | Sample size | Comorbidities |
|---|---|---|---|---|---|---|
| Halle [43] | 2009 | Urban | Patients with CKD | 50.1 | 140 | Hypertension (62.1%); diabetes mellitus (25.0%); gout (7.1%); HIV (6.4%) |
| Halle [35] | 2014 | Urban | ESRD patients on dialysis | 49.4 | 113 | Mid-arm muscle circumference (23.9%); heart failure (22.1%); diabetes mellitus (20.3%); HIV (4.4%) |
| Kaze [36] | 2014 | Urban | Patients on maintenance hemodialysis | 52.7 | 45 | Hypertension (95.6%); anemia (42%); left ventricular hypertrophy (60%); valvular heart disease (51.1%); heart failure (33.3%); dyslipidemia (33.3%); diabetes mellitus (24%); tobacco use (22.2%); obesity (4%) |
| Kaze [38] | 2015 | Urban | Patients with CKD | 44.8 | 139 | Hypertension (81.3%); diabetes mellitus (32.2%); tobacco use (15.1%); HIV (10.1%) |
| Mbouemboue [44] | 2016 | Semi-urban | ESRD | 45.0 | 35 | Anemia (Females [100%]; Males [92%]) |
| Halle [40] | 2016 | Urban | Maintenance hemodialysis | 51.0 | 97 | Hypertension (25.8%); Diabetes mellitus (17.5%); HCV (20.6%); HIV (8.2%); HBV (6.2%) |
| Kouotou [45] | 2016 | Urban | Hemodialyzed patients | 48.6 | 112 | Hypertension (66.1%); Diabetes mellitus (25.9%); HCV (26.8%) |
| Hamadou [27] | 2017 | Urban | Patients diagnosed with CKD | 54.2 | 400 | Anemia (44.5%), Obesity (39.75%), Diabetes mellitus (32%); hyperuricemia (10.75%); tobacco use (0.8%) |
| Moor [42] | 2017 | Urban | Patients on maintenance hemodialysis | 55.0 | 44 | Hypertension (59.1%); Diabetes mellitus (11.4%); alcohol use (11.4%); tobacco use (4.5%) |
| Luma [41] | 2017 | Semi-urban | Patients on maintenance hemodialysis | 48.0 | 104 | Hypertension (84.6%); HCV (19.2%); HIV (13.5%); HBV (10.6%) |
| Lemogoum [46] | 2018 | Urban | Patients with CKD | 52.0 | 150 | Hypertension (87.3%); dyslipidemia (62.0%); overweight/obesity (53.3%); abdominal obesity (34.0%); Diabetes mellitus (32.7%); previous cardiovascular event (18.0%) |
| Doualla [47] | 2018 | Urban | Non-dialysed CKD patients | 55.8 | 103 | Hypertension (87.4%); Diabetes mellitus (34.0%); gout (21.4%); HIV (12.6%) |
| Halle [34] | 2019 | Urban | Patients with CKD | 53.1 | 130 | Hypertension (70.77%); diabetes mellitus (41.54%); HIV (8.5%); gout (6.9%) |
CKD Chronic kidney disease, ESRD End-stage renal disease, CRF Chronic renal failure, HIV Human immunodeficiency syndrome, HBV Hepatitis B, HCV Hepatitis C
Treatment of CKD in Cameroon
Most of the CKD patients required hospitalization and eventual dialysis. The hospitalization rate was 42.2% in patients who were referred late to the nephrologist, and 33.6% of these late referrals were proposed emergency dialysis [43]. Emergency unplanned dialysis on a temporary catheter was required in 88.3% of 863 adult patients with CKD [37].
Cost of CKD management in Cameroon
Data on the economic burden of CKD is scarce in Cameroon. In a one-month retrospective cost analysis of non-dialysis CKD stage 3–5 patients, the total cost for management of CKD was 163 USD, 86.4% of which was from direct medical cost [33]. Meanwhile, only 1.4% of the 69 participants, with a median monthly salary of 162 USD, had full health insurance coverage [33].
Mortality of CKD in Cameroon
The mortality rate of CKD in Cameroon ranged between 26.8 and 58.0% during a period of 1 to 10 years of follow up, Table 6 [39, 48, 49]. An audit of 661 medical records reported a 10-year mortality rate of 44.9% [39]. The highest mortality rate of 58.0% was reported in a 15 months’ prospective study in 197 ESRD patients. Furthermore, the one-year mortality rate of hemodialyzed patients in a retrospective study was 29.8% [49]
Table 6.
Mortality of CKD in Cameroon
| First author, publication year | Study area | Study Design | Study setting | Study population | Median age | Sample size | Mortality rate |
|---|---|---|---|---|---|---|---|
| Halle 2016 [41] | Urban | Retrospective cohort | Hospital-based | ESRD patients on hemodialysis | 46.3 | 661 | 12-month mortality = 26.8% 10-year mortality = 44.9% |
| Fouda 2017 [48] | Urban | Prospective cohort | Hospital-based | ESRD patients on dialysis | 48.0 | 197 | 15-month mortality = 58.0% |
| Halle 2018 [43] | Urban | Retrospective cohort | Hospital-based | PLHIV with ESRD on hemodialysis | 46.0 | 57 | 12-month mortality = 38.6% |
NR Not Reported, ESRD End-stage renal disease, PLHIV People living with Human Immunodeficiency Virus
Discusssion
This scoping review systematically summarizes data on the prevalence, associated factors, etiology, comorbidities, treatment and its cost, and mortality of CKD in Cameroon. The prevalence of CKD was high, ranging from about 1 in every 10 people in the general population to about 1 in every 2 persons in high-risk groups. Hypertension, diabetes mellitus and chronic glomerulonephritis were the most common causes of CKD, while the cause was unknown in a significant proportion of patients. Hypertension, diabetes mellitus, obesity, advanced age and female gender were some factors associated with developing CKD in Cameroon. The treatment of CKD involved the management of comorbidities, progression factors, and hemodialysis in those with ESRD. Despite these treatment measures, mortality from CKD remains high with a 1-year mortality rate of more than 25% among hemodialyzed patients. The cost of non-dialysis treatment was high relative to the monthly income of patients with CKD.
The prevalence of CKD was reported in both the general population and in high-risk populations (persons with hypertension, diabetes mellitus, obesity, and HIV) applying various estimators of GFR. The prevalence of CKD in the general population ranged from 10 to 14.2%, which is similar to the overall prevalence of 15.8% in the African adult population [13]. In rural areas, the prevalence was higher compared to urban areas which was similar to the findings of Stanifer and colleagues [15]. This could be due to the low awareness of CKD risk factors such as consumption of nephrotoxic herbal concoctions and alcohol in rural settings. Compared to the general population, the prevalence of CKD was higher in high-risk populations, which was similar to the findings of Kaze and colleagues [13].
About a third to half of patients with hypertension in Cameroon had CKD [27], this was higher than the prevalence reported by Bahrey and colleagues (about 1 in 5 hypertensives had CKD) in Ethiopia [50]. This discrepancy might be due to differences in the characteristics of the study population. The prevalence of CKD in newly diagnosed patients with hypertension in Cameroon was 12.4% [28], which was much lower than the prevalence among known hypertensives on treatment (32.3%) [27] and those not on treatment (52.1%) [30]. Compared to treated hypertensive patients, those newly diagnosed with hypertension are more likely to have lived with the disease for a shorter duration; and as a result, are less likely to develop CKD. The prevalence of CKD in PLWHA was 3%, which was comparable to the findings of Kabore and colleagues [51]. We observed a prevalence of CKD among persons living with diabetes mellitus of 18.7%. In a systematic review in Africa, the prevalence of CKD among patients with diabetes was found to vary widely between 11 and 83.7% [52]. The duration of diagnosis and comorbidities played a significant role on the prevalence of CKD among patients with diabetes mellitus [52].
Advanced age and hypertension were common factors associated with CKD in Cameroon and this was similar to other African settings [14, 53]. Overweight and diabetes were independently associated with CKD, which is in line with the findings of Bahrey and colleagues [50]. Females were more likely to develop CKD, and this was coherent with the findings in a study in Uganda [54]. Hypertension, diabetes mellitus, and chronic glomerulonephritis were identified as the most common causes of CKD in our study. No cause for CKD was identified in about 15% of cases.
The most common comorbidities among patients with CKD in our review were hypertension, diabetes mellitus, anemia, obesity, and cardiac diseases. This was similar to the findings of Fraser and colleagues who highlighted hypertension, diabetes mellitus, anemia and ischemic heart disease as common comorbidities associated with CKD [55]. Whether hypertension is a consequence or an etiology of CKD, depends on which was diagnosed first as hypertension is an established cause and consequence of CKD. Anemia is almost always associated in the course of kidney disease because of the kidney’s role in erythropoiesis. Cardiovascular diseases are established comorbidities of CKD likely due to other cardiovascular risk factors such as hypertension and diabetes mellitus in these patients. Cardiovascular diseases were twice more common among CKD patients compared to the general population and advances at twice the rate [56]. Additionally, hyperuricemia was identified in non-dialyzed CKD patients followed in referral centers and as a factor of progression of CKD [47].
Hepatitis B virus, hepatitis C virus, and HIV were found to be common comorbidities in hemodialysed CKD patients [40, 41]. The hemodialysis procedure raises an issue of safety coupled with disturbances in both innate and adaptive immunity in those on maintenance hemodialysis. Hence, rendering these patients more susceptible to these blood-borne viral infections.
The growing burden of CKD is paralleled by the need to curtail those who end up in ESRD requiring renal replacement therapy (RRT). Effective and practical therapies for CKD remain a challenge even in developed countries [57]. Little is known about the cost of management of CKD in Cameroon. Nevertheless, it is estimated that these patients have to pay about 12 US Dollars per dialysis session [22]. The management of CKD was costly, especially in a population with low health insurance coverage as discussed in another study in non-dialysed stage 3–5 CKD patients [33]. In the USA, the cost of medical care of CKD patients even doubled when there were comorbid conditions [58]. Chronic kidney disease is associated with a huge economic burden in low-income settings, and limited access to treatment centers, essentially hemodialysis centers that are located sparingly in urban areas.
Chronic kidney disease has a high mortality rate among patients with ESRD. Over a quarter of patients starting hemodialysis die within the first year, with about half within the first six months. Chronic kidney disease patients with co-existing hypertension and diabetes mellitus conveyed the poorest prognosis. Late presentation of CKD and affordability were cited as major drivers of high early mortality [39]. Slowing the progression of CKD to ESRD is significantly hampered in our setting by the late presentation of these patients at nephrology centres. In developed settings, there are more effective referral strategies to nephrologists for a prompt management of CKD, and CKD-related complications or comorbidities [59]. Instituting a screening program and national CKD registry, and improving the availability, accessibility, and affordability of dialysis care in Cameroon are crucial to reducing the burden of CKD in Cameroon.
Our review had some key limitations. The prevalence of CKD reported by studies using a single time-point assessment of kidney function or damage are likely to be biased estimates of the true prevalence. Since serum creatinine has a high intraperson variability, a single time point measurement would lead to random misclassification of participants as cases or non-cases of CKD. This error is worse in small studies. Having a large enough sample size with control measurement of serum creatinine levels after three months is important to account for this random error by regression to the mean. The fact that the formulae used to estimate glomerular filtration rate have not been validated in the African population further complicates efforts to estimate the incidence and prevalence of CKD in this population. In addition, limited financial and human resources are major barriers to ascertain the diagnosis of CKD in epidemiological studies, especially in Cameroon. There was a substantial degree of heterogeneity across study participants in the studies included in this review. Studies reporting on the causes of CKD were cross-sectional, which do not provide evidence of temporality. Therefore, it is impossible to know if, for example, hypertension in a given patient is a cause or consequence of CKD. These limitations highlight the need for collaborative efforts to better understand the epidemiological profile of CKD in Cameroon.
Conclusion
Chronic kidney disease represents a significant cause of morbidity and mortality in Cameroon. The prevalence of CKD was highest among patients with hypertension, diabetes mellitus, and HIV. The main causes include hypertension, diabetes mellitus, chronic glomerulonephritis, HIV and unknown in some cases. Potential actions to curb the burden of CKD in Cameroon include raising public awareness about the disease, encouraging timely referral from general practitioners to nephrologists, increasing the availability of treatment centers, and encouraging health insurance to cover some of the cost of care.
Research perspective
There is limited data on the incidence and prevalence of CKD in the general population. Factors associated with CKD has been generated mostly from cross-sectional studies with possibility of reverse causation. There is a need for population-based cohort studies to assess the incidence and risk factors of CKD in Cameroon. A less costly approach to assess the risk factors of CKD would be to conduct a case-control study using population-based controls. In addition, more research is needed to assess the mortality rate of CKD and its predictors in patients with ESRD. Studies evaluating the economic burden of CKD in Cameroon are warranted. Creation of a national registry for CKD patients may help foster research in CKD in Cameroon and improve on its management and survival rate.
Abbreviations
- CKD
Chronic Kidney Disease
- DALYs
Disability-adjusted life years
- ESRD
End Stage Renal Disease
- GFR
Glomerular Filtration Rate
- HDP
Hemodialysis patient
- HAART
Highly active anti-retroviral therapy
- NCD
Non-communicable disease
- PLWHA
People living with HIV/AIDS
- RRT
Renal Replacement Therapy
Authors’ contributions
VNA conceived the study. VNA did the literature search. JBA, SM and VNA selected studies. JBA and SM collected data. JBA and VNA summarized and interpreted the data. JBA and VNA drafted the manuscript. JBA, SM, NME, DSME, BAK and VNA revised the manuscript. All authors read and approved the final manuscript. VNA is the guarantor of this manuscript.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Availability of data and materials
Available data can be obtained by contacting the corresponding author.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jerry Brown Aseneh, Email: jbaseneh@gmail.com.
Ben-Lawrence A. Kemah, Email: kbenlawrence@gmail.com
Stephane Mabouna, Email: stephanemabouna@gmail.com.
Mbeng Emmanuel Njang, Email: njangjr@gmail.com.
Domin Sone Majunda Ekane, Email: dominekane@gmail.com.
Valirie Ndip Agbor, Email: nvagbor@gmail.com.
References
- 1.Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2):104–114. doi: 10.1038/nrneph.2016.163. [DOI] [PubMed] [Google Scholar]
- 2.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FDR. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4(5):e307–19. 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed]
- 4.Coresh J. Update on the burden of CKD. J Am Soc Nephrol. 2017;28(4):1020–1022. doi: 10.1681/ASN.2016121374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Lond Engl. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY-M, Yang C-W. Chronic kidney disease: global dimension and perspectives. Lancet Lond Engl. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 7.Neuen BL, Chadban SJ, Demaio AR, Johnson DW, Perkovic V. Chronic kidney disease and the global NCDs agenda. BMJ Glob Health. 2017;2(2):e000380. doi: 10.1136/bmjgh-2017-000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonelli M, Agarwal S, Cass A, Garcia GG, Jha V, Naicker S, Wang H, Yang C-W, O’Donoghue D. How to advocate for the inclusion of chronic kidney disease in a national noncommunicable chronic disease program. Kidney Int. 2014;85(6):1269–1274. doi: 10.1038/ki.2012.488. [DOI] [PubMed] [Google Scholar]
- 9.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao M, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet Lond Engl. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 10.Bamgboye EL. The challenges of ESRD care in developing economies: sub-Saharan African opportunities for significant improvement. Clin Nephrol. 2016 Supplement;86 (2016)(13):18–22. [DOI] [PubMed]
- 11.Kaze FF, Halle M-P, Mopa HT, Ashuntantang G, Fouda H, Ngogang J, Kengne A-P. Prevalence and risk factors of chronic kidney disease in urban adult Cameroonians according to three common estimators of the glomerular filtration rate: a cross-sectional study. BMC Nephrol. 2015;16:96. doi: 10.1186/s12882-015-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrington WG, Smith M, Bankhead C, Matsushita K, Stevens S, Holt T, Hobbs FDR, Coresh J, Woodward M. Body-mass index and risk of advanced chronic kidney disease: Prospective analyses from a primary care cohort of 1.4 million adults in England. PLoS One. 2017;12(3):e0173515. doi: 10.1371/journal.pone.0173515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaze AD, Ilori T, Jaar BG, Echouffo-Tcheugui JB. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):125. doi: 10.1186/s12882-018-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George JA, Brandenburg J-T, Fabian J, Crowther NJ, Agongo G, Alberts M, Ali S, Asiki G, Boua PR, Gómez-Olivé FX, Mashinya F, Micklesfield L, Mohamed SF, Mukomana F, Norris SA, Oduro AR, Soo C, Sorgho H, Wade A, Naicker S, Ramsay M. Kidney damage and associated risk factors in rural and urban sub-Saharan Africa (AWI-gen): a cross-sectional population study. Lancet Glob Health. 2019;7(12):e1632–e1643. doi: 10.1016/S2214-109X(19)30443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, Patel U. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(3):e174–e181. doi: 10.1016/S2214-109X(14)70002-6. [DOI] [PubMed] [Google Scholar]
- 16.Matsha TE, Erasmus RT. Chronic kidney disease in sub-Saharan Africa. Lancet Glob Health. 2019;7(12):e1587–e1588. doi: 10.1016/S2214-109X(19)30467-X. [DOI] [PubMed] [Google Scholar]
- 17.Kaze FF, Meto DT, Halle M-P, Ngogang J, Kengne A-P. Prevalence and determinants of chronic kidney disease in rural and urban Cameroonians: a cross-sectional study. BMC Nephrol. 2015;16:117. doi: 10.1186/s12882-015-0111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuate Defo B, Mbanya JC, Kingue S, Tardif J-C, Choukem SP, Perreault S, Fournier P, Ekundayo O, Potvin L, D’Antono B, Emami E, Cote R, Aubin M-J, Bouchard M, Khairy P, Rey E, Richard L, Zarowsky C, Mampuya WM, Mbanya D, Sauvé S, Ndom P, da Silva RB, Assah F, Roy I, Dubois C-A. Blood pressure and burden of hypertension in Cameroon, a microcosm of Africa. J Hypertens. 2019;37(11):2190–2199. doi: 10.1097/HJH.0000000000002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigna JJ, Nansseu JR, Katte J-C, Noubiap JJ. Prevalence of prediabetes and diabetes mellitus among adults residing in Cameroon: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;137:109–118. doi: 10.1016/j.diabres.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Nansseu JR, Noubiap JJ, Bigna JJ. Epidemiology of overweight and obesity in adults living in Cameroon: a systematic review and meta-analysis. Obes Silver Spring Md. 2019;27(10):1682–1692. doi: 10.1002/oby.22566. [DOI] [PubMed] [Google Scholar]
- 21.UNAIDS. HIV in Cameroon. Available from: https://www.unaids.org/en/regionscountries/countries/cameroon. [cited 2020 May 26].
- 22.Kaze FF, Kengne AP, Choukem SP, Dzudie A, Halle MP, Dehayem MY, Ashuntantang G. Dialysis in Cameroon. Am J Kidney Dis. 2008;51(6):1072–1074. doi: 10.1053/j.ajkd.2008.02.366. [DOI] [PubMed] [Google Scholar]
- 23.Arsksey, O’Malley. Methodological framework. 2005.
- 24.FolefackKaze F, Kengne A-P, Pefura Yone EW, NdamFemben NS, Ashuntantang G. Renal function, urinalysis abnormalities and correlates among HIV-infected Cameroonians naive to antiretroviral therapy. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transpl Saudi Arab. 2013;24(6):1291–1297. doi: 10.4103/1319-2442.121280. [DOI] [PubMed] [Google Scholar]
- 25.Feteh VF, Choukem S-P, Kengne A-P, Nebongo DN, Ngowe-Ngowe M. Anemia in type 2 diabetic patients and correlation with kidney function in a tertiary care sub-Saharan African hospital: a cross-sectional study. BMC Nephrol. 2016:17 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4799843/[cited 2020 6 Feb]. [DOI] [PMC free article] [PubMed]
- 26.Ekiti ME, Zambo J-B, Assah FK, Agbor VN, Kekay K, Ashuntantang G. Chronic kidney disease in sugarcane workers in Cameroon: a cross-sectional study. BMC Nephrol. 2018;19. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5769452/ [cited 2020 6 Feb]. [DOI] [PMC free article] [PubMed]
- 27.Hamadou B, Boombhi J, Kamdem F, Fitame A, Amougou SN, Mfeukeu LK, Nganou CN, Menanga A, Ashuntantang G. Prevalence and correlates of chronic kidney disease in a group of patients with hypertension in the Savanah zone of Cameroon: a cross-sectional study in sub-Saharan Africa. Cardiovasc Diagn Ther. 2017;7(6):581–588. doi: 10.21037/cdt.2017.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamdem F, Lekpa FK, Doualla MS, Nouga YN, Sontsa OD, Temfack E, Kingue S. Prevalence and risk factors of chronic kidney disease in newly diagnosed and untreated hypertensive patients in Cameroon: A cross-sectional study. Saudi J Kidney Dis Transpl. 2017;28(5):1144-49. [DOI] [PubMed]
- 29.Temgoua M, Ashuntantang G, Essi MJ, Tochie JN, Oumarou M, Abongwa AF, Mbonda A, Kingue S. Prevalence and risk factors for chronic kidney disease in family relatives of a Cameroonian population of hemodialysis patients: a cross-sectional study. Hosp Pract Res. 2019;4(1):12–17. [Google Scholar]
- 30.Kaze FF, Kengne A-P, Magatsing CT, Halle M-P, Yiagnigni E, Ngu KB. Prevalence and determinants of chronic kidney disease among hypertensive Cameroonians according to three common estimators of the glomerular filtration rate. J Clin Hypertens Greenwich Conn. 2016;18(5):408–414. doi: 10.1111/jch.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prevalence and determinants of chronic kidney disease in urban adults’ populations of northern Cameroon: a cross-sectional study. 2019 Aug 15 [cited 2020 May 9]; Available from: https://www.researchsquare.com/article/rs-3746/v1.
- 32.Patrice HM, Moussa O, Francois KF, Yacouba M, Hugo MNB, Henry LN. Prevalence and associated factors of chronic kidney disease among patients infected with human immunodeficiency virus in Cameroon. Iran J Kidney Dis. 2018;12(5):268–274. [PubMed] [Google Scholar]
- 33.Ngeugoue FT, Njoumemi Z, Kaze FF. Monthly direct and indirect costs of management of CKD 3–5 non-dialysis patients in an out-of-pocket expenditure system: The Case of Yaoundé. Clin Nephrol. 2019 ; Available from: https://www.dustri.com/index.php?id=8&artId=185664&doi=10.5414/CNP92S117 [cited 2020 2 Apr]. [DOI] [PubMed]
- 34.Marie Patrice H, Joiven N, Hermine F, Jean Yves B, Folefack François K, Enow GA. Factors associated with late presentation of patients with chronic kidney disease in nephrology consultation in Cameroon-a descriptive cross-sectional study. Ren Fail. 2019;41(1):384–392. doi: 10.1080/0886022X.2019.1595644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halle MP, Zebaze PN, Mbofung CM, Kaze F, Mbiatat H, Ashuntantang G, Kengne AP. Nutritional status of patients on maintenance hemodialysis in urban sub-Saharan Africa: evidence from Cameroon. J Nephrol. 2014;27(5):545–553. doi: 10.1007/s40620-014-0047-2. [DOI] [PubMed] [Google Scholar]
- 36.Kaze FF, Kengne A-P, AMA D, Ashuntantang G, Halle MP, Menanga AP, Kingue S. Pattern and correlates of cardiac lesions in a group of sub-Saharan African patientson maintenance hemodialysis. Pan Afr Med J. 2014;17(3) Available from: https://www.panafrican-med-journal.com/content/article/17/3/full/ [cited 2020 9 Feb]. [DOI] [PMC free article] [PubMed]
- 37.Halle MP, Takongue C, Kengne AP, et al. Epidemiological profile of patients with end stage renal disease in a referral hospital in Cameroon. BMC Nephrol. 2015;16(1):59. [DOI] [PMC free article] [PubMed]
- 38.Kaze FF, Ekokobe FE, Halle MP, Fouda H, Menanga AP, Ashuntantang G. The clinical pattern of renal diseases in the nephrology in-patient unit of the Yaounde general Hospital in Cameroon: a five-year audit. Pan Afr Med J. 2015;21:205. doi: 10.11604/pamj.2015.21.205.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halle MP, Ashuntantang G, Kaze FF, Takongue C, Kengne A-P. Fatal outcomes among patients on maintenance haemodialysis in sub-Saharan Africa: a 10-year audit from the Douala General Hospital in Cameroon. BMC Nephrol. 2016;17(1):165. doi: 10.1186/s12882-016-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halle M-P, Choukem S-P, Kaze FF, Ashuntantang G, Tchamago V, Mboue-Djieka Y, Temfack E, Luma HN. Hepatitis B, Hepatitis C, and human immune deficiency virus Seroconversion positivity rates and their potential risk factors among patients on maintenance hemodialysis in Cameroon. Iran J Kidney Dis. 2016;10(5):304–309. [PubMed]
- 41.Luma HN, Halle MP, Eloumou SAFB, Azingala F, Kamdem F, Donfack-Sontsa O, Ashuntantang G. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses among haemodialysis patients in two newly opened centres in Cameroon. Pan Afr Med J. 2017;27:235. doi: 10.11604/pamj.2017.27.235.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ama Moor VJ, Nansseu JRN, Azingni DBT, Kaze FF. Assessment of the 10-year risk of cardiovascular disease among a group of patients on maintenance hemodialysis: a cross-sectional study from Cameroon. JRSM Cardiovasc Dis. 2017;6:2048004017705273. doi: 10.1177/2048004017705273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halle MPE, Kengne AP, Ashuntantang G. Referral of patients with kidney impairment for specialist care in a developing country of sub-Saharan Africa. Ren Fail. 2009;31(5):341–348. doi: 10.1080/08860220902882014. [DOI] [PubMed] [Google Scholar]
- 44.Pancha Mbouemboue O, Danbe OD, Tangyi Tamanji M, Ngoufack JO. Frequency of specific cardiovascular disease risk factors among Cameroonian patients on Dialysis: the cases of Anaemia, inflammation, phosphate, and calcium. Cardiol Res Pract. 2016;2016:5031927. doi: 10.1155/2016/5031927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kouotou EA, Folefack FK, Tatsa JT, Sieleunou I, Njingang JRN, Ashuntantang G, CZ-K BA. Profil épidémio-clinique des atteintes dermatologiques chez le noir Africain en hémodialyse chronique. Pan Afr Med J. 2016:25 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5326034/ [cited 2020 9 Feb]. [DOI] [PMC free article] [PubMed]
- 46.Lemogoum D, Halle MP, Mboule RD, Van de Borne P, Bika Lele EC, Kamdem F, Doualla MS, Luma H, Hermans MP, Van Bortel L. Arterial stiffness in black African ancestry patients with chronic kidney disease living in Cameroon. Cardiovasc Diagn Ther. 2018;8(4):450–459. doi: 10.21037/cdt.2018.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doualla M, Halle MP, Moutchia J, Tegang S, Ashuntantang G. Determinants of hyperuricemia in non-dialysed chronic kidney disease patients in three hospitals in Cameroon. BMC Nephrol. 2018;19(1):169. doi: 10.1186/s12882-018-0959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fouda H, Ashuntantang G, Kaze F, Halle M-P. La survie en hémodialyse chronique au Cameroun. Pan Afr Med J. 2017:26 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5409996/ [cited 2020 10 Feb. [DOI] [PMC free article] [PubMed]
- 49.Halle MP, Edjomo AM, Fouda H, Djantio H, Essomba N, Ashuntantang GE. Survival of HIV infected patients on maintenance hemodialysis in Cameroon: a comparative study. BMC Nephrol. 2018;19(1):166. doi: 10.1186/s12882-018-0964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bahrey D, Gebremedhn G, Mariye T, Girmay A, Aberhe W, Hika A, Teklay G, Tasew H, Zeru T, Gerensea H, Demoz GT. Prevalence and associated factors of chronic kidney disease among adult hypertensive patients in Tigray teaching hospitals: a cross-sectional study. BMC Res Notes. 2019;12(1):562. doi: 10.1186/s13104-019-4610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaboré NF, Poda A, Zoungrana J, Da O, Ciaffi L, Semdé A, Yaméogo I, Sawadogo AB, Delaporte E, Meda N, Limou S, Cournil A. Chronic kidney disease and HIV in the era of antiretroviral treatment: findings from a 10-year cohort study in a west African setting. BMC Nephrol. 2019;20(1):155. doi: 10.1186/s12882-019-1335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noubiap JJN, Naidoo J, Kengne AP. Diabetic nephropathy in Africa: a systematic review. World J Diabetes. 2015;6(5):759–773. doi: 10.4239/wjd.v6.i5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mwenda V. Prevalence and factors associated with chronic kidney disease among medical inpatients at the Kenyatta National Hospital, Kenya, 2018: a cross-sectional study. Pan Afr Med J. 2019; Available from: https://pesquisa.bvsalud.org/gim/resource/en/afr-200909 [cited 2020 13 Apr]. [DOI] [PMC free article] [PubMed]
- 54.Kalyesubula R, Nankabirwa JI, Ssinabulya I, Siddharthan T, Kayima J, Nakibuuka J, Salata RA, Mondo C, Kamya MR, Hricik D. Kidney disease in Uganda: a community based study. BMC Nephrol. 2017;18(1):116. [DOI] [PMC free article] [PubMed]
- 55.Fraser SDS, Roderick PJ, May CR, McIntyre N, McIntyre C, Fluck RJ, Shardlow A, Taal MW. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol. 2015;16(1):193. doi: 10.1186/s12882-015-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins AJ, Li S, Gilbertson DT, Liu J, Chen S-C, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population: management of comorbidities in kidney disease in the 21st century: Anemia and bone disease. Kidney Int. 2003;64:S24–S31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 57.Turner JM, Bauer C, Abramowitz MK, Melamed ML, Hostetter TH. Treatment of chronic kidney disease. Kidney Int. 2012;81(4):351–362. doi: 10.1038/ki.2011.380. [DOI] [PubMed] [Google Scholar]
- 58.Smith DH. Cost of medical Care for Chronic Kidney Disease and Comorbidity among enrollees in a large HMO population. J Am Soc Nephrol. 2004;15(5):1300–1306. doi: 10.1097/01.asn.0000125670.64996.bb. [DOI] [PubMed] [Google Scholar]
- 59.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–1304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available data can be obtained by contacting the corresponding author.