Abstract
Diabetes mellitus and angina pectoris are important conditions in older persons. The utility of pre-diabetes mellitus, diabetes mellitus and other risk factors as predictors of incident angina pectoris among older adults has not been characterized. We examined incident angina pectoris rates by sex and diabetes mellitus status in 4511 adults aged ⩾65 years without coronary heart disease at baseline from the Cardiovascular Health Study. Cox regression examined predictors of incident angina pectoris, including pre-diabetes mellitus or diabetes mellitus adjusted for sociodemographic characteristics and other risk factors, over 12.2 ± 6.9 years of follow-up. Overall, 39.1% of participants had pre-diabetes mellitus, 14.0% had diabetes mellitus and 532 (11.8%) had incident angina pectoris. Incident angina pectoris rates per 1000 person-years in those with neither condition, pre-diabetes mellitus, and diabetes mellitus were 7.9, 9.0 and 12.3 in women and 10.3, 11.2 and 14.5 in men, respectively. Pre-diabetes mellitus and diabetes mellitus were not independently associated with incident AP; however, key predictors of AP were male sex, low-density lipoprotein-cholesterol, triglycerides, systolic blood pressure, antihypertensive medication and difficulty performing at least one instrumental activity of daily living (all p < 0.05 to p < 0.01). In our cohort of older adult participants, while the incidence of AP is greater in those with diabetes mellitus, neither diabetes mellitus nor pre-diabetes mellitus independently predicted incident angina pectoris.
Keywords: Angina pectoris, diabetes, older age, sex differences
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among adults in the United States and is increasing in prevalence worldwide.1 While diabetes mellitus (DM) has been characterized as a coronary heart disease (CHD) ‘risk equivalent’,2,3 a large meta-analysis shows this may not be the case, because patients with DM without prior myocardial infarction (MI) have a 43% lower risk of CHD events compared to patients without DM and with clinically evident CHD or prior MI.4 Symptomatic angina pectoris (AP) can be a debilitating consequence of CHD5 in patients with and without DM alike, and is often the initial presenting symptom.6,7 Demographically, in patients with DM, AP has been reported to be more prevalent in women than men.8,9 The relationship between type 2 DM, documented stable CHD, and AP symptoms in relation to all-cause and CVD mortality has been previously investigated using the results of the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. In patients with DM and stable CHD, a similar risk of all-cause mortality and CVD death has been demonstrated over 5 years of follow up in those with asymptomatic CHD compared to those with symptomatic CHD (i.e. AP or angina-equivalent symptoms, such as dyspnea on exertion) at baseline.10 In addition, impaired glucose tolerance (pre-DM) and increased serum creatinine have also been described as important factors that predict increased CV events in younger patients with stable angina.11 Not examined, however, is the temporal relationship between pre-DM, DM and incident AP, as well as other factors that predict incident AP in older adults. Early population studies have sought to estimate the incidence and prognosis of AP in the general population among adults without previously known CHD;7,12 however, prior studies have been performed primarily in younger patient groups.7,8,10,12 The Cardio-vascular Health Study (CHS) is a population-based observational study of adults aged 65 years and older, designed to evaluate the risk factors associated with CVD incidence, morbidity and mortality. In this study, we aimed to examine the factors, including potential sex differences, associated with the development of incident AP among older adults without prior CHD, and to examine whether DM (and to a lesser extent pre-DM) is an independent predictor of incident AP in this population.
Methods
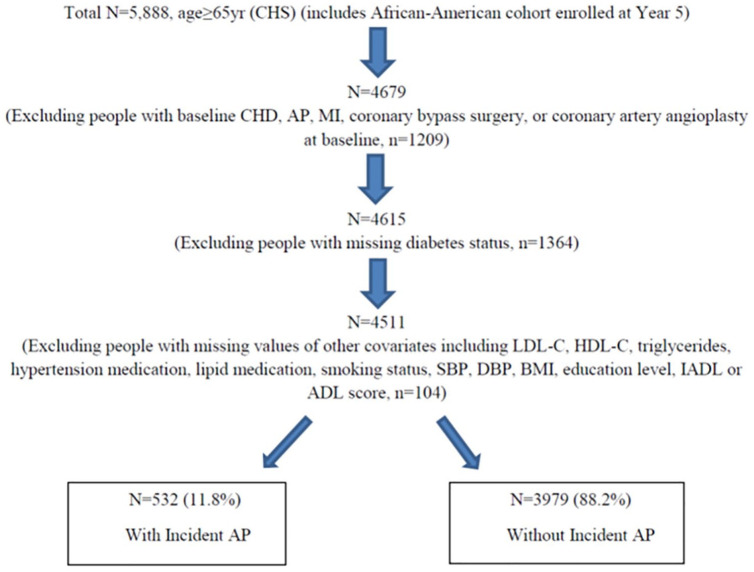
The CHS is a prospective, population-based observational study of CVD in older adults. Detailed CHS design and methodology have been published.13 5201 participants were enrolled in the initial recruitment during 1989–1990, and 3 years later, 687 African-American participants were enrolled for a total combined cohort of 5888 participants. Participants were recruited from four US regions: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland and Pittsburgh, Pennsylvania, and sampled using Health Care Financing Administration Medicare eligibility data. Consent was obtained at the time of the initial examination. Extensive history and physical examination and fasting laboratory studies were performed at baseline, and participants were followed over time with semi-annual contacts, including telephone calls and scheduled clinic visits. Specifically, the current analysis was derived from 4511 participants without known CHD at baseline (1989–1990 in the original cohort and 1992–1993 in the African-American cohort) but with known DM status and complete covariates for analysis. A flow diagram showing the derivation of the study sample is shown in Figure 1.
Figure 1.
Flow diagram showing derivation of study sample.
ADL: activities of daily living; AP: angina pectoris; BMI: body mass index; CHD: coronary heart disease; CHS: Cardiovascular Health Study; DBP: diastolic blood pressure; DM: diabetes mellitus; HDL-C: high density lipoprotein cholesterol; IADL: instrumental activities of daily living; LDL-C: low density lipoprotein cholesterol; pre-DM: pre-diabetes mellitus; SBP: systolic blood pressure.
Adults with prevalent CHD at baseline, including prior MI, AP, coronary bypass surgery or coronary artery angioplasty, were excluded. CHS criteria for AP required a physician diagnosis along with current medical therapy for AP (nitrates, beta-blockers or calcium-channel blockers), or one of the following: coronary artery bypass surgery, ⩾70% luminal diameter blockage of a coronary artery, or ST depression >1 mm on exercise stress testing with a positive medical history using the Rose questionnaire.13 Time to incident AP was defined as the adjudicated first occurrence of AP during follow up after the baseline examination (1989–1990 for the original cohort and 1992–1993 for the African-American cohort) through the end of 2004, with a mean follow-up time of 12.2 ± 6.9 years.
DM was defined as having a fasting plasma glucose level ⩾126 mg/dL, use of either oral hypoglycemic medications or insulin, or both in combination. Pre-DM was defined as having a fasting plasma glucose level between 100 and 125 mg/dL without use of oral hypoglycemic medication or insulin. Those with neither condition were defined as non-DM. The average of two systolic blood pressure (SBP) and diastolic blood pressure (DBP) measures were taken. Serum total cholesterol, high density lipoprotein-cholesterol (HDL-C) and triglycerides were measured as previously described13 with low density lipoprotein-cholesterol (LDL-C) calculated by the Friedewald equation. Physical functioning was assessed by the number of reported blocks walked in the week prior to the 1992–1993 examination and by self-reported exercise intensity level. Exercise intensity level was categorized as low, moderate or high intensity versus no reported exercise activity. The modified Mini-Mental State (3MS) exam was used to assess cognitive function (maximum score of 100). The 3MS has been validated in prior studies defining cognitive impairment as a score less than 77.14 Additional assessment of physical, cognitive and sensory input functioning was performed using the modified version of the Health Interview Survey Supplement on Aging questionnaire,13,15 which evaluated self-reported difficulty with any task of Instrumental Activities of Daily Living (IADL) and Activities of Daily Living (ADL).16 Education level was categorically defined as those with no prior schooling, high school or vocational school graduate, college graduate, and graduate or professional studies completion.
CVD outcomes, including MI, AP, congestive heart failure, claudication as a result of peripheral artery disease, stroke and transient ischemic attack were ascertained through June 2014 for the overall CHS study. During follow up, suspected CVD events and the cause of all non-stroke fatalities were reviewed and adjudicated by a 5-member cardiovascular events committee.13,17 Persons who were concurrently classified as having MI at the time of AP were censored. The current investigation was limited to examining the incidence of AP in persons free of AP at baseline, and the predictors of the development of AP.
The bivariate association of pre-DM and DM, as well as other potential risk factors, with incident AP was examined using the Student’s t-test and Chi-square test of proportions for continuous and categorical predictors, respectively. Incident AP event rates per 1000 person-years were calculated by sex and DM status. Kaplan Meier curves were used to describe the cumulative incidence and time to incident AP by DM status and sex. Cox proportional hazards regression was used to determine the hazard ratios (HRs) for the risk of incident AP according to presence of pre-DM or DM, compared to neither condition. Participants who developed incident non-fatal MI or coronary death during the follow up period were censored in the analysis.
Models were adjusted for age, sex, race, smoking status, HDL-C, LDL-C, triglyceride levels, body mass index (BMI), SBP and DBP, any antihypertensive use, use of a lipid-lowering medication, aspirin use, difficulty with more than one IADL or ADL, cognitive function, exercise intensity level, and education level. Secondary analyses were done stratified by sex and DM status and interaction terms with sex as an effect modifier in the relationship of pre-DM and DM as predictors of incident AP. SAS version 9.3 was used for all statistical analysis.18 An α level of <0.05 was deemed to be statistically significant.
Results
Table 1 shows baseline characteristics of the entire cohort and among participants with and without AP. Of the 4511 older adults without CHD at baseline in our study sample, the mean age was 72.6 ± 5.5 years, with 60.4% women and 15.3% African-Americans. In total, 11.7% of women and 12.0% of men had incident AP. Overall, 39.1% had pre-DM, 14.0% had DM, 40.3% were former smokers, 12.4% current smokers, 43.9% had completed a high school education, and 82.5% reported engaging in either low- or moderate-intensity level exercise activity at baseline. In the overall cohort, 29.3% of participants reported aspirin use at baseline, while 40.3% and 4.5% reported use of antihypertensive and lipid medications, respectively. Those with incident AP, as compared to those without AP, were slightly younger (72.5 vs 72.6 years, p < 0.05), more likely to be on antihypertensive medication (47.6% vs 39.4%, p < 0.001), have difficulty with one or more IADLs (27.1% vs 21.6%) or ADLs (6.8% vs 4.0%) (both p < 0.01), and have higher BMI, waist circumference, SBP, total and LDL-C, lower HDL-C, and higher triglycerides.
Table 1.
Demographic and risk factor characteristics among participants with and without incident angina.
| All participants (n = 4511) | Incident AP (n = 532) | No AP (n = 3979) | p value | |
|---|---|---|---|---|
| Age, years | 72.6 ± 5.5 | 72.5 ± 5.0 | 72.6 ± 5.6 | 0.0499 |
| Sex | ||||
| Women | 2723 (60.4) | 318 (59.8) | 2405 (60.4) | 0.7674 |
| Men | 1788 (39.6) | 214 (40.2) | 1574 (39.6) | |
| Ethnicity | ||||
| White | 3795 (84.1) | 451 (84.8) | 3344 (84.0) | 0.7208 |
| African American | 689 (15.3) | 80 (15.0) | 609 (15.3) | |
| American Indian/Alaskan Native | 11 (0.2) | 0 (0) | 11 (0.3) | |
| Asian/Pacific Islander | 2 (0.1) | 0 (0) | 2 (0.1) | |
| Other | 14 (0.3) | 1 (0.2) | 13 (0.3) | |
| Body mass index, kg/m2 | 26.6 ± 4.7 | 27.3 ± 4.7 | 26.5 ± 4.7 | 0.0002 |
| Waist circumference, cm | 93.9 ± 13.2 | 96.0 ± 12.6 | 93.7 ± 13.3 | 0.0002 |
| Diabetes status | ||||
| No diabetes | 2116 (46.9) | 237 (44.6) | 1879 (47.2) | 0.4832 |
| Pre-diabetes | 1764 (39.1) | 215 (40.4) | 1549 (38.9) | |
| Diabetes mellitus | 631 (14.0) | 80 (15.0) | 551 (13.9) | |
| Systolic blood pressure, mm Hg | 136.4 ± 21.5 | 138.6 ± 21.7 | 136.1 ± 21.5 | 0.0129 |
| Diastolic blood pressure, mm Hg | 71.1 ± 11.3 | 71.2 ± 11.1 | 71.1 ± 11.3 | 0.8370 |
| Total cholesterol, mg/dL | 211.7 ± 38.7 | 216.5 ± 39.2 | 211.1 ± 38.6 | 0.0026 |
| HDL, mg/dL | 55.5 ± 15.7 | 53.8 ± 15.4 | 55.8 ± 15.7 | 0.0064 |
| LDL, mg/dL | 129.6 ± 35.6 | 133.5 ± 37.9 | 129.1 ± 35.2 | 0.0108 |
| Triglyceride, mg/dL | 132.9 ± 59.3 | 145.8 ± 64.3 | 131.1 ± 58.3 | <0.0001 |
| Aspirin use | 1323 (29.3) | 167 (31.4) | 1156 (29.1) | 0.2658 |
| Antihypertensive meds | 1819 (40.3) | 253 (47.6) | 1566 (39.4) | 0.0003 |
| Lipid meds | 204 (4.5) | 27 (5.1) | 177 (4.5) | 0.5134 |
| Smoking status | ||||
| Current smoker | 558 (12.4) | 58 (10.9) | 500 (12.6) | 0.2103 |
| Former smoker | 1818 (40.3) | 232 (43.6) | 1586 (39.9) | |
| Physical activity (blocks walked in the prior week) | 39.4 ± 54.1 | 36.5 ± 48.0 | 39.8 ± 54.9 | 0.1348 |
| Difficulty with IADLs (⩾1) | 1002 (22.2) | 144 (27.1) | 858 (21.6) | 0.0041 |
| Difficulty with ADLs (⩾1) | 196 (4.3) | 36 (6.8) | 160 (4.0) | 0.0035 |
| Modified mini-mental state (3MS) exam (maximum score of 100) | 89.9 ± 8.9 | 90.4 ± 8.1 | 89.9 ± 9.0 | 0.1287 |
| Exercise intensity level | ||||
| No exercise | 360 (8.0) | 43 (8.1) | 317 (8.0) | 0.9504 |
| Low intensity | 2154 (47.8) | 248 (46.6) | 1906 (47.9) | |
| Moderate intensity | 1567 (34.7) | 188 (35.3) | 1379 (34.6) | |
| High intensity | 430 (9.5) | 53 (10.0) | 377 (9.5) | |
| Education level | ||||
| <High school | 2531 (56.1) | 299 (56.2) | 2232 (56.1) | 0.5015 |
| Vocational school | 389 (8.6) | 51 (9.6) | 338 (8.5) | |
| College graduate | 1135 (25.2) | 137 (25.7) | 998 (25.1) | |
| Graduate or professional | 456 (10.1) | 45 (8.5) | 411 (10.3) | |
AP: angina pectoris; HDL: high density lipoprotein; LDL: low density lipoprotein; IADL: instrumental activities of daily living; ADL: activities of daily living.
Data presented as mean ± SD, n (%).
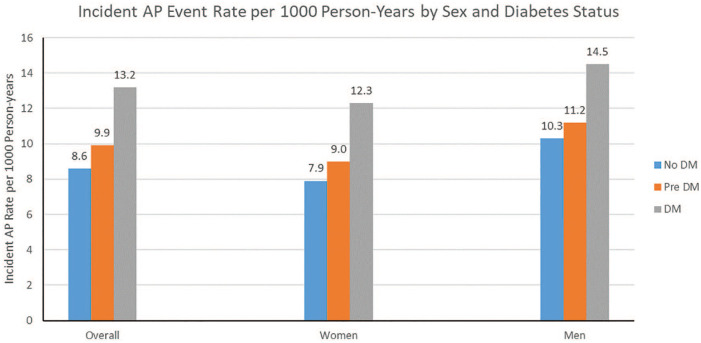
Among those with incident AP, those with DM (compared to pre-DM and no DM) were less likely to be White, had higher BMI and waist circumference, higher systolic BP, lower HDL-C and higher triglycerides (Table 2). Incident AP event rates generally increased across DM status categories and were greater in men than in women. Overall, incident AP event rates per 1000 person-years were 8.6, 9.9 and 13.2 in those with no-DM, pre-DM and DM, respectively. In those with no DM, pre-DM, and DM, incident AP event rates were 7.9, 9.0 and 12.3 in women and 10.3, 11.2 and 14.5 in men, per 1000 person-years, respectively (Figure 2).
Table 2.
Demographic and risk factor characteristics among participants with and without incident angina by diabetes status.
| Incident angina
(n = 532) |
p value | No angina
(n = 3979) |
p value | |||||
|---|---|---|---|---|---|---|---|---|
| No DM (n = 237) | Pre-DM (n = 215) | DM (n = 80) | No DM (n = 1879) | Pre-DM (n = 1549) | DM (n = 551) | |||
| Age, years | ||||||||
| 65–74 | 159 (67.1) | 151 (70.2) | 61 (76.3) | 0.4485 | 1299 (69.1) | 1059 (68.4) | 357 (64.8) | 0.1440 |
| 75–84 | 75 (31.6) | 60 (27.9) | 19 (23.7) | 502 (26.7) | 440 (28.4) | 174 (31.6) | ||
| ⩾85 | 3 (1.3) | 4 (1.9) | 0 (0) | 78 (4.2) | 50 (3.2) | 20 (3.6) | ||
| Sex | ||||||||
| Women | 155 (65.4) | 120 (55.8) | 43 (53.7) | 0.0570 | 1263 (67.2) | 854 (55.1) | 288 (52.3) | <0.0001 |
| Men | 82 (34.6) | 95 (44.2) | 37 (46.3) | 616 (32.8) | 695 (44.9) | 263 (47.7) | ||
| Ethnicity | ||||||||
| White | 199 (84.0) | 194 (90.2) | 58 (72.5) | 0.0007 | 1593 (84.8) | 1347 (87.0) | 404 (73.3) | <0.0001 |
| Other | 38 (16.0) | 21 (9.8) | 22 (27.5) | 286 (15.2) | 202 (13.0) | 147 (26.7) | ||
| Body mass index, kg/m2 | 26.2 ± 4.3 | 27.9 ± 4.9 | 29.2 ± 4.6 | <0.0001 | 25.3 ± 4.3 | 27.2 ± 4.6 | 28.7 ± 5.0 | <0.0001 |
| Waist circumference, cm | 92.7 ± 11.7 | 97.5 ± 12.3 | 101.6 ± 13.2 | <0.0001 | 90.0 ± 12.7 | 95.7 ± 12.8 | 100.8 ± 12.6 | <0.0001 |
| Systolic blood pressure, mm Hg | 136.7 ± 22.1 | 138.7 ± 21.0 | 143.7 ± 21.9 | 0.0462 | 133.3 ± 21.2 | 137.6 ± 21.2 | 141.7 ± 21.6 | <0.0001 |
| Diastolic blood pressure, mm Hg | 71.0 ± 11.4 | 71.0 ± 10.6 | 72.3 ± 11.1 | 0.6325 | 70.1 ± 11.1 | 72.0 ± 11.2 | 72.0 ± 12.0 | <0.0001 |
| Total cholesterol, mg/dL | 215.9 ± 38.1 | 218.5 ± 40.3 | 212.6 ± 39.9 | 0.4934 | 212.2 ± 37.3 | 211.7 ± 38.4 | 205.5 ± 43.0 | 0.0012 |
| HDL, mg/dL | 57.7 ± 17.3 | 51.3 ± 13.0 | 48.7 ± 12.4 | <0.0001 | 59.3 ± 16.3 | 54.0 ± 14.9 | 48.8 ± 12.7 | <0.0001 |
| LDL, mg/dL | 131.2 ± 38.3 | 137.4 ± 37.9 | 130.1 ± 36.2 | 0.1494 | 129.0 ± 34.5 | 130.5 ± 34.7 | 125.6 ± 38.6 | 0.0181 |
| Triglyceride, mg/dL | 135.0 ± 62.0 | 149.1 ± 62.5 | 169.2 ± 69.5 | 0.0001 | 120.1 ± 52.0 | 135.8 ± 57.1 | 155.7 ± 71.7 | <0.0001 |
| Aspirin use | 65 (27.4) | 74 (34.4) | 28 (35.0) | 0.2092 | 535 (28.5) | 454 (29.3) | 167 (30.3) | 0.6778 |
| Antihypertensive meds | 104 (43.9) | 103 (47.9) | 46 (57.5) | 0.1072 | 589 (31.4) | 664 (42.9) | 313 (56.8) | <0.0001 |
| Lipid meds | 8 (3.4) | 12 (5.6) | 7 (8.8) | 0.1512 | 82 (4.4) | 67 (4.3) | 28 (5.1) | 0.7384 |
| Smoking status | ||||||||
| Current smoker | 24 (10.1) | 24 (11.2) | 10 (12.5) | 0.9491 | 249 (13.3) | 192 (12.4) | 59 (10.7) | 0.0016 |
| Former smoker | 105 (44.3) | 95 (44.2) | 32 (40.0) | 690 (36.7) | 670 (43.3) | 226 (41.0) | ||
| Physical activity (blocks walked in the prior week) | 34.0 ± 41.2 | 39.8 ± 56.6 | 34.7 ± 41.2 | 0.4130 | 41.2 ± 55.5 | 40.2 ± 55.1 | 34.2 ± 51.8 | 0.0315 |
| Difficulty with IADLs (⩾1) | 68 (28.7) | 58 (27.0) | 18 (22.5) | 0.5590 | 395 (21.0) | 309 (20.0) | 154 (28.0) | 0.0003 |
| Difficulty with ADLs (⩾1) | 13 (5.5) | 13 (6.1) | 10 (12.5) | 0.0837 | 69 (3.7) | 57 (3.7) | 34 (6.2) | 0.0217 |
| Modified mini-mental state (3MS) exam (maximum score of 100) | 90.7 ± 8.2 | 90.5 ± 8.6 | 89.5 ± 6.4 | 0.5448 | 90.3 ± 9.1 | 90.1 ± 8.5 | 87.8 ± 9.7 | <0.0001 |
| Exercise intensity level | ||||||||
| No exercise | 20 (8.4) | 18 (8.4) | 5 (6.2) | 123 (6.6) | 129 (8.3) | 65 (11.8) | <0.0001 | |
| Low intensity | 104 (43.9) | 111 (51.6) | 33 (41.3) | 0.4243 | 870 (46.3) | 756 (48.8) | 280 (50.8) | |
| Moderate intensity | 91 (38.4) | 64 (29.8) | 33 (41.2) | 674 (35.9) | 530 (34.2) | 175 (31.8) | ||
| High intensity | 22 (9.3) | 22 (10.2) | 9 (11.3) | 212 (11.2) | 134 (8.7) | 31 (5.6) | ||
| Education level | ||||||||
| ⩽High school | 125 (52.7) | 122 (56.7) | 52 (65.0) | 984 (52.4) | 889 (57.4) | 359 (65.2) | <0.0001 | |
| Vocational school | 28 (11.8) | 16 (7.4) | 7 (8.8) | 0.1738 | 175 (9.3) | 121 (7.8) | 42 (7.6) | |
| College graduate | 68 (28.7) | 53 (24.7) | 16 (20.0) | 510 (27.1) | 377 (24.3) | 111 (20.2) | ||
| professional | 16 (6.8) | 24 (11.2) | 5 (6.2) | 210 (11.2) | 162 (10.5) | 39 (7.0) | ||
DM: diabetes mellitus; pre-DM: pre-diabetes; HDL: high density lipoprotein; LDL: low density lipoprotein; IADL: instrumental activities of daily living; ADL: activities of daily living.
Data presented as mean + SD or n (%).
Figure 2.
Incident AP event rates overall in the entire cohort and by sex and DM status. Overall, incident AP event rates per 1000 person-years were 8.6, 9.9 and 13.2 in those with no-DM, pre-DM and DM, respectively. In those with neither, pre-DM or DM, incident AP event rates were 7.9, 9.0 and 12.3 in women and 10.3, 11.2 and 14.5 in men, per 1000 person-years, respectively.
AP: angina pectoris; DM: diabetes mellitus; pre-DM: pre-diabetes.
For those with no DM, pre-DM or DM, women had lower AP event rates than did men. In fully adjusted multivariable Cox regression analysis, only male sex (HR = 1.40, 95% CI = 1.11–1.78), LDL-C, triglycerides, SBP, antihypertensive medication use, and difficulty with at least one IADL were independently associated with an increased likelihood of incident AP; in men, only HDL-C (inversely), LDL-C, and difficulty with at least one IADL, and in women, only triglycerides, SBP, antihypertensive medication use, difficulty with at least one IADL and difficulty with at least one ADL were independently associated with incident AP (Table 3).
Table 3.
Multivariable Cox regression of predictors of incident angina.
| Overall | Men | Women | |
|---|---|---|---|
| Age | 1.16 (0.94–1.51) | 1.23 (0.89–1.69) | 1.14 (0.86–1.51) |
| Sex | |||
| Men (Ref = Women) | 1.40 (1.11–1.78)** | – | – |
| Race | |||
| White | Ref | Ref | Ref |
| Other | 0.88 (0.55–1.42) | 1.11 (0.51–2.43) | 0.77 (0.42–1.41) |
| Diabetic status | |||
| Normal glycaemic | Ref | Ref | Ref |
| Pre-DM | 0.99 (0.80–1.23) | 1.02 (0.73–1.44) | 1.03 (0.78–1.35) |
| DM | 1.09 (0.79–1.49) | 1.37 (0.85–2.23) | 1.04 (0.68–1.60) |
| Smoking status | |||
| Never | Ref | Ref | Ref |
| Former | 1.14 (0.92–1.40) | 1.28 (0.92–1.79) | 1.05 (0.79–1.38) |
| Current | 1.15 (0.82–1.61) | 1.16 (0.64–2.10) | 1.13 (0.75–1.72) |
| HDL-C | 0.97 (0.86–1.10) | 0.75 (0.59–0.97)* | 1.05 (0.91–1.22) |
| LDL-C | 1.13 (1.02–1.24)* | 1.39 (1.19–1.63)** | 1.02 (0.90–1.15) |
| Triglycerides | 1.16 (1.05–1.28)** | 1.11 (0.95–1.31) | 1.16 (1.02–1.32)* |
| BMI | 1.07 (0.96–1.19) | 0.94 (0.76–1.16) | 1.11 (0.98–1.26) |
| Systolic blood pressure | 1.09 (1.03–1.15)** | 1.03 (0.94–1.11) | 1.12 (1.04–1.20)** |
| Diastolic blood pressure | 0.92 (0.83–1.02) | 0.99 (0.85–1.18) | 0.88 (0.77–1.00) |
| Antihypertensive medication use | 1.32 (1.08–1.61)** | 1.05 (0.76–1.46) | 1.53 (1.18–1.99)** |
| Lipid medication use | 1.10 (0.71–1.69) | 0.87 (0.38–1.98) | 1.16 (0.70–1.94) |
| Aspirin use | 1.04 (0.85–1.28) | 1.13 (0.82–1.55) | 1.01 (0.77–1.33) |
| IADLs | |||
| No difficulty with IADLs | Ref | Ref | Ref |
| Difficulty with IADLs (⩾1) | 1.48 (1.17–1.87)** | 1.60 (1.05–2.43)* | 1.42 (1.07–1.89)* |
| ADLs | |||
| No difficulty with ADLs | Ref | Ref | Ref |
| Difficulty with ADLs (⩾1) | 1.20 (0.77–1.88) | 0.32 (0.08–1.35) | 1.65 (1.01–2.68)* |
| Cognitive function (modified mini-mental state exam score) | 0.98 (0.87–1.12) | 0.98 (0.80–1.19) | 0.99 (0.83–1.17) |
| Exercise intensity level | |||
| No exercise | Ref | Ref | Ref |
| Low intensity | 0.93 (0.64–1.35) | 0.95 (0.80–1.19) | 0.91 (0.58–1.44) |
| Moderate intensity | 0.94 (0.64–1.38) | 0.93 (0.47–1.82) | 0.93 (0.58–1.51) |
| High intensity | 1.03 (0.65–1.62) | 0.84 (0.39–1.82) | 1.20 (0.68–2.11) |
| Education level | |||
| ⩽High school | Ref | Ref | Ref |
| Vocational school | 0.99 (0.71–1.38) | 1.11 (0.65–1.88) | 0.93 (0.60–1.43) |
| College graduate | 0.99 (0.78–1.25) | 0.99 (0.68–1.44) | 1.02 (0.76–1.38) |
| Graduate or professional | 0.83 (0.58–1.19) | 0.96 (0.58–1.58) | 0.78 (0.46–1.33) |
pre-DM: pre-diabetes; DM: diabetes mellitus; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; BMI: body mass index; IADL: instrumental activities of daily living; ADL: activities of daily living.
Values presented are hazard ratios (HRs) with 95% confidence limits in parenthesis. HRs for age, systolic blood pressure and diastolic blood pressure expressed per 10 years and 10 mmHg, respectively. All other continuous variables expressed per standard deviation.
p < 0.05.
p < 0.01.
Kaplan Meier curves of time to incident angina by DM status among men and women are shown in Figures 3 to 5. A significant difference was seen in time to incident AP overall and among men and women, respectively, stratified by DM status. In the overall sample, there was significantly greater incident AP across disease groups (p < 0.01) (Figure 3); however, these differences across DM status groups did not reach significance in sex-stratified analyses (Figures 4 and 5).
Figure 3.
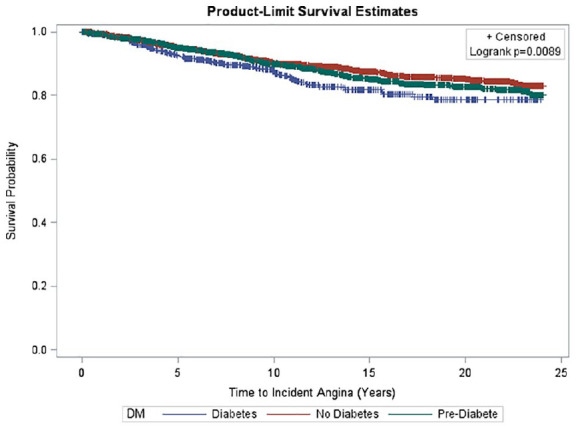
Survival curve of time to incident angina by diabetes status. Kaplan Meier curves depicting time to incident AP by DM status categories overall. Mean time to incident AP overall was 12.2 ± 6.9 years. A significant difference was seen in time to incident AP overall with the lowest survival probability seen in people with DM (p = 0.0089).
Figure 5.
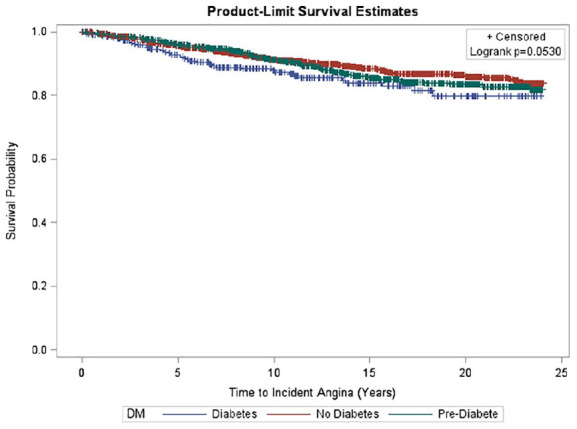
Survival curve of time to incident angina by diabetes status. Kaplan Meier curves depicting time to incident AP by DM status categories among older women. Mean time to incident AP was 13.3 ± 6.9 years. No significant difference was seen in time to incident AP among DM categories (p = 0.0530).
Figure 4.
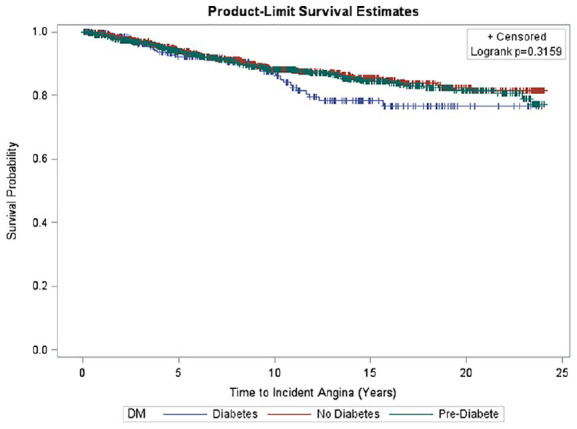
Survival curve of time to incident angina by diabetes status. Kaplan Meier curves depicting time to incident AP by DM status categories among older men. Mean time to incident AP was 10.6 ± 6.7 years in men. No significant difference was seen in time to incident AP among DM categories (p = 0.3159).
Discussion
In this study, approximately 12% of community dwelling older adults, who were free of CHD at baseline, developed AP over 12.2 years of follow-up. While the incidence of AP was greater in persons with DM, after adjustment for age and other risk factors, important predictors of AP were limited to male sex, higher LDL-C, triglycerides, SBP, antihypertensive medication, and difficulty with at least one IADL.
Prior studies have found AP to be more prevalent in women, as compared to men, particularly among younger patients and those with DM.8,9 However, in the general population of US adults over age 40 years, AP prevalence has ranged from between 8% and 11% for men, and 5%–9% for women over the age of 65 years.19 Our study further shows incidence rates of AP to be consistently higher in older men compared to older women among those with pre-DM, DM, or neither condition, with adjusted risks showing men overall to be 40% more likely to develop incident AP than women. The lower incidence rates of AP among women may reflect a sex-specific difference in AP symptom recognition, reporting, and evaluation,6,20 or may reflect sex-specific differences in the development of atherosclerosis.
Our findings further demonstrate a disparity in sex-specific AP incidence among older adults during long-term follow up. Furthermore, the difference between known AP prevalence and incidence is noteworthy. A retrospective study from the National Health and Nutrition Examination Survey (NHANES) from 1998 to 2012 reported a decline in the prevalence of AP in US adults, particularly in individuals aged ⩾65 years (decline from 9.4% in 2001–2004 to 5.1% in 2009–2012 among women; and from 11.0% in 2001–2004 to 7.8% in 2009–2012 among men).19 NHANES data on AP prevalence were derived from Rose Questionnaires of AP symptoms and self-reported survey information, which can lead to an underestimation of AP prevalence. Our study, with physician-adjudicated criteria for incident AP from the CHS, offers unique insight into the incidence of AP in older adults over longer term follow up not reflected in prior studies.
Although incident AP event rates were greater with more profound glucometabolic derangement, neither DM nor pre-DM were independent predictors of AP in our study after adjustment for age, sex and risk factors. This suggests that those with DM or pre-DM who did not experience AP, therefore possibly being inadequately treated due to the absence of AP, could be at higher risk of worse prognosis, especially women. While it was beyond the scope of our study to investigate this, it may in part explain the worse cardiovascular prognosis in those with DM, especially among women.21 A recent study from the Duke Databank for Cardiovascular Diseases reported that, in a comparatively younger patient population (median age 64 years),22 prevalent AP was associated with a similar risk of CV hospitalization, revascularization, and all-cause mortality, regardless of DM status. The authors concluded that there was insufficient evidence to support an association between different clinical outcomes in patients with AP based solely on DM status.22 Moreover, Zellweger and colleagues23 have shown the extent of objective evidence of coronary disease as well as MI or cardiac death were similar in those with and without AP. However, data from the Rancho Bernardo study among older adults showed that women with AP and DM had a 3–4 times higher risk of CHD mortality, independent of other CVD risk factors, than women with DM but no AP.24 This sex-specific difference in CHD mortality among those with DM with AP versus DM without AP was not seen in men.24 Although the incidence of AP was found to be lower among older women compared to men in our study, studies have shown that women tend to have a poorer prognosis and higher morbidity once AP develops.6 Despite the limitation in assessing duration of DM and pre-DM among the cohort in our study, on univariate and multivariate analysis (including controlling for fasting plasma glucose levels, oral hypoglycemic agents, and insulin use), there was no independent association between DM and incident AP in either men or women in our sample.
The association between global cognitive function and CVD has been previously examined.25-27 Prior findings have suggested that decreased cognitive function may lead to an increased risk of CVD and CVD death,26,27 likely given the overlap of risk factors related to CVD and vascular-disease-mediated cognitive decline.28 In a recent analysis from the Women’s Health Initiative Memory Study (WHIMS),27 the link between cognitive impairment and incident CVD was examined in older post-menopausal women who were free of cardiovascular disease at baseline. The 3MS score was used as the measure of cognitive function at baseline and prospectively, with higher scores indicating better cognitive function.27 Our results are consistent with these findings: Cognitive function, similarly measured by the 3MS score, was not an independent predictor of incident AP among older men or women in the CHS cohort. Interestingly, when stratified by DM status, those with AP and DM were less likely to be college graduates as compared to those without AP and with DM. Further investigation regarding the association between cognitive function and incident AP in a more ethnically diverse cohort of patients with lower cognitive function scores and education level at baseline may reveal an association between lower cognitive function and incident AP not captured by our specific cohort of predominantly White older adults.
Prior reports have assessed the association between AP and physical activity,8 showing that community-based adults with AP have significantly more limitations in physical functioning compared to those without AP, with worse limitations in physical activity observed in adults with AP who also have DM. In our cohort of older adults, we further assessed the impact of baseline physical functioning, by determining specific exercise intensity levels, on the development of incident AP. In our study, we showed that in women, those with difficulty with at least one ADL had a 65% greater risk of developing AP. We found that levels of baseline exercise intensity were not independently associated with development of AP. The possibility that more intense physical activity could have precipitated angina in some persons cannot be excluded.
Strengths of our study include the large sample size, the prospective study design of the Cardiovascular Health Study with long term follow up, and the availability of well-characterized and standardized CVD and socio-demographic risk factors. Notably, all self-reported CHD was verified by medical records and baseline EKG findings, and CHS criteria for adjudicated baseline and incident AP also included physician diagnosis along with a positive medical history obtained using the Rose questionnaire, AP medical therapy, or the development of an ischemic end point such as coronary artery bypass surgery, ⩾70% blockage of a coronary artery, or ST depression >1 mm on exercise stress testing.13,28 The Rose questionnaire has previously been validated as a tool for use in AP determination in epidemiological studies.19,20 In addition, participants who developed incident non-fatal MI and coronary death were censored in the analysis, thus reducing the likelihood of misclassification and validating the results of our cohort vis-a-vis participants with incident AP. Our study is one of the few that provide further insight into the predictors of incident AP in older community-dwelling adults, while uniquely assessing the impact of DM and pre-DM, along with sex differences, as independent risk factors in this special population over a long-term follow up period.
There are several limitations of our study. First, the Cardiovascular Health Study was comprised of White and African-American participants, but a limited number of participants of other ethnicities, which may limit the generalizability of our findings among more diverse populations. Also, since subjects did not routinely receive ischemic testing or coronary angiography at baseline or in conjunction with the development of AP, we cannot exclude the possibility of significant coronary disease or ischaemia being present in some included persons at baseline. Furthermore, although atherosclerotic obstructive disease of the epicardial coronary arteries is the most common cause of AP, other causes may include coronary artery spasm or endothelial dysfunction. In addition, duration of DM and pre-DM could not be ascertained at baseline; thus, potential variations in disease severity could not be controlled for within our multivariate analysis. Further investigation that combines a temporal assessment of the relationship of incident DM, and pre-DM, to the development of incident AP, with extension to subsequent cardiovascular mortality, would enhance the findings of our study.
Our results show that while persons with DM have a greater incidence of AP, this is largely explained by other CHD risk factors – in particular, male sex, higher LDL-C, triglycerides, SBP, antihypertensive medication, and difficulty with at least one IADL. Moreover, there are sex-specific differences in predictors of incident AP risk in older adults which may be considerations for sex-specific strategies aimed at preventing AP in these individuals.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The analysis for this manuscript was supported by a contract from Gilead Sciences to the University of California, Irvine.
ORCID iD: Nathan D Wong  https://orcid.org/0000-0003-1102-7324
https://orcid.org/0000-0003-1102-7324
References
- 1. National Center for Health Statistics. Heath, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville, MD: National Center for Health Statistics, 2016. [PubMed] [Google Scholar]
- 2. Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in non-diabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234. [DOI] [PubMed] [Google Scholar]
- 3. Carnethon MR, Biggs ML, Barzilay J, et al. Diabetes and coronary heart disease as risk factors for mortality in older adults. Am J Med 2010; 123: 556.e1–556e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bulugahapitiya U, Siyambalapitiya S, Sithole J, et al. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med 2009; 26: 142–148. [DOI] [PubMed] [Google Scholar]
- 5. Jespersen L, Abildstrøm SZ, Hvelplund A, et al. Persistent angina: highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol 2013; 102: 571–581. [DOI] [PubMed] [Google Scholar]
- 6. Hemingway H, McCallum A, Shipley M, et al. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA 2006; 295: 1404–1411. [DOI] [PubMed] [Google Scholar]
- 7. Frank CW, Weinblatt E, Shapiro S. Angina pectoris in men: prognostic significance of selected medical factors. Circulation 1973; 47: 509–517. [DOI] [PubMed] [Google Scholar]
- 8. Hui G, Koch B, Calara F, et al. Angina in coronary artery disease patients with and without diabetes: US National Health and Nutrition Examination Survey 2001-2010. Clin Cardiol 2016; 39: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tamis-Holland JE, Lu J, Korytkowski M, et al. Sex differences in presentation and outcome among patients with type 2 diabetes and coronary artery disease treated with contemporary medical therapy with or without prompt revascularization: a report from the BARI 2D Trial (Bypass Angioplasty Revascularization Investigation 2 Diabetes). J Am Coll Cardiol 2013; 61: 1767–1776. [DOI] [PubMed] [Google Scholar]
- 10. Dagenais GR, Lu J, Faxon DP, et al. Prognostic impact of the presence and absence of angina on mortality and cardiovascular outcomes in patients with type 2 diabetes and stable coronary artery disease: results from the BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial. J Am Coll Cardiol 2013; 61: 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kahan T, Forslund L, Held C, et al. Risk prediction in stable angina pectoris. Eur J Clin Invest 2013; 43: 141–151. [DOI] [PubMed] [Google Scholar]
- 12. Gandhi MM, Lampe FC, Wood DA. Incidence, clinical characteristics, and short-term prognosis of angina pectoris. Br Heart J 1995; 73: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1: 263–276. [DOI] [PubMed] [Google Scholar]
- 14. Bland RC, Newman SC. Mild dementia or cognitive impairment: the modified mini-mental state examination (3MS) as a screen for dementia. Can J Psychiatry 2001; 46: 506–510. [DOI] [PubMed] [Google Scholar]
- 15. Fitti JE, Kovar MG. The supplement on aging to the 1984 National Health Interview Survey. Vital Health Stat January 1987; 21: 1–115. [PubMed] [Google Scholar]
- 16. Fried LP, Ettinger WH, Lind B, et al. Physical disability in older adults: a physiological approach. J Clin Epidemiol 1994; 47: 747–760. [DOI] [PubMed] [Google Scholar]
- 17. Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. Ann Epidemiol 1995; 5: 278–285. [DOI] [PubMed] [Google Scholar]
- 18. SAS Institute Inc. Base SAS 9.3 procedures guide: statistical procedures. Cary, NC: SAS Institute Inc, 2011. [Google Scholar]
- 19. Will JC, Yuan K, Ford E. National trends in the prevalence and medical history of angina: 1988 to 2012. Circ Cardiovasc Qual Outcomes 2014; 7: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sorlie PD, Cooper L, Schreiner PJ, et al. Repeatability and validity of the Rose questionnaire for angina pectoris in the Atherosclerosis Risk in Communities Study. J Clin Epidemiol 1996; 49: 719–725. [DOI] [PubMed] [Google Scholar]
- 21. Roche MM, Wang PP. Sex differences in all-cause and cardiovascular mortality, hospitalization for individuals with and without diabetes, and patients with diabetes diagnosed early and late. Diabetes Care 2013; 36: 2582–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banks A, Broderick S, Chiswell K, et al. Comparison of clinical characteristics and outcomes of patients with versus without diabetes mellitus and with versus without angina pectoris (from the Duke Databank for Cardiovascular Disease). Am J Cardiol 2017; 119: 1703–1709. [DOI] [PubMed] [Google Scholar]
- 23. Zellweger MJ, Hachamovitch R, Kang X, et al. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J 2004; 25: 543–550. [DOI] [PubMed] [Google Scholar]
- 24. Carpiuc KT, Wingard DL, Kritz-Silverstein D, et al. The association of angina pectoris with heart disease mortality among men and women by diabetes status: the Rancho Bernardo Study. J Womens Health (Larchmt) 2010; 19: 1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc 2005; 53: 1101–1107. [DOI] [PubMed] [Google Scholar]
- 26. Elkins JS, Knopman DS, Yaffe K, et al. Cognitive function predicts first-time stroke and heart disease. Neurology 2005; 64: 1750–1755. [DOI] [PubMed] [Google Scholar]
- 27. Leng X, Espeland MA, Manson JE, et al. Cognitive function and changes in cognitive function as predictors of incident cardiovascular disease: the Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci 2018; 73: 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol 1995; 5: 270–277. [DOI] [PubMed] [Google Scholar]