Abstract
Activation of the prostaglandin E2 receptor EP4 alters polarization of adipose tissue macrophages towards the anti-inflammatory M2 phenotype to suppress chronic inflammation. However, the role of EP4 signalling in pancreatic macrophages that affect insulin secretion is unclear. We examined the role of EP4 signalling in islet inflammation in vitro and in vivo. Obese diabetic db/db mice were treated with an EP4-selective agonist or vehicle for 4 weeks. Islet morphology did not significantly differ and glucose-stimulated insulin secretion was increased, whereas the pancreatic M1/M2 ratio was decreased in the EP4 agonist-treated group compared to the vehicle group. Because EP4 activation in MIN6 cells did not affect insulin secretion, we used a MIN6/macrophage co-culture system to evaluate the role of EP4 signalling in islet inflammation and subsequent inhibition of insulin release. Co-culture with M1-polarized macrophages markedly suppressed insulin expression in MIN6 cells; however, modulation of M1 polarization by the EP4 agonist significantly reversed the negative impact of co-cultivation on insulin production. The enhanced expression levels of pro-inflammatory cytokines in co-cultured MIN6 cells were markedly inhibited by EP4 agonist treatment of M1 macrophages. Thus, EP4 activation may suppress islet inflammation and protect β-cell function by altering inflammatory macrophages in the diabetic pancreas.
Keywords: Prostaglandin E2, EP4 receptor, islet inflammation, macrophage, glucose-stimulated insulin secretion, type 2 diabetes mellitus
Introduction
Macrophages invade pancreatic islets and activated pancreatic macrophages cause islet inflammation and β-cell dysfunction.1,2 Macrophages exhibit two main phenotypes: the ‘pro-inflammatory M1’ and ‘anti-inflammatory M2’ macrophage. Numerous studies have shown that macrophages can switch from the M1 to the M2 phenotype to improve insulin sensitivity.3
Prostaglandin E2 (PGE2) is a major prostanoid and has four receptors, EP1–EP4. It has been suggested that PGE2 can suppress the pro-inflammatory activation of macro-phages via EP4-receptor signalling.4 Previously we showed that EP4 activation suppresses obesity-related adipose tissue inflammation and increases insulin sensitivity. Besides, EP4 agonist treatment promoted the polarization of M2 macrophage both in vivo and in vitro.5
This study was conducted to evaluate whether activation of EP4 signalling mitigates pancreatic inflammation and affect β-cell function in type 2 diabetes mellitus (T2DM). The results obtained revealed that EP4 activation increases glucose-stimulated insulin secretion (GSIS) from β-cells by suppressing inflammatory activation of macrophages in diabetic pancreas.
Methods
Animal experimental protocol
ONO-AE1-329 (Ono Pharmaceutical, Osaka, Japan), an EP4-selective agonist, was suspended in the vehicle (0.3% ethanol and 0.1% Tween 80 in phosphate-buffered saline (PBS)), and 0.3 mg/kg of the reagent or vehicle was administered subcutaneously to 7-week-old db/db mice (Oriental BioService, Kyoto, Japan), twice a day for 4 weeks. All animal experiments were conducted according to the guidelines for animal experiments at Kyoto University.
Cell culture
A pancreatic β-cell line, MIN6, was maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 100 µg/mL ampicillin, 100 µg/mL streptomycin and 10% foetal bovine serum.
Peritoneal macrophages were extracted from the peritoneal lavage of 12- to 15-week-old ddY mice (Shimizu Laboratory Supplies Co., Ltd., Kyoto, Japan). The induction of M1/M2 macrophage polarization was performed as described previously.6 Briefly, together with 1 μM ONO-AE1-329 or vehicle, peritoneal macrophages were treated with 1 μg/mL of lipopolysaccharide (LPS) (Calbiochem, La Jolla, CA, USA) or 20 ng/mL of interleukin (IL)-4 and IL-13 (R&D Systems, Minneapolis, MN, USA) for 8 h.
For non-contact co-culture of pancreatic β-cells and macrophages, we used Transwell Permeable Supports (Corning, Inc., Corning, NY, USA). The details are described in the Supplemental Materials.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from the pancreas, MIN6 cells, and peritoneal macrophages using the RNeasy Mini kit (Qiagen, Hilden, Germany). Subsequently, we generated first-strand cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). All experiments were performed in duplicate, and the results obtained were normalized to the β-actin expression levels. The primers used for amplification are described in the Supplemental Materials (Table S1).
Statistical analysis
Data are presented as the mean scores ± standard error of the mean (mean ± SEM) for all groups. Differences between two value sets were determined using the unpaired, two-tailed Student’s t test. A one-way analysis of variance, followed by Tukey–Kramer analysis, was used for multiple comparisons between groups. For all comparisons, significance was defined as a p value less than 0.05.
Results
EP4 activation increased GSIS in diabetic db/db mice
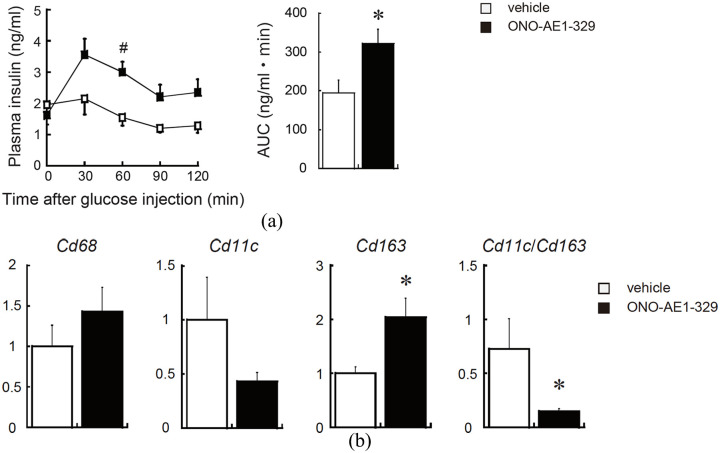
To clarify the role of EP4 signalling in β-cell function, we treated db/db mice with the EP4-selective agonist or vehicle. After 4 weeks, we observed no significant difference in the body weight between both groups. However, glucose tolerance was improved and GSIS was enhanced in the EP4 agonist-treated group compared to the control (Figure 1(a)).
Figure 1.
EP4 activation increases glucose-stimulated insulin secretion and alters the polarization of pancreatic macrophages in obese diabetic mice. (a) After intraperitoneally injecting glucose (1.5 g/kg of d-glucose) into the mice, blood was drawn every 30 min, and the plasma insulin levels (ng/mL) were measured. Net area under the curve (AUC, mg/dL) was calculated from the data shown in the graph (right panel) and (b) expression of macrophage (Cd68), M1-type (Cd11c) and M2-type (Cd163) mRNA levels in the pancreas of db/db mice was analysed by quantitative real-time PCR. All values are the mean scores ± SEM (n = 5–6 for each group). *p < 0.05; #p < 0.01.
Immunofluorescence analyses revealed no significant difference in the insulin-positive area and islet size between the two groups (Figure S1). However, the mRNA level of Cd163 (a marker of M2 macrophages) and ratios of Cd11c (a marker of M1 macrophages) to Cd163 were significantly increased in the pancreas from db/db mice treated with the EP4-selective agonist, suggesting that EP4 activation shifted the polarization of pancreatic macrophages from the M1 to the M2 status (Figure 1(b)).
EP4 activation caused functional changes in M1 macrophages and recovered GSIS from β-cells
To investigate the direct effect of EP4 signalling on insulin secretion from pancreatic β-cells, we incubated MIN6 cells with an EP4 agonist. However, EP4 activation does not alter glucose-induced insulin secretion in MIN6 cells (Figure S2).
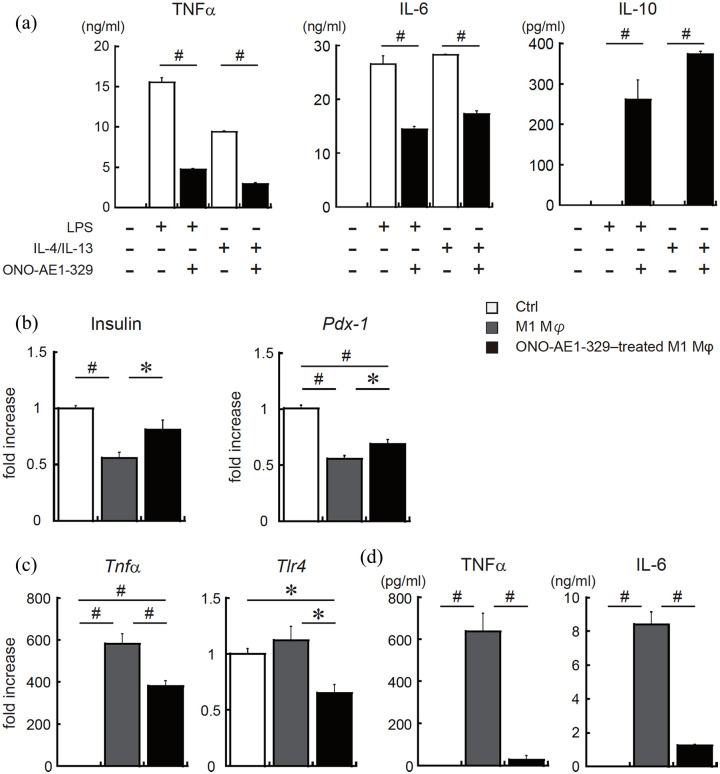
Next, we performed in vitro M1/M2 polarization assay in primary mouse macrophages with or without EP4 agonist treatment. Both in M1 and M2 macrophages, EP4 activation significantly suppressed the production of tumour necrosis factor alpha (TNFα) and IL-6 compared to the control. Notably, EP4 agonist-treated M1 macrophages can produce considerable amount of IL-10 (Figure 2(a)). These results indicate that EP4 activation not only causes a shift in polarization but also functional changes in pancreatic macrophages.
Figure 2.
EP4 receptor-activation in M1 macrophages inhibits inflammatory activation and recovers impaired insulin production of pancreatic β-cells. (a) Peritoneal macrophages from wild type (WT) mice were incubated with 1 μg/mL of LPS or with 20 ng/mL of IL-4 and IL-13, as well as with 1 μM of EP4 agonist (ONO-AE1-329) or vehicle. After 8 h, the secretion of TNFα, IL-6 and IL-10 was measured. (b)–(d) MIN6 cells were co-cultured with M1-polarized peritoneal macrophages with or without EP4 agonist. After 14 h of incubation, macrophages were removed and MIN6 cells were incubated for another 24 h. The mRNA levels of (b) insulin, Pdx1, (c) TNFα, TLR4 in MIN6 cells and the protein levels of (d) TNFα and IL-6 in culture supernatant were measured. All values are the mean scores ± SEM (n = 6 for each condition). *p < 0.05; #p < 0.01 versus control.
To investigate the correlation between pancreatic macrophages and β-cell function, MIN6 cells were incubated with M1 macrophages with or without EP4 agonist treatment using non-contact co-culture system. The results showed that, in pancreatic β-cells, gene expressions of insulin and pancreas–duodenum homeobox-1 (Pdx1), a key transcription factor for pancreatic development and β-cell function, was improved in the EP4 receptor-activated M1 macrophage co-culture group compared to in the vehicle-treated M1 macrophage co-culture group (Figure 2(b)).
Furthermore, the mRNA expression of TNFα and TLR4 was significantly decreased in MIN6 cells incubated with EP4 receptor-activated M1 macrophages (Figure 2(c)). Accordingly, secretion of TNFα and IL-6 protein markedly reduced in MIN6 cells co-cultured with EP4 agonist–treated macrophages (Figure 2(d)). These results suggest that activation of EP4 signalling affects the function of M1 macrophages in the pancreas and can lead to reduced islet inflammation.
Discussion
In this study, administration of an EP4-specific agonist did not affect pancreatic islet morphology in vivo and did not directly regulate GSIS in the β-cells in vitro, suggesting that EP4 signalling maintains pancreatic function in an indirect manner, such as by controlling the local micro-environment.
As in adipose tissue, macrophages infiltrate into the pancreatic islets and switch from the anti-inflammatory (M2) to the pro-inflammatory (M1) type, causing β-cell dysfunction and type 2 diabetes.7 Previously, we demonstrated that activation of EP4 signalling increases the susceptibility to M2 macrophage polarization in obese adipose tissue probably via the peroxisome proliferator–activated receptor (PPAR)γ and the PPARδ pathways.5 The EP4 and PPARs pathways may participate significantly in macrophage polarization and islet inflammation in obese islets, because numerous studies have shown that PPARs are the major transcriptional factors involved in regulating macrophage polarization and insulin resistance.8,9
Our non-contact co-culture of pancreatic β-cells and macrophages revealed that pretreatment of M1-polarized macrophages with an EP4 receptor agonist suppressed pro-inflammatory activation of β-cells. Thus, EP4 receptor activation in M1 macrophages can indirectly inhibit inflammatory activation of pancreatic β-cells, and certain humoral factors may mediate mitigating inflammatory activation of pancreatic β-cells. Regarding the anti-inflammatory mediator, in this study, EP4 signalling strongly enhanced the secretion of the anti-inflammatory cytokine IL-10, in both M1 and M2 macrophages. Indeed, overexpression of IL-10 has been shown to protect β-cells from cytokine-induced dysfunction and apoptosis.10
In summary, administration of an EP4-selective agonist enhanced GSIS in mice with T2DM, at least in part by enhancing macrophage polarization towards the M2 subtype and by inhibiting the inflammatory activation of M1 macrophages. Our findings indicate that the EP4 agonist negatively regulates immune inflammation in obese islets and is a good therapeutic drug target for T2DM and obesity.
Key messages.
EP4 activation increases glucose-stimulated insulin secretion in diabetic mice.
EP4 signalling shifted the polarization of pancreatic macrophages from the M1 to the M2 status in diabetic mice.
EP4 activation mitigates inflammatory activation of M1 macrophages infiltrated into the diabetic pancreas and can lead to reduced islet inflammation.
Supplemental Material
Supplemental material, Supplemental_Materials_DVDRes-Mar-2020-00026.R1_20200705 for EP4 signalling is essential for controlling islet inflammation by causing a shift in macrophage polarization in obesity/type 2 diabetes by Mika Yasui-Kato, Suthomwong Patlada, Masayuki Yokode, Kaeko Kamei and Manabu Minami in Diabetes & Vascular Disease Research
Acknowledgments
The authors thank Ono Pharmaceutical for supplying the EP4 agonist, ONO-AE1-329.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported in part by the grants from the Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research (grant numbers 26460338, 15K08230 and 17K08592), Takeda Science Foundation and SENSHIN Medical Research Foundation (to M.M.).
ORCID iD: Manabu Minami  https://orcid.org/0000-0003-0052-398X
https://orcid.org/0000-0003-0052-398X
Supplemental material: Supplemental material for this article is available online.
References
- 1. Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab 2012; 15: 518–533. [DOI] [PubMed] [Google Scholar]
- 2. Cucak H, Grunnet LG, Rosendahl A. Accumulation of M1-like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. J Leukoc Biol 2014; 95: 149–160. [DOI] [PubMed] [Google Scholar]
- 3. Fujisaka S, Usui I, Bukhari A, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009; 58: 2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minami M, Shimizu K, Okamoto Y, et al. Prostaglandin E receptor type 4-associated protein interacts directly with NF-kappaB1 and attenuates macrophage activation. J Biol Chem 2008; 283: 9692–9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yasui M, Tamura Y, Minami M, et al. The prostaglandin E2 receptor EP4 regulates obesity-related inflammation and insulin sensitivity. PLoS ONE 2015; 10: e0136304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakanishi Y, Nakatsuji M, Seno H, et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis 2011; 32: 1333–1339. [DOI] [PubMed] [Google Scholar]
- 7. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246. [DOI] [PubMed] [Google Scholar]
- 8. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPAR gamma controls alternative activation and improves insulin resistance. Nature 2007; 447: 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab 2008; 7: 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu A-J, Zhu W, Tian F, et al. Recombinant adenoviral expression of IL-10 protects beta cell from impairment induced by pro-inflammatory cytokine. Mol Cell Biochem 2010; 344: 163–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Materials_DVDRes-Mar-2020-00026.R1_20200705 for EP4 signalling is essential for controlling islet inflammation by causing a shift in macrophage polarization in obesity/type 2 diabetes by Mika Yasui-Kato, Suthomwong Patlada, Masayuki Yokode, Kaeko Kamei and Manabu Minami in Diabetes & Vascular Disease Research