Abstract
Our aim was to examine the seasonal variations in home blood pressure measurements and the relationship of ambient temperature or room temperature with the seasonal variations in home blood pressure measurements using a home blood pressure telemonitoring system in patients with type 2 diabetes. The home blood pressure measurements of 41 patients with type 2 diabetes were self-measured. Patients performed triplicate morning and evening blood pressure measurements at least 5 days per month for 12 consecutive months. The lowest values of both systolic blood pressure and diastolic blood pressure were observed in August (126.3 and 70.4 mmHg, respectively), and the highest systolic and diastolic blood pressure values were observed in January (140.3 and 76.9 mmHg, respectively). The root mean squared error between the mean systolic blood pressure and room temperature was 6.50 mmHg and between mean systolic blood pressure and ambient temperature was 6.55 mmHg. Using a home blood pressure telemonitoring system, this study revealed for the first time that home blood pressure varied seasonally, with the highest values observed in January and the lowest values observed in August, and that the seasonal variations in home blood pressure were related to room temperature as well as ambient temperature.
Keywords: Room temperature, seasonal variation in home blood pressure, type 2 diabetes, telemedicine system
Introduction
In epidemiological studies, a high prevalence of concomitant diabetes and hypertension, which have insulin resistance as a common factor,1 has been observed. Thus, it is important to control blood pressure (BP) as well as blood glucose for the prevention of target organ damage in patients with diabetes.2 With respect to BP control, the self-measurement of BP at home (HBP) has been shown to have better prognostic value for predicting mortality and cardiovascular events than BP measured in the clinic.3–5 Moreover, several studies have reported that BP measured in the morning provided more prognostic power than that measured in the evening.6
Previous studies of the relationship between seasonality and HBP have shown winter peaks and summer nadirs in HBP. This phenomenon is largely attributable to the change in ambient temperature.7–9 On the other hand, room temperature also affects seasonal variations in HBP, although it has less seasonal change than the ambient temperature.10 However, no study has investigated whether ambient temperature or room temperature is more related to seasonal variations in HBP in patients with diabetes. Thus, we aimed to examine the seasonal variations in morning HBP and the relationship of ambient temperature or room temperature with seasonal variations in morning HBP in patients with type 2 diabetes.
Methods
Study patients
We sequentially recruited 132 patients with type 2 diabetes who regularly attended the diabetes outpatient clinic at the Hospital of the Kyoto Prefectural University of Medicine from 2013 to 2015. The inclusion criteria were as follows: 40–80 years of age and type 2 diabetes. No BP-level criterion was used for study inclusion. The exclusion criteria were as follows: secondary hypertension or malignant hypertension, history of myocardial infarction, cerebrovascular disease or hospitalization for angina pectoris within 6 months prior to inclusion, changes in antihypertensive medication and/or antidiabetic medication within 1 month prior to inclusion, advanced renal dysfunction (serum creatinine equal to or more than 2.0 mg/dL or current treatment by dialysis), atrial fibrillation or severe arrhythmia, life-threatening conditions such as malignant tumours or judgement from a supervising physician to be an unsuitable study patient. We could not determine the sample size before the study because previous reports were not available regarding the relationship between room temperature and seasonal variation in HBP in patients with type 2 diabetes. The diagnosis of type 2 diabetes was based on the American Diabetes Association criteria.11 All procedures were approved by the local research ethics committee and were conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all patients (RBMR-E-349-4).
HBP monitoring
HBP was self-measured using an HEM-7251G automated BP monitor (Omron Healthcare Co., Ltd, Kyoto, Japan), which uses the cuff-oscillometric method to measure systolic BP (SBP) or diastolic BP (DBP). The HEM-7251G was previously validated and satisfied the criteria of the European Society of Hypertension protocol.12 The device is capable of automatically transmitting measurement results, including room temperature at the time of measurement, immediately after each measurement via a mobile phone line to the server of the Medical LINK® (Omron Healthcare) BP management system, saving the patients the trouble of record-taking and automatically providing reliable data for physicians. The HBP readings were visible to the patients. The patients were instructed to perform triplicate morning BP measurements, with at least 1 min between recordings, at least 5 days per week during the study period. The patients were also instructed to perform the morning BP measurements within 1 h of waking up, before eating breakfast or taking any drugs, and after sitting and resting for at least 5 min.13 The cuff was placed around the non-dominant arm, and the position of the cuff was maintained at the level of the heart. The patients used a proper cuff size based on their arm circumference. We calculated the means of the three morning BP measurements each day. The morning HBP values for a month were defined as the average of the morning HBP measurements taken on the 5 measurement days of the month.
Data collection
Blood samples were collected in the morning for biochemical measurements at the time of study entry. Haemoglobin A1C, serum lipid profile (total cholesterol, low-density lipoprotein cholesterol, triglycerides and high-density lipoprotein cholesterol), and other biochemical data were determined using standard laboratory measurements. Urinary albumin excretion (UAE) was measured with an immunoturbidimetric assay. Haemoglobin A1C was expressed as a National Glycohemoglobin Standardization Program unit. Each patient’s data, including age, duration of diabetes, smoking and alcohol consumption status (assessed by an interview), and antihypertensive medication use were assessed concurrently with the HBP measurements. Retinopathy was assessed from chart reviews and was graded as follows: no diabetic retinopathy (NDR), simple diabetic retinopathy (SDR) and proliferative diabetic retinopathy (PDR). Nephropathy was graded into three stages depending on UAE as follows: normoalbuminuria, UAE less than 30 mg/g Cr; microalbuminuria, 30–300 mg/g Cr; or macroalbuminuria, greater than 300 mg/g Cr. Neuropathy was defined as the diagnostic criterion for diabetic neuropathy proposed by the Diagnostic Neuropathy Study Group.14 Briefly, in the absence of peripheral neuropathies, other than diabetic neuropathy in patients with diabetes, diabetic neuropathy was diagnosed when there were two or more abnormalities in the following three neurological examinations: sensory symptoms, decreased or absent ankle reflex (bilateral) and decreased vibratory sensation on bilateral medial malleoli evaluated using a c128-Hz tuning fork. A macrovascular complication was defined as the presence of a previous cardiovascular disease, cerebrovascular disease or arteriosclerosis obliterans based on the clinical history of the patient or a physical examination. The mean monthly ambient temperature data were obtained from the Japan Meteorological Agency.15 The mean room temperature was obtained from the server of the Medical LINK® (Omron Healthcare) BP management system described above.
Statistical analysis
The baseline characteristics are summarized by median and interquartile ranges for continuous variables and as numbers for categorical variables. Pearson’s correlation analysis was used to investigate the relationship between morning HBP and room temperature or ambient temperature. The prediction models were constructed by a generalized linear model of BP by either room temperature or ambient temperature. We built unadjusted and adjusted models that included haemoglobin A1C, sex, age, body mass index, smoking status, drinking status and antihypertensive medication. Because the BP data were from the same patient, both models included the subject-specific factor as a fixed effect. To reveal the predictive ability, we calculated the root mean squared error (RMSE) in each model. The RMSE was defined as the square root of the mean squared difference between actual BP and predicted BP. A smaller RMSE indicates a better predictive ability. The SPSS statistical package, version 19.0J (SPSS, Inc., Chicago, IL, United States) and JMP software version 13.2.0 (SAS Institute Inc., Cary, NC, United States) were used for all statistical analyses. All tests were two-sided, and p values <0.05 were considered statistically significant.
Results
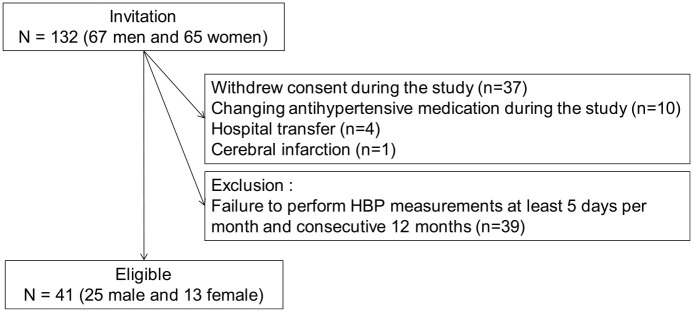
Of the 132 patients, 52 patients were excluded from the study primarily due to withdrawal of their consent for the following reasons: uncomfortable and/or troublesome feelings regarding transmitting their results automatically after each measurement to the server of the BP management system, changing antihypertensive medication, hospital transfer and cerebral infarction (Figure 1). Consequently, 80 patients remained enrolled in the study. Among these 80 patients, 41 (25 male and 16 female) patients who performed triplicate morning HBP measurements at least 5 days per month for 12 consecutive months composed the study population.
Figure 1.
Study flow diagram for the enrolment of patients.
The baseline characteristics at study entry are shown in Table 1. The median (interquartile range) age and median haemoglobin A1C were 73.0 years (67.5–76.0 years) and 6.8% (6.4%–7.4%), respectively.
Table 1.
Baseline characteristics of the patients.
| Variables | n = 41 |
|---|---|
| Sex | |
| Male | 24 (58.5) |
| Female | 17 (41.5) |
| Age (years) | 73.0 (67.5–76.0) |
| Duration of diabetes mellitus (years) | 13.0 (9.0–24.0) |
| Body mass index (kg/m2) | 22.5 (20.8–23.8) |
| Haemoglobin A1C (%) | 6.8 (6.4–7.4) |
| Haemoglobin A1C (mmol/mol) | 52.8 (48.3–60.6) |
| Total cholesterol (mmol/L) | 4.5 (4.2–5.2) |
| Triglycerides (mmol/L) | 1.3 (0.9–1.8) |
| Creatinine (mg/dL) | 0.7 (0.6–0.9) |
| eGFR (mL/min/1.73 m2) | 68.8 (59.5–83.1) |
| Smoking status | |
| Current smoker | 5 (12.2) |
| Past smoker | 19 (46.3) |
| Alcohol consumption status | |
| Daily | 8 (19.5) |
| Social | 10 (24.4) |
| Diabetes complications | |
| Nephropathy (microalbuminuria) | 13 (31.7) |
| Nephropathy (macroalbuminuria) | 5 (12.2) |
| Retinopathy | 10 (24.4) |
| Neuropathy | 17 (41.5) |
| Macrovascular complication | 10 (24.4) |
| Hypoglycaemic treatment (diet/OHA/GLP-1 receptor agonist/insulin) | 4/36/1/12 |
| Use of antihypertensive medication | 26 (63.4) |
| RAS inhibitors/CCB/diuretics/others | 24/14/4/3 |
eGFR: estimated glomerular filtration rate; NDR: no diabetic retinopathy; SDR: simple diabetic retinopathy; PDR: proliferative diabetic retinopathy; OHA: oral hypoglycaemic agent; GLP: glucagon-like peptide; RAS: renin–angiotensin–aldosterone system; CCB: calcium channel blocker.
For categorical variables, n (%) is presented. For continuous variables, the median (interquartile range) is presented.
The variations in morning HBP of all patients in this study are shown in Figure 2 according to the measurement month. Both SBP and DBP showed apparent seasonal variations. After March, morning HBP showed a noticeable decrease and reached its nadir in August; after August, morning HBP increased continuously and reached its peak in January. The lowest value of both SBP and DBP was observed in August (126.3 and 70.4 mmHg, respectively) and the highest values were observed in January (140.3 and 76.9 mmHg, respectively). The mean monthly ambient temperature in Japan was highest in August (28.4°C) and lowest in January (5.0°C) [Figure 3(a)]. The mean monthly room temperature was highest in August (28.2°C) and lowest in January (13.2°C) [Figure 3(b)].
Figure 2.
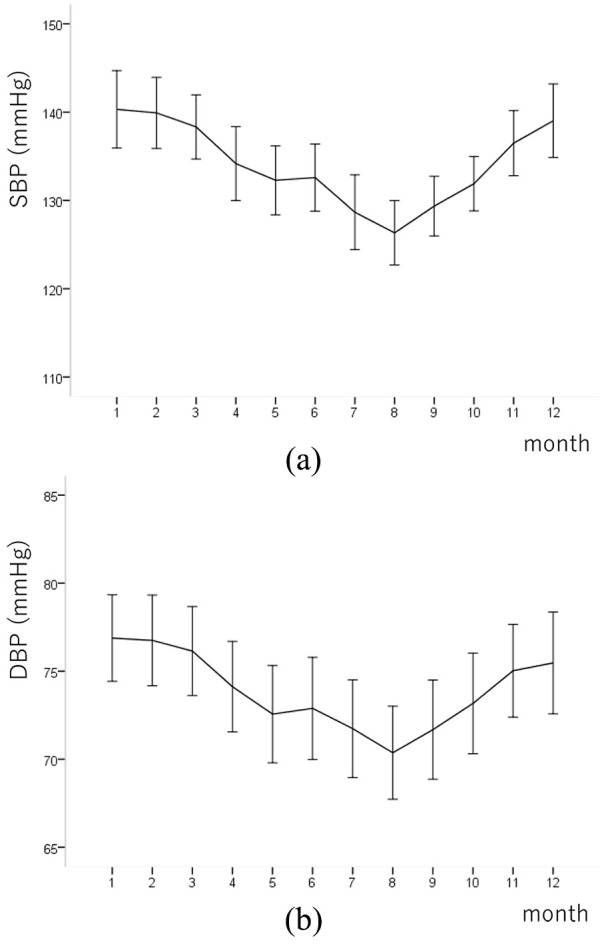
Seasonal variation in the (a) systolic and (b) diastolic blood pressure of the patients. Values are expressed as the means ± SDs.
Figure 3.
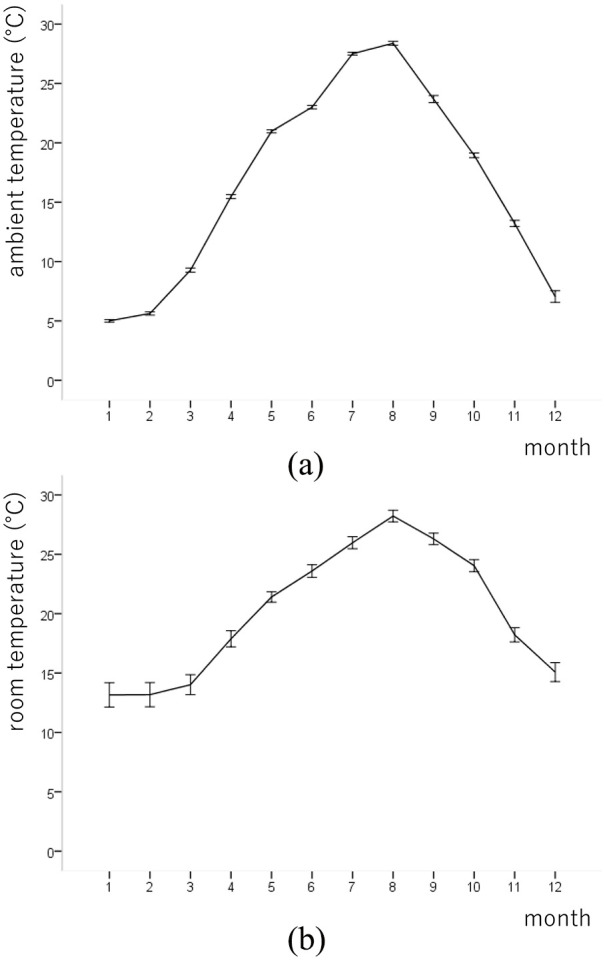
Monthly average (a) ambient and (b) room temperature of the locations of the patients. Values are expressed as the means ± SDs.
In Pearson’s correlation analysis, mean SBP was associated with room temperature (r = −0.335, p < 0.001) and ambient temperature (r = −0.344, p < 0.001) and mean DBP was associated with room temperature (r = −0.160, p < 0.001) and ambient temperature (r = −0.230, p < 0.001). The RMSE and mean difference (95% confidence interval) between mean SBP and room temperature were 6.24 mmHg and −0.813 (−0.917, −0.709), respectively, and between mean SBP and ambient temperature were 6.29 mmHg and −0.547 (−0.618, −0.475), respectively. In analyses adjusted for various potential confounders, including haemoglobin A1C, sex, age, body mass index, smoking status, drinking status and antihypertensive medication, the RMSE and mean difference (95% confidence interval) between mean SBP and room temperature were 6.11 mmHg and −0.812 (−0.914, −0.710), respectively, and between mean SBP and ambient temperature were 6.14 mmHg and −0.547 (−0.617, −0.477), respectively.
Discussion
Using an HBP telemonitoring system for patients with type 2 diabetes, this study revealed that morning HBP varied seasonally, with the highest values in January and the lowest values in August. This study also revealed that the seasonal variation in morning HBP was related to room temperature as well as ambient temperature. To our knowledge, this study is the first to examine the relationship between seasonal changes in morning HBP and room temperature or ambient temperature in patients with type 2 diabetes. Few studies have shown seasonal variations in clinic-measured BP in patients with diabetes, observing higher values in the winter months and lower values in the summer months.9,16 These studies also demonstrated that both SBP and DBP were inversely correlated with the mean monthly ambient temperature, resulting in higher BP values in the winter. On the other hand, Hattori and Munakata17 examined the seasonal changes in clinic-measured BP in 104 untreated men at a standardized room temperature and suggested that typical seasonal BP changes could be masked in cases of mildly elevated BP measured at a standardized indoor temperature. Conversely, the availability of indoor temperature control at home or in the working place has been shown to significantly attenuate the increase in BP from summer to winter.18 Regarding seasonal variations in HBP, Stergiou et al. investigated seasonal BP changes by assessing HBP measurements in patients with treated hypertension in relation to changes in several meteorological parameters and weather-induced discomfort.19 They showed that HBP was lower in the summer than in the winter and that seasonal changes in temperature and meteorological parameters that reflected weather-induced patient discomfort were correlated with HBP changes.
Although seasonal BP variations have been described in previous studies, the mechanisms regarding the effects of meteorological parameters are not fully understood. The effect of ambient temperature on the sympathetic nervous system has been studied.20 Sympathetic tone, which increases both heart rate and BP, increases in the cold. Several previous studies also demonstrated the simultaneous elevation of BP and plasma noradrenaline concentrations in response to exposure to cold.21,22 Increased seasonal BP variations are also attributed to impaired baroreflex control and enhanced vasoreactivity, which are associated with advanced age.23 In the summer, the warm weather induces vasodilatation and decreases peripheral vascular resistance, thereby reducing BP.24 Moreover, a previous study of 20 individuals with hypertension suggested that an increased sodium load in the kidneys, assessed by urinary sodium, may be a factor that contributes to the rise in BP in the winter in patients with essential hypertension.25
In our study, the summer–winter difference in morning HBP was 14.0/6.5 mmHg. Hanazawa et al.26 showed that small-to-moderate seasonal variations in HBP (0–9.1/0–4.5 mmHg) were associated with better cardiovascular outcomes and that earlier adjustments of antihypertensive medications according to the coming season reduced the summer–winter difference in HBP. Thus, the early adjustment of antihypertensive medication may also contribute to better outcomes in patients with type 2 diabetes.
We aimed to investigate whether ambient temperature or room temperature is more related to seasonal variations in HBP by constructing the prediction models. In adjusted models, the RMSE between mean SBP and room temperature was 6.11 mmHg and between mean SBP and ambient temperature was 6.14 mmHg. The difference of RMSE is only 0.03 mmHg. Thus, predictive ability would not differ between room temperature and ambient temperature in this study.
The strengths of the present study are as follows: the same patients were included in all seasonal assessments and our use of the HBP telemonitoring system, which transmitted measurement results including BP and room temperature automatically and immediately to the BP management system server, provided reliable aggregated data rather than trusting poorly recorded patient logbooks.27
This study has several limitations. First, the small sample size limits the statistical power. However, we included the same patients in all seasonal assessments mentioned above and could detect a statistically significant relationship between seasonal changes in morning HBP and room temperature or ambient temperature. Second, a previous study showed that the magnitude of the seasonal BP increase from summer to winter may be affected by an increase in salt intake and a reduction in physical activity and vegetable intake.28 However, we do not have data from the patients related to these factors. Third, the generalizability of our study results to other ethnic populations is uncertain. Fourth, the ambient temperature data reflected the mean monthly ambient temperature in the nearest area, which was obtained from the Japan Meteorological Agency. Thus, the ambient temperature data were not perfect measurements of the ambient temperature at the time of HBP measurements or of the temperature to which the individual had recently been exposed. Therefore, it is difficult to compare the relationship of the exact ambient temperature or room temperature with the seasonal variation in HBP. Finally, the devise used for the HBP measurements in this study was not validated in patients with diabetes. Hyperglycaemia is known to affect large arteries, changing the physical properties of the arterial wall, which may invalidate the results of the BP measurements, especially the oscillometric measurements. This possibility means that the devices used for BP measurement in patients with diabetes should be validated by studying patients with diabetes. However, the device was validated in patients with stage 3–5 chronic kidney disease, which is also known to affect macroangiopathy.29 Thus, it is expected that the systematic error due to altered arterial wall properties was substantially small.
In conclusion, this study suggested that morning HBP varied seasonally in patients with type 2 diabetes. Morning HBP values were inversely correlated with room temperature and ambient temperature. The results might also help clinicians decide the therapeutic approach for patients with type 2 diabetes, taking seasonal HBP variation into account.
Acknowledgments
We would like to thank Naoko Higo, Machiko Hasegawa and Terumi Kaneko at the Kyoto Prefectural University of Medicine for teaching the patients how to measure their BP and Sayoko Tanaka, also at the Kyoto Prefectural University of Medicine, for her secretarial assistance. We would like to thank Editage (www.editage.com) for the English language editing.
Footnotes
Research ethics and patient consent: This study was performed with the approval of the local research ethics committee and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all the participants (RBMR-E-349-4).
Declaration of conflicting interests: Masahiro Yamazaki received personal fees from AstraZeneca plc, which were unrelated to the submitted work. Michiaki Fukui received grants from the Japan Society for the Promotion of Science; AstraZeneca plc; Astellas Pharma Inc.; Nippon Boehringer Ingelheim Co., Ltd.; Daiichi Sankyo Co., Ltd.; Eli Lilly Japan K.K.; Kyowa Hakko Kirin Company Ltd.; Kissei Pharmaceutical Co., Ltd.; MSD K.K.; Mitsubishi Tanabe Pharma Corporation; Novo Nordisk Pharma Ltd.; Sanwa Kagaku Kenkyusho Co., Ltd.; Sanofi K.K.; Ono Pharmaceutical Co., Ltd.; and Takeda Pharmaceutical Co., Ltd., which were unrelated to the submitted work. The sponsors were not involved in the study design; in the collection, analysis or interpretation of the data; in the writing of this article; or in the decision to submit the article for publication. The authors, their immediate families and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article. The authors declare that although they are affiliated with a department that is supported financially by a pharmaceutical company, the authors received no current funding for this study, and their department affiliation does not alter their adherence to all the full journal policies on sharing data and materials.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: E.U. received grant support from the Japanese Study Group for Physiology and Management of Blood Pressure and the Astellas Foundation for Research on Metabolic Disorders (grant number: 4024).
ORCID iDs: Emi Ushigome  https://orcid.org/0000-0003-1031-4380
https://orcid.org/0000-0003-1031-4380
Nobuko Kitagawa  https://orcid.org/0000-0001-6624-0101
https://orcid.org/0000-0001-6624-0101
References
- 1. Iimura O. Insulin resistance and hypertension in Japanese. Hypertens Res 1996; 19: 1–8. [DOI] [PubMed] [Google Scholar]
- 2. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 3. Niiranen TJ, Hänninen M-R, Johansson J, et al. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension 2010; 55: 1346–1351. [DOI] [PubMed] [Google Scholar]
- 4. Ushigome E, Oyabu C, Tanaka T, et al. Impact of masked hypertension on diabetic nephropathy in patients with type II diabetes: a KAMOGAWA-HBP study. J Am Soc Hypertens 2018; 12: 364–371.e1. [DOI] [PubMed] [Google Scholar]
- 5. Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens 1998; 16: 971–975. [DOI] [PubMed] [Google Scholar]
- 6. Hoshide S, Yano Y, Haimoto H, et al. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese general practice population: The Japan Morning Surge-Home Blood Pressure Study. Hypertension 2016; 68: 54–61. [DOI] [PubMed] [Google Scholar]
- 7. Sega R, Cesana G, Bombelli M, et al. Seasonal variations in home and ambulatory blood pressure in the PAMELA population. Pressione Arteriose Monitorate E Loro Associazioni. J Hypertens 1998; 16: 1585–1592. [DOI] [PubMed] [Google Scholar]
- 8. Iwahori T, Miura K, Obayashi K, et al. Seasonal variation in home blood pressure: findings from nationwide web-based monitoring in Japan. BMJ Open 2018; 8: e017351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hermann JM, Rosenbauer J, Dost A, et al. Seasonal variation in blood pressure in 162,135 patients with Type 1 or Type 2 diabetes mellitus. J Clin Hypertens 2016; 18: 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Modesti PA, Morabito M, Massetti L, et al. Seasonal blood pressure changes: an independent relationship with temperature and daylight hours. Hypertension 2013; 61: 908–914. [DOI] [PubMed] [Google Scholar]
- 11. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003; 26: 5–20. [Google Scholar]
- 12. O’Brien E, Atkins N, Stergiou G, et al. Working group on blood pressure monitoring of the European Society of Hypertension. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit 2010; 15: 23–38. [DOI] [PubMed] [Google Scholar]
- 13. Imai Y, Kario K, Shimada K, et al. ; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese society of hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hyperten Res 2012; 35: 777–795. [DOI] [PubMed] [Google Scholar]
- 14. Yasuda H, Sanada M, Kitada K, et al. Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res Clin Pract 2007; 77: S178–S183. [DOI] [PubMed] [Google Scholar]
- 15.http://www.data.jma.go.jp/obd/stats/etrn/select/prefecture.php (accessed 19 November 2018).
- 16. Liang WW. Seasonal changes in preprandial glucose, A1C, and blood pressure in diabetic patients. Diabetes Care 2007; 30: 2501–2502. [DOI] [PubMed] [Google Scholar]
- 17. Hattori T, Munakata M. Blood pressure measurement under standardized indoor condition may mask seasonal blood pressure variation in men with mildly elevated blood pressure. Clin Exp Hypertens 2015; 37: 317–322. [DOI] [PubMed] [Google Scholar]
- 18. Kristal-Boneh E, Harari G, Green MS, et al. Seasonal changes in ambulatory blood pressure in employees under different indoor temperatures. Occup Environ Med 1995; 52: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stergiou GS, Myrsilidi A, Kollias A, et al. Seasonal variation in meteorological parameters and office, ambulatory and home blood pressure: predicting factors and clinical implications. Hypertens Res 2015; 38: 869–875. [DOI] [PubMed] [Google Scholar]
- 20. Rosenthal T. Seasonal variations in blood pressure. Am J Geriatr Cardiol 2004; 13: 267–272. [DOI] [PubMed] [Google Scholar]
- 21. Winer N, Carter C. Effect of cold pressor stimulation on plasma norepinephrine, dopamine-beta-hydroxylase, and renin activity. Life Sci 1977; 20: 887–893. [DOI] [PubMed] [Google Scholar]
- 22. Palmer GJ, Ziegler MG, Lake CR. Response of norepinephrine and blood pressure to stress increases with age. J Gerontol 1978; 33: 482–487. [DOI] [PubMed] [Google Scholar]
- 23. Brennan PJ, Greenberg G, Miall WE, et al. Seasonal variation in arterial blood pressure. Br Med J 1982; 285: 919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rowell LB. Cardiovascular aspects of human thermoregulation. Circ Res 1983; 52: 367–379. [DOI] [PubMed] [Google Scholar]
- 25. Hata T, Ogihara T, Maruyama A, et al. The seasonal variation of blood pressure in patients with essential hypertension. Clin Exp Hypertens A 1982; 4: 341–354. [DOI] [PubMed] [Google Scholar]
- 26. Hanazawa T, Asayama K, Watabe D, et al. ; HOMED-BP (Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure) Investigators. Association between amplitude of seasonal variation in self-measured home blood pressure and cardiovascular outcomes: HOMED-BP (Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure) Study. J Am Heart Assoc 2018; 7: e008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsumoto S, Fukui M, Hamaguchi M, et al. Is home blood pressure reporting in patients with type 2 diabetes reliable. Hypertens Res 2014; 37: 741–745. [DOI] [PubMed] [Google Scholar]
- 28. Goodwin J, Pearce VR, Taylor RS, et al. Seasonal cold and circadian changes in blood pressure and physical activity in young and elderly people. Age Ageing 2001; 30: 311–317. [DOI] [PubMed] [Google Scholar]
- 29. Akpolat T, Erdem E, Aydogdu T. Validation of the Omron M3 Intellisense (HEM-7051-E) upper arm blood pressure monitor, for self-measurement, according to the European Society of Hypertension International Protocol revision 2010 in a stage 3-5 chronic kidney disease population. Kidney Blood Press Res 2012; 35: 82–88. [DOI] [PubMed] [Google Scholar]