Abstract
Purpose:
To investigate the association between high myopia and diabetic retinopathy, and its possible mechanism, in a northeastern Chinese population with type 2 diabetic mellitus.
Methods:
Patients were included from Fushun Diabetic Retinopathy Cohort Study. High myopia was defined as spherical equivalent of autorefraction less than −5D.
Results:
A total of 1817 patients [688 (37.9%) diabetic retinopathy, 102 (5.6%) high myopia] were included. Compared to eyes without high myopia, the frequency of diabetic retinopathy and non-proliferative diabetic retinopathy was significantly less in eyes with high myopia (23.5% vs 38.7%, p = 0.002; 22.5% vs 35.3%, p = 0.005). Eyes with high myopia were less likely to have diabetic retinopathy (multivariate odds ratio, 95% confidence interval: 0.39, 0.22–0.68) or non-proliferative diabetic retinopathy (odds ratio, 95% confidence interval: 0.40, 0.23–0.70). High myopia was negatively associated with central retinal venular equivalent (multivariate β, 95% confidence interval: −37.1, −42.3 to −31.8, p < 0.001). Furthermore, central retinal venular equivalent (per 10 μm increase) had a significant association with diabetic retinopathy (odds ratio, 95% confidence interval: 1.24, 1.17–1.31) as well as non-proliferative diabetic retinopathy (odds ratio, 95% confidence interval: 1.24, 1.18–1.31).
Conclusions:
High myopia was negatively associated with both diabetic retinopathy and non-proliferative diabetic retinopathy in this northeastern Chinese population. This protective effect may have been partially achieved via thinning retinal veins.
Keywords: High myopia, diabetic retinopathy, central retinal vein
Introduction
Diabetic retinopathy (DR) is the leading cause of blindness in working-age adults.1 Previous epidemiological studies, inclusive of our community-based study of Fushun Diabetic Retinopathy Cohort Study (FS-DIRECT), have shown the prevalence of DR to be 11.9%–44.3% in the Chinese population.2–7 In addition, these studies have identified certain risk factors, such as duration of diabetes, hyperglycaemia and hypertension,2,4–6,8 which were consistent with results from other populations.9,10
Myopia was suggested as a protective factor for DR in population-based studies, clinical studies and meta-analysis.11–18 However, several studies have denied the protective effect for myopia against DR.2,19–21 Regarding the association in Chinese population, data are limited and remain controversial.2,13,21 In the Beijing Eye Study (BES) with a moderate sample size (n = 362), Xie et al. found that neither the prevalence of DR nor the stage of DR was associated with refractive error.2 Although the 10-year longitudinal results of BES found that the incidence of DR was associated with a shorter axial length [odds ratio (OR), 95% confidence interval (CI): 0.48, 0.33–0.71], no such association was found for refractive error.21 Also from Beijing, a clinical study showed that myopia was negatively associated with DR.13
Though several studies assessed the protective relationship of myopia against DR, very few studies directly explored the underlying mechanisms. As the eye elongates, the retinal vessels stretch and become thinner. The thinner or less dilated vessels may reduce leakage of lipids, erythrocytes and serum through a vessel wall, thus relieving the progression of DR. This phenomenon was supported by several studies.22–25 For example, in the Blue Mountains Eye Study, the authors reported that the mean retinal venular calibre was wider in participants with moderate–severe non-proliferative diabetic retinopathy (NPDR) than in non-diabetic participants or participants with diabetes but no DR.23 Hence, we propose the hypothesis that high myopia can be protective against DR via thinning retinal vasculature.
We therefore conducted this study in patients with type 2 diabetes mellitus (T2DM) living in Fushun, northeast of China to (1) verify the protective role of myopia against DR in diabetic patients with Chinese ethnicity, (2) assess the relationship between myopia and retinal vessel diameters and (3) verify the underlying effect of thinner retinal vessels.
Methods
The FS-DIRECT is an ongoing community-based cohort study. Residents aged 30 years and above in Jiangjun Street, Fushun City, with T2DM were recruited between July 2012 and May 2013. Ethics Committee approval was obtained from the Fushun Eye Hospital. Written informed consent was obtained from all subjects. The details of the rationale, study design and methodology of FS-DIRECT have been previously described elsewhere.7 The inclusion criteria for this study were available data of patients with gradable fundus photography. The exclusion criteria were (1) history of refractive surgery, such as laser-assisted in-situ keratomileusis, (2) aphakic or pseudophakic eyes and (3) history of penetrating ocular trauma.
Definition of DM
DM was diagnosed according to the criteria of American Diabetes Association:26 fasting plasma glucose (FPG) ⩾7.0 mmol/L or 2-h oral glucose-tolerance test (2-h OGTT) ⩾11.1 mmol/L or self-reported diabetes medication use. DM was considered to be type 1 if the participant was aged less than 30 years when diagnosed with diabetes and was receiving insulin therapy. Otherwise, DM was considered to be type 2.
Evaluation of DR and macular edema
Six fields of colour fundus photography with stereoscopic macula image of each subject were taken by certified photographers using a 45° non-mydriatic retinal camera (Kowa, VK-2, Tokyo, Japan) after pupil dilation. Fundus photographs were graded in a masked manner by two graders according to the modified Airlie House Classification system.27 The level of retinopathy was graded according to the following criteria: (1) no DR (levels 10–20); (2) NPDR [mild (levels 31–37), moderate (levels 43–47) or severe (levels 53)] or (3) proliferative DR (levels 60–85). ME was defined as the presence of retinal thickening within 1 disc diameter from the foveal centre or presence of focal photocoagulation scars in the macular area. Clinically significant macular edema (CSME) was considered present when one of the following situations occurs: (1) retinal thickening within 500 μm from centre of macula or focal photocoagulation scars were present, (2) hard exudates within 500 μm from centre of macula with adjacent retinal thickening and (3) retinal thickening of more than one optic disc area within one optic disc diameter from centre of macula.
Measurement of central retinal vessel diameters
The central retinal vessel diameters were measured using the Integrative Vessel Analysis (IVAN) software version 1.3 (Department of Ophthalmology and Visual Science, University of Wisconsin, Madison, WI, USA) from the fundus photographs. The details of the measurement for rest vertical dimension have been described elsewhere.28 Briefly, six largest arterioles and venules passing completely through a circumferential zone 0.5–1 disc diameter from the optic disc margin were identified automatically by IVAN programme. Three summary variables were calculated by the software automatically: the central retinal arteriolar equivalent (CRAE), the central retinal venular equivalent (CRVE) and the ratio of the two variables (arteriole-to-venule ratio), using the revised Parr–Hubbard formula.29
Slit lamp examination
The anterior and posterior segments were examined at the slit lamp. The status of the lens was recorded as phakic, pseudophakic and aphakic.
Refractive error
All participants received a non-cycloplegic, monocularly (right followed left eye), autorefraction (NIDEK, AR-610/630A, Japan). The data of autorefraction were converted to spherical equivalent calculated as the spherical value plus half of the astigmatic value. High myopia was defined as spherical equivalent less than −5D.16
Statistical analysis
Since the level of DR and value of spherical equivalent of the right and left eyes were highly correlated (Pearson correlation coefficient was 0.87 and 0.73, respectively), only information of right eyes were used for further analyses for simplicity.
The normally distributed parameters were presented as the mean ± standard deviation and compared by independent t-tests. Chi-square tests or Fisher’s exact tests (if necessary) were performed for the comparison of the discrete categorized data. Risk factors, including age, gender, presence of high myopia, spherical equivalent, education level, income level, duration of DM, FPG, HbA1c, body mass index, waist hip ratio, systolic blood pressure (SBP), diastolic blood pressure (DBP), serum creatinine, blood urea nitrogen, blood uric acid, total cholesterol, total triglycerides, low-density lipoprotein (LDL), high-density lipoprotein (HDL) and urine protein level, were primarily assessed in the univariate regression for the association with DR, as well as with CRAE/CRVE. Age, gender as well as risk factors with statistical p value less than 0.1 were further analysed in multivariate regressions. Statistical analyses were performed using Statistical Analysis System for Windows version 9.1.3 (SAS Inc., Cary, NC, USA). A p value less than 0.05 was considered to be statistically significant.
Results
There were 1849 patients with gradable fundus photography and refraction data. Among them, 32 patients with aphakic or pseudophakic eyes were excluded. No patients with history of refractive surgery or penetrating ocular trauma were excluded. Hence, 1817 patients (male = 751, 41.3%), including 102 (5.6%) high myopia patients, were enrolled for further analysis. The mean age and duration of DM for the overall patients were 61.3 ± 8.6 years and 7.5 ± 5.8 years, respectively. Of them, 688 (37.9%) patients had DR, with 629 (34.6%) and 59 (3.3%) patients were classified as non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR), respectively. Table 1 presented the characteristics of patients with and without high myopia.
Table 1.
Characteristics of subjects with and without high myopia.
| Absence of high myopia (n = 1715) | High myopia (n = 102) | p value | |
|---|---|---|---|
| Age (years) | 61.4 ± 8.5 | 59.5 ± 9.9 | 0.06 |
| Male, n (%) | 725 (42.3) | 26 (25.5) | <0.001 |
| Spherical equivalent (diopter) | 0.18 ± 1.55 | −9.33 ± 4.96 | <0.001 |
| Duration of diabetes (years) | 7.6 ± 5.8 | 6.5 ± 4.9 | 0.03 |
| FPG (mmol/L) | 9.3 ± 3.4 | 9.6 ± 3.9 | 0.41 |
| HbA1C (%) | 7.8 ± 2.0 | 7.8 ± 1.9 | 0.96 |
| BMI (kg/m2) | 26.4 ± 3.4 | 26.5 ± 3.3 | 0.80 |
| Waist/hip ratio | 0.96 ± 0.06 | 0.97 ± 0.06 | 0.48 |
| SBP (mmHg) | 147.1 ± 23.2 | 149.7 ± 24.1 | 0.27 |
| DBP (mmHg) | 77.3 ± 11.2 | 79.3 ± 13.4 | 0.14 |
| Serum creatinine (μmol/L) | 84.6 ± 19.6 | 83.0 ± 17.0 | 0.42 |
| BUN (mmol/L) | 6.3 ± 3.3 | 5.9 ± 1.8 | 0.047 |
| BUA (μmol/L) | 309.6 ± 88.2 | 312.7 ± 82.6 | 0.73 |
| Total cholesterol (mmol/L) | 5.5 ± 1.3 | 5.5 ± 1.1 | 0.94 |
| Total triglycerides (mmol/L) | 2.2 ± 1.7 | 2.1 ± 1.6 | 0.56 |
| LDL (mmol/L) | 3.2 ± 0.9 | 3.2 ± 0.8 | 0.50 |
| HDL (mmol/L) | 1.5 ± 0.4 | 1.5 ± 0.4 | 0.36 |
| Current smoker, n (%) | 385 (22.4) | 19 (18.6) | 0.37 |
| Current drinker, n (%) | 384 (22.4) | 18 (17.6) | 0.26 |
| Proteinuria, n (%) | 1154 (67.3) | 67 (65.7) | 0.74 |
FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; BUN: blood urea nitrogen; BUA: blood uric acid; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
Table 2 showed the frequency and pathological features of DR between eyes with and without high myopia. The frequency of DR was significantly less in eyes with high myopia than that in eyes without high myopia (23.5% vs 38.7%, p = 0.002). Similar differences between the groups were also found for the NPDR (22.5% vs 35.3%, p = 0.005), but not for the PDR. It showed that the eyes with high myopia had significantly less severity of microaneurysms and spot haemorrhages (p = 0.04). It also showed that the presence of hard exudates (11.8% vs 21.7%, p = 0.02) and intraretinal microvascular abnormality (IRMA; none vs 4.2%, p = 0.03) were less frequent in eyes with high myopia. However, no significant difference was found for cotton wool spots, venous beading or diabetic macular edema (DME).
Table 2.
Frequency of diabetic retinopathy (DR) and its pathological features in eyes with and without high myopia.
| Absence of high myopia (n, %) | High myopia (n, %) | p value | |
|---|---|---|---|
| DR | 664 (38.7) | 24 (23.5) | 0.002 |
| NPDR | 606 (35.3) | 23 (22.5) | 0.005 |
| PDRa | 58 (3.4) | 1 (1.0) | 0.25 |
| MA and spot haemorrhage | |||
| None | 674 (39.3) | 53 (52.0) | 0.038 |
| Mild | 909 (53.0) | 42 (41.2) | |
| Severe | 132 (7.7) | 7 (6.9) | |
| Hard exudates | |||
| None | 1343 (78.3) | 90 (88.2) | 0.017 |
| Presence | 372 (21.7) | 12 (11.8) | |
| Cotton wool spot | |||
| None | 1479 (86.2) | 93 (91.2) | 0.16 |
| Presence | 236 (13.8) | 9 (8.8) | |
| IRMAa | |||
| None | 1643 (95.8) | 102 (100.0) | 0.031 |
| Presence | 72 (4.2) | 0 (0.0) | |
| VBa | |||
| None | 1692 (98.7) | 102 (100.0) | 0.66 |
| Presence | 23 (1.3) | 0 (0.0) | |
| DME | 215 (12.5) | 10 (9.8) | 0.42 |
| Non-CSME | 95 (5.5) | 5 (4.9) | 0.68 |
| CSME | 120 (7.0) | 5 (4.9) | |
NPDR: non-proliferative diabetic retinopathy; PDR: proliferative DR; MA: microaneurysm; IRMA: intraretinal microvascular abnormality; VB: venous beading; DME: diabetic macular edema; CSME: clinically significant macular edema.
Calculated by Fisher’s exact test, while others calculated by chi-square test.
In a multivariate logistic regression, adjusting for age, gender, income level, duration of DM, FPG, HbA1c, SBP, total cholesterol, HDL and urine protein level, selected from univariate regressions, high myopia had significantly negative association with the presence of DR (OR, 95% CI: 0.39, 0.22–0.68). When high myopia was changed to spherical equivalent, the model showed that for every 1 diopter increase, there was a 1.08 (1.03–1.12) increase in OR for DR. Similar associations between high myopia/spherical equivalent and NPDR were found. However, no associations were found for PDR (Table 3).
Table 3.
Logistic regression analysis for the diabetic retinopathy (DR).
| Univariate OR (95% CI) | Multivariate OR (95% CI)a | |
|---|---|---|
| High myopia (vs not high myopia) | ||
| DR | 0.49 (0.31–0.78) | 0.39 (0.22–0.68) |
| NPDR | 0.51 (0.32–0.82) | 0.40 (0.23–0.70) |
| PDR | 0.28 (0.04–2.06) | 0.33 (0.04–2.51) |
| Per 1 diopter increase | ||
| DR | 1.04 (1.00–1.07) | 1.08 (1.03–1.12) |
| NPDR | 1.04 (1.00–1.08) | 1.08 (1.03–1.13) |
| PDR | 1.00 (0.92–1.10) | 1.05 (0.93–1.19) |
OR: odds ratio; CI: confidence interval; NPDR: non-proliferative diabetic retinopathy; PDR: proliferative DR.
Adjusted for age, gender, income level, duration of diabetes mellitus, fasting plasma glucose, HbA1c, systolic blood pressure, total cholesterol, high-density lipoprotein and urine protein level.
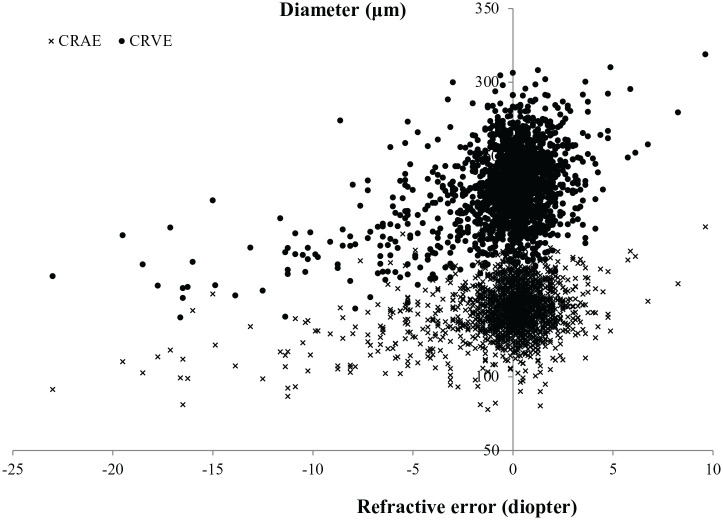
Among the 1817 patients, 1782 with available data (1117 no DR, 614 NPDR, 51 PDR) of CRAE and CRVE were used for further corresponding analyses. Scatter plot between CRAE/CRVE and refractive error is shown in Figure 1. The multivariate regression, adjusting for age, gender, education level, income level, duration of DM, FPG, HbA1c, body mass index, SBP, DBP, serum creatinine, blood uric acid, total triglycerides, HDL and urine protein level, showed significant associations between CRVE and high myopia (β = −37.1, 95% CI: −42.3 to −31.8, p < 0.001), CRVE and diopters (per 1 diopter increase, β = 4.3, 95% CI: 3.9–4.7, p < 0.001). Similar results were found for CRAE and high myopia/diopter (Table 4).
Figure 1.
Scatter plot between central retinal arteriolar/venular equivalent (CRAE, CRVE) and refractive error.
Table 4.
Association between CRAE/CRVE and high myopia/diopter.
| CRAE |
CRVE |
|||
|---|---|---|---|---|
| β (95% CI)a | p value | β (95% CI)a | p value | |
| High myopia (vs not high myopia) | −18.8 (−22.3 to −15.2) | <0.001 | −37.1 (−42.3 to −31.8) | <0.001 |
| Per 1 diopter increase | 2.0 (1.7–2.3) | <0.001 | 4.3 (3.9–4.7) | <0.001 |
CRAE: central retinal arteriolar equivalent; CRVE: central retinal venular equivalent; CI: confidence interval.
Adjusted for age, gender, education level, income level, duration of diabetes mellitus, fasting plasma glucose, HbA1c, body mass index, systolic blood pressure, diastolic blood pressure, serum creatinine, blood uric acid, total triglycerides and high-density lipoprotein.
When including both CRAE and CRVE into the multivariate logistic regression, adjusting for the same risk factors for DR, CRVE (per 10 μm increase) had significant association with the presence of DR (OR, 95% CI: 1.24, 1.17–1.31) and NPDR (OR, 95% CI: 1.24, 1.18–1.31), but not associated with PDR. Contrarily, CRAE (per 10 μm increase) had significantly negative association with the presence of PDR (OR, 95% CI: 0.67, 0.56–0.82), but not with DR or NPDR. The refraction was shown to be not significant in these models (Table 5).
Table 5.
Logistic regression analysis for the diabetic retinopathy (DR).
| Univariate OR (95% CI) | Multivariate OR (95% CI)a | |
|---|---|---|
| CRAE (per 10 μm increase) | ||
| DR | 1.08 (1.02–1.14) | 0.99 (0.92–1.07) |
| NPDR | 1.12 (1.06–1.19) | 1.02 (0.95–1.10) |
| PDR | 0.71 (0.61–0.82) | 0.67 (0.56–0.82) |
| CRVE (per 10 μm increase) | ||
| DR | 1.25 (1.20–1.30) | 1.24 (1.17–1.31) |
| NPDR | 1.26 (1.21–1.32) | 1.24 (1.18–1.31) |
| PDR | 1.01 (0.91–1.13) | 1.00 (0.87–1.15) |
OR: odds ratio; CI: confidence interval; CRAE: central retinal arteriolar equivalent; CRVE: central retinal venular equivalent; CI: confidence interval; NPDR: non-proliferative diabetic retinopathy; PDR: proliferative DR.
Adjusted for age, gender, spherical equivalent, income level, duration of diabetes mellitus, fasting plasma glucose, HbA1c, systolic blood pressure, total cholesterol, high-density lipoprotein and urine protein level.
Discussion
Both myopia and diabetes have become prominent public health diseases in the Chinese population within the last few decades. A 2-year longitudinal study reported that the prevalence of myopia and high myopia in junior high school students increased from 67.4% and 3.2% to 79.4% and 7.0%, respectively.30 The prevalence of diabetes and prediabetes among the Chinese population was 9.7% and 15.5%, respectively, accounting for 92.4 million adults with diabetes and 148.2 million adults with prediabetes.31 Our previous study also showed a very high prevalence (44.3%) of DR in the population of this study.7 Hence, given the high and increasing prevalence of both conditions, a better understanding of the relationship between myopia and DR is paramount for informing and directing public health policy making.
There were several important findings in this study. First, in this study cohort with a large sample size of Chinese ethnicity, we found a negative association between high myopia and DR/NPDR, as well as an increasing OR of diopter for DR and NPDR. Previous studies of other ethnicities have been reported this association.11,12,14,15 For example, in a population-based study of Indians in Singapore (the Singapore Indian Eye Study), myopic eyes were less likely to have DR compared with emmetropic eyes (OR, 0.68; 95% CI, 0.46–0.98).14 Similar results were found in Singapore Malay Eye Study12 and Korea National Health and Nutrition Examination Survey.15 Cohort data from Wisconsin Epidemiological Study of Diabetic Retinopathy also found that myopia reduced the risk for progression to PDR to 40% in patients with younger-onset DM (OR, 95% CI: 0.40, 0.18–0.86).11 However, date from other epidemiology studies, including Chinese population-based studies, did not find this protective effect of myopia.2,19–21,32,33 Although the longitudinal results of BES found that the incidence of DR was associated with shorter axial length, the refraction was never found to be associated with prevalence/incidence of DR.2,21 Similarly, Man et al. found that the longer axial length rather than myopia was the main contributor for the protective effect.32,33 The inconsistent findings may be due to different definitions or classifications of myopia, methodology of fundus photography and definitions of DR. Besides, it should be noted that the sample size of baseline of BES (n = 362) and Diabetes Management Project Melbourne (DMP; n = 630) was moderate, which may relatively reduce the statistical power to find a significant association.
The second finding of this study was that a further comparison of pathological features of DR between eyes with and without high myopia, for the first time. For eyes with high myopia, the presence of the microaneurysm and spot haemorrhage was less severe compared to the eyes without high myopia. In addition, the presence of hard exudation, and IRMA, was also less frequent. This finding further supports the protective effect of high myopia.
The third and most important finding in this study was that the thinning retinal venular diameter played a significant role on the protective effect of high myopia against DR. In this study, we found that the refraction was positively associated with both CRAE and CRVE in patients with diabetes, after adjusting for the confounders (e.g. age, blood pressure, which was reported to be associated with retinal vessel diameters).22 Moreover, in a further model, taking account of the refraction, CRAE and CRVE, we found that the CRVE was also positively associated with the presence of DR and NPDR. Hence, taken together, the results of this study suggested that the protective effect of myopia on DR may be partially achieved through thinning retinal vein. Epidemiological studies have also reported the association between CRVE and DR.22–25 For example, in Wisconsin Epidemiologic Study of Diabetic Retinopathy, Klein et al. found that larger CRVE was associated with the more severe of DR,22 progression of DR (OR, 95% CI: 1.21, 1.12–1.30) and 6-year incidence of DR (OR, 95% CI: 1.26, 1.10–1.43).25 This finding underscores the value of clinical observation of retinal venular calibre when monitoring patients with retinopathy.
The explanation may lie in the decreasing capillary hydrostatic pressure and decreasing leakage as well as rupture of compromised retinal capillaries in diabetes due to thinner venular calibre (Starling and Laplace’s law).34 An alternative explanation was that thinner retinal vessel would have a reduction in retinal blood flow (law of Hagen–Poiseuille),35 and the latter has been demonstrated to be associated with less severity and less progression of DR by clinical studies.36–38 For example, Dimitrova et al. found an increasing central retinal vein peak systolic velocity and end diastolic velocity in patients with DR progression.38 However, other studies found no such association39 or a reverse association.40,41 The contradictory results may be due to different areas of blood flow assessed and DR assessment methodology.37,39 As most of the clinical studies were conducted with small samples and did not adjust for the confounders, further studies are warranted. It should also be noted that in patients with thinner retinal thickness and reduced capillary density, light from the apparatus may be easier to hit and scatter from the moving red blood cells in the choriocapillaries instead of retinal capillaries, hence skewing the results.
Regarding the PDR, we found a negative association with CRAE in this study. This was understandable that a larger arterial vessel with more blood flow may provide more oxygen, resulting in a more blunted hypoxic response (e.g. less vascular endothelial growth factor release). It should be noted that 22 out of the 51 PDR eyes had pan retinal photocoagulation (PRP) or vitrectomy (also PRP during surgery), which may influence their natural pathological process. Furthermore, we did not find an association between either high myopia or refraction and PDR in this study, suggesting other mechanisms for this pathological change.
Study strengths of this study included a relatively large sample size with high response rate (91.4%), standard and comprehensive clinical examinations, standard protocols of retinal photograph grading and retinal vessel diameter measurement. However, some limitations remained in this study. First, the axial length or vitreous chamber length, which are important parameters accompanied with myopia and reported to be associated with DR or diabetic ME,12,21,32,33,42–44 were not obtained in this study. Second, although the association between myopia and DR has been assessed, the cross-sectional design limited our ability to determine the causal effect between exposures and outcomes, as well as its potential mechanism.
In summary, we found that high myopia was negatively associated with the presence of DR and NPDR, after adjusting for the potential confounders, in a northeastern Chinese population aged 30 years and above. The potential mechanism for this association may be partially as a result of thinning retinal vasculature.
Acknowledgments
The authors thank Dr Nived Moonasar (Caribbean Eye Institute, Valsayn, Trinidad and Tobago) for his invaluable assistance in revising the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The study was supported by Zhejiang Provincial Natural Science Foundation of China (LQ18H120004), Wenzhou Basic Medical and Health Technology Project (Y20190632) and Liaoning Provincial Natural Science Foundation of China (20170540328).
ORCID iD: Zhong Lin  https://orcid.org/0000-0002-4828-7157
https://orcid.org/0000-0002-4828-7157
References
- 1. Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol 2007; 14: 179–183. [DOI] [PubMed] [Google Scholar]
- 2. Xie XW, Xu L, Wang YX, et al. Prevalence and associated factors of diabetic retinopathy. The Beijing eye study 2006. Graefes Arch Clin Exp Ophthalmol 2008; 246: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 3. Wang FH, Liang YB, Zhang F, et al. Prevalence of diabetic retinopathy in rural China: the Handan eye study. Ophthalmology 2009; 116: 461–467. [DOI] [PubMed] [Google Scholar]
- 4. Xu J, Wei WB, Yuan MX, et al. Prevalence and risk factors for diabetic retinopathy: the Beijing communities diabetes study 6. Retina 2012; 32: 322–329. [DOI] [PubMed] [Google Scholar]
- 5. Hu Y, Teng W, Liu L, et al. Prevalence and risk factors of diabetes and diabetic retinopathy in Liaoning province, China: a population-based cross-sectional study. PLoS One 2015; 10: e0121477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li YY, Yang XF, Gu H, et al. The Beijing Desheng diabetic eye study: rationale, design, methodology and baseline data. Int J Ophthalmol 2018; 11: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Lin Z, Wen L, et al. Rationale, design, methodology and baseline data of Fushun Diabetic Retinopathy Cohort Study (FS-DIRECT). Ophthalmic Epidemiol 2020; 27: 73–82. [DOI] [PubMed] [Google Scholar]
- 8. Wang FH, Liang YB, Peng XY, et al. Risk factors for diabetic retinopathy in a rural Chinese population with type 2 diabetes: the Handan eye study. Acta Ophthalmol 2011; 89: e336–343. [DOI] [PubMed] [Google Scholar]
- 9. Varma R, Torres M, Pena F, et al. Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology 2004; 111: 1298–1306. [DOI] [PubMed] [Google Scholar]
- 10. Wong TY, Cheung N, Tay WT, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay eye study. Ophthalmology 2008; 115: 1869–1875. [DOI] [PubMed] [Google Scholar]
- 11. Moss SE, Klein R, Klein BE. Ocular factors in the incidence and progression of diabetic retinopathy. Ophthalmology 1994; 101: 77–83. [DOI] [PubMed] [Google Scholar]
- 12. Lim LS, Lamoureux E, Saw SM, et al. Are myopic eyes less likely to have diabetic retinopathy. Ophthalmology 2010; 117: 524–530. [DOI] [PubMed] [Google Scholar]
- 13. Jiang JJ, Li XX, Yuan L, et al. [Ocular biological structures and relevant risk factors in the occurrence of diabetic retinopathy in diabetes mellitus patients]. Zhonghua Yan Ke Za Zhi 2012; 48: 898–902. [PubMed] [Google Scholar]
- 14. Pan CW, Cheung CY, Aung T, et al. Differential associations of myopia with major age-related eye diseases: the Singapore Indian eye study. Ophthalmology 2013; 120: 284–291. [DOI] [PubMed] [Google Scholar]
- 15. Chao DL, Lin SC, Chen R, et al. Myopia is inversely associated with the prevalence of diabetic retinopathy in the South Korean population. Am J Ophthalmol 2016; 172: 39–44. [DOI] [PubMed] [Google Scholar]
- 16. Bazzazi N, Akbarzadeh S, Yavarikia M, et al. HIGH MYOPIA AND DIABETIC RETINOPATHY: a contralateral eye study in diabetic patients with high myopic anisometropia. Retina 2017; 37: 1270–1276. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Tang L, Gao L, et al. Myopia and diabetic retinopathy: a systematic review and meta-analysis. Diabetes Res Clin Pract 2016; 111: 1–9. [DOI] [PubMed] [Google Scholar]
- 18. Fu Y, Geng D, Liu H, et al. Myopia and/or longer axial length are protective against diabetic retinopathy: a meta-analysis. Acta Ophthalmol 2016; 94: 346–352. [DOI] [PubMed] [Google Scholar]
- 19. Ganesan S, Raman R, Reddy S, et al. Prevalence of myopia and its association with diabetic retinopathy in subjects with type II diabetes mellitus: a population-based study. Oman J Ophthalmol 2012; 5: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jee D, Lee WK, Kang S. Prevalence and risk factors for diabetic retinopathy: the Korea national health and nutrition examination survey 2008-2011. Invest Ophthalmol Vis Sci 2013; 54: 6827–6833. [DOI] [PubMed] [Google Scholar]
- 21. Xu J, Xu L, Wang YX, et al. Ten-year cumulative incidence of diabetic retinopathy. PLoS One 2014; 9: e111320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klein R, Klein BE, Moss SE, et al. Retinal vascular caliber in persons with type 2 diabetes: the Wisconsin epidemiological study of diabetic retinopathy: XX. Ophthalmology 2006; 113: 1488–1498. [DOI] [PubMed] [Google Scholar]
- 23. Kifley A, Wang JJ, Cugati S, et al. Retinal vascular caliber, diabetes, and retinopathy. Am J Ophthalmol 2007; 143: 1024–1026. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen TT, Wang JJ, Sharrett AR, et al. Relationship of retinal vascular caliber with diabetes and retinopathy: the multi-ethnic study of atherosclerosis (MESA). Diabetes Care 2008; 31: 544–549. [DOI] [PubMed] [Google Scholar]
- 25. Klein R, Myers CE, Lee KE, et al. Changes in retinal vessel diameter and incidence and progression of diabetic retinopathy. Arch Ophthalmol 2012; 130: 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2005; 28: S37–S42. [DOI] [PubMed] [Google Scholar]
- 27. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie house classification. ETDRS report number 10. Early treatment diabetic retinopathy study research group. Ophthalmology 1991; 98: 786–806. [PubMed] [Google Scholar]
- 28. Wong TY, Knudtson MD, Klein R, et al. Computer-assisted measurement of retinal vessel diameters in the Beaver dam eye study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 2004; 111: 1183–1190. [DOI] [PubMed] [Google Scholar]
- 29. Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 2003; 27: 143–149. [DOI] [PubMed] [Google Scholar]
- 30. Wang SK, Guo Y, Liao C, et al. Incidence of and factors associated with myopia and high myopia in Chinese children, based on refraction without cycloplegia. JAMA Ophthalmology 2018; 136: 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. [DOI] [PubMed] [Google Scholar]
- 32. Man RE, Sasongko MB, Sanmugasundram S, et al. Longer axial length is protective of diabetic retinopathy and macular edema. Ophthalmology 2012; 119: 1754–1759. [DOI] [PubMed] [Google Scholar]
- 33. Man RE, Gan AT, Gupta P, et al. Is myopia associated with the incidence and progression of diabetic retinopathy. Am J Ophthalmol 2019; 208: 226–233. [DOI] [PubMed] [Google Scholar]
- 34. Quigley M, Cohen S. A new pressure attenuation index to evaluate retinal circulation. Arch Ophthalmol 1999; 117: 84–89. [DOI] [PubMed] [Google Scholar]
- 35. Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Research 2000; 32: 249–256. [DOI] [PubMed] [Google Scholar]
- 36. Patel V, Rassam S, Newsom R, et al. Retinal blood flow in diabetic retinopathy. BMJ 1992; 305: 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cuypers MH, Kasanardjo JS, Polak BC. Retinal blood flow changes in diabetic retinopathy measured with the Heidelberg scanning laser Doppler flowmeter. Graefe’s Arch Clin Exp Ophthalmol 2000; 238: 935–941. [DOI] [PubMed] [Google Scholar]
- 38. Dimitrova G, Kato S, Yamashita H, et al. Relation between retrobulbar circulation and progression of diabetic retinopathy. Br J Ophthalmol 2003; 87: 622–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Man RE, Sasongko MB, Xie J, et al. Decreased retinal capillary flow is not a mediator of the protective myopia-diabetic retinopathy relationship. Invest Ophthalmol Vis Sci 2014; 55: 6901–6907. [DOI] [PubMed] [Google Scholar]
- 40. Burgansky-Eliash Z, Nelson DA, Bar-Tal OP, et al. Reduced retinal blood flow velocity in diabetic retinopathy. Retina 2010; 30: 765–773. [DOI] [PubMed] [Google Scholar]
- 41. Srinivas S, Tan O, Nittala MG, et al. Assessment of retinal blood flow in diabetic retinopathy using Doppler Fourier-domain optical coherence tomography. Retina 2017; 37: 2001–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pierro L, Brancato R, Robino X, et al. Axial length in patients with diabetes. Retina 1999; 19(5): 401–404. [DOI] [PubMed] [Google Scholar]
- 43. Yang KJ, Sun CC, Ku WC, et al. Axial length and proliferative diabetic retinopathy. Optom Vis Sci 2012; 89(4): 465–470. [DOI] [PubMed] [Google Scholar]
- 44. Man RE, Lamoureux EL, Taouk Y, et al. Axial length, retinal function, and oxygen consumption: a potential mechanism for a lower risk of diabetic retinopathy in longer eyes. Invest Ophthalmol Vis Sci 2013; 54: 7691–7698. [DOI] [PubMed] [Google Scholar]