Abstract
Background:
Multiple studies demonstrate an albuminuria-lowering impact of Roux-en-Y gastric bypass surgery, but neither evaluation of its penetrance across different baseline levels of albuminuria nor its association with alterations in podocyte phenotype has previously been reported.
Methods:
We profiled changes in body weight, glycaemic control and urinary albumin excretion following Roux-en-Y gastric bypass surgery in 105 patients with type 2 diabetes, albuminuria of varying degrees of severity and classified as being at moderate or high risk of chronic kidney disease progression according to the Kidney Disease: Improving Global Outcomes 2012 criteria. In parallel pre-clinical studies, the impact of Roux-en-Y gastric bypass surgery on markers of podocyte injury was assessed in the Zucker diabetic fatty rat model of diabetic kidney disease.
Results:
At 12- to 18-month post-operative follow-up in patients at moderate or high risk of chronic kidney disease, significant reductions in albuminuria were observed across all tertiles of baseline albumin–creatinine ratio, with remission of albuminuria occurring in 78% of patients. Relative to sham-operated control animals, weight loss and improvements in glycaemia following Roux-en-Y gastric bypass surgery in Zucker diabetic fatty rats were paralleled by normalisation of glomerular tuft-size, reductions in podocyte expression of desmin, and preservation of podocyte foot process morphology.
Conclusion:
Improvements in podocyte differentiation likely underpin the reductions in albuminuria observed following Roux-en-Y gastric bypass surgery.
Keywords: Diabetes, albuminuria, kidney, podocyte, surgery
Introduction
Periodic assessment of urinary albumin excretion is useful in the long-term monitoring of renal and cardiovascular risk in patients with diabetic kidney disease (DKD). Moreover, early changes in microalbuminuria [urinary albumin–creatinine ratio (ACR) = 30–300 mg/g] as a measure of short-term treatment response predict preservation of renal function at follow-up.1
Podocyte injury and dedifferentiation are recognised as an integral element of the loss of selective permeability at the glomerular barrier in DKD. Stress-induced podocyte dedifferentiation fundamentally alters the differentiated morphology of podocytes and is associated with the effacement of podocyte foot processes, in turn compromising size-selective sieving at the slit diaphragm and promoting excess filtration of albumin. De novo acquisition of desmin positivity is a sensitive early marker of podocyte injury and dedifferentiation in DKD and cytoskeletal adaption to glomerular hypertension arising from the diabetic milieu.2
Roux-en-Y gastric bypass (RYGB) improves multiple recognised risk factors for DKD progression.3 Coherent with this, beneficial renal effects of RYGB are increasingly being reported in long-term follow-up of high-quality case–control series such as The Swedish Obese Subjects Study.4
The Zucker diabetic fatty rat (ZDF fa/fa) develops high levels of urinary albumin in the context of marked insulin resistance, progressive hyperglycaemia, dyslipidaemia, obesity, and inflammation.5 Consistent with reports in humans, we have previously shown that RYGB reduces albuminuria in the ZDF rat, with improvements not replicated by matched dietary weight-loss alone.6
The current brief report highlights that RYGB effectively reduces albuminuria across the spectrum of baseline grades of severity of microalbuminuria and in parallel pre-clinical studies, we demonstrate that RYGB-induced reductions in albuminuria coincide with evidence of reduced cellular stress and improvements in differentiation in podocytes.
Methods
Human DKD cohort
We prospectively audited 105 consecutive cases of RYGB surgery in patients with baseline microalbuminuria/low-range macroalbuminuria. The clinical audit was approved by Oswaldo Cruz German Hospital, São Paulo, Brazil (reference 20190404). Measures of body weight, glycaemic control, and urine ACR (mg/g) in spot morning urine samples were recorded before and 12–18 months after surgery. Chronic kidney disease (CKD)-Epi equation estimated glomerular filtration rate (eGFR) was calculated, and the combination of baseline eGFR and urine ACR values used to classify patients according to the Kidney Disease: Improving Global Outcomes (KDIGO) CKD staging criteria.7 Albumin was measured by immunoturbidimetric assay (RocheTM reagents), and creatinine was assessed by kinetic colorimetric assay (RocheTM reagents).
ZDF rat studies
Obese, diabetic ZDF rats (fa/fa), and control rats (fa/+) (Charles River Laboratories, France and UK) were included in a parallel study, focussed on the impact of RYGB on podocyte injury under licence from Health Products Regulatory Authority Ireland (AE18692-P009), Age- and weight-matched ZDF rats underwent (1) SHAM surgery involving laparotomy (n = 8) or (2) RYGB (n = 15) at 12 weeks of age and were euthanised at 20 weeks of age. Body weight and glycaemia (ACCU-CHEK®-Roche) were assessed weekly. Insulin degludec (Novo Nordisk, Denmark) was used for 7 days prior to surgery to achieve morning glucose readings of <12 mmol/L. Under isoflurane anaesthesia, a midline laparotomy and either SHAM surgery or RYGB surgery were performed as previously described.8 Urinary albumin excretion rates were calculated as ACR from overnight urine collections in metabolic cages. Urinary albumin and creatinine were assessed using an autoanalyzer (Roche/Hitachi Cobas c 502 Modular analyser) and resulting values used to derive the ACR (mg/g). Thick sections (4 µm) of paraffin-embedded tissue were used for immunohistochemistry of Wilms’ Tumour (WT-1) (antibody C-19, sc-192; Santa Cruz Biotechnology, 1:250 dilution) and desmin (Antibody Clone Dako; Cytomation, 1:100 dilution).
Glomerular morphometry was performed using Image J 1.48v software analysis of scanned 20× images of WT-1-stained sections (Digital Slide Scanner; Hamamatsu Photonics). Twenty glomeruli per sample were analysed. Podocyte number per glomerulus was determined using the cell counter function on Image J. Glomerular volume (μm3) was estimated using the Weibel and Gomez formula.9 Glomerular volume served per podocyte (podocyte density) was estimated using enumeration of WT-1-positive glomerular cells and expression as a ratio of calculated glomerular volume. Twenty glomeruli were analysed per sample. Semi-quantitative scoring of desmin staining was carried out in 20 individual glomeruli per sample at 40× magnification. Scoring was based on the product of both distribution [0 = absent, 1 = segmental (<50%) or 2 = global (>50%)] and intensity (1 = low, 2 = intermediate and 3 = high) of staining.
For transmission electron microscopy (TEM) analysis, ultra-thin sections of epoxy resin-embedded tissue were imaged (Technai 12). Glomerular basement membrane (GBM) thickness was measured according to the Haas10 method at 22,500×. Twenty GBM thickness measurements were recorded per specimen, and podocyte foot process frequency (PFPF) was estimated at 9900× by determining the number of podocyte foot processes per unit length (8 µm) of peripheral GBM.11
Statistical analysis
Data were analysed using GraphPad Prism® version 6 (CA, USA), SPSS® version 20 (IL, USA) and RStudio® version 3.5.1. Paired t test and Wilcoxon signed-rank test were performed for within-group comparisons of parametric and non-parametric data at baseline and follow-up, respectively. Analysis of variance (ANOVA) with post hoc Holm Sidak test was used for comparing data between ⩾3 groups. The value of p < 0.05 was considered statistically significant.
Results
Impact of RYGB on albuminuria in humans
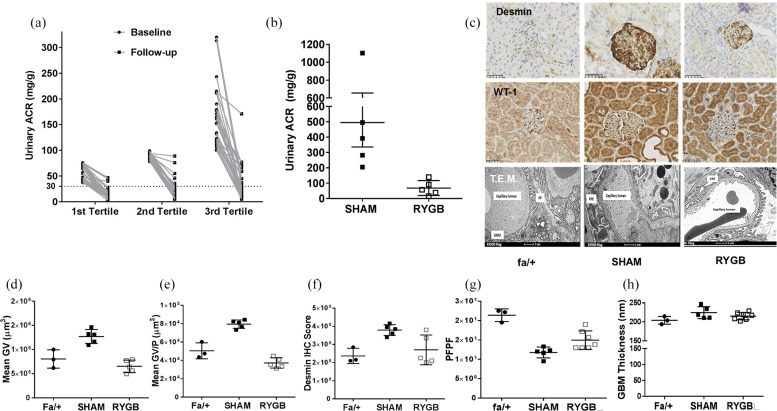
The cohort of 105 patients reported in the study was 54.0 ± 7.0 years of age, 49% were female, 89% were Caucasian, with a mean diabetes duration of 12.7 ± 3.1 years and 13.4 ± 2.3 months of follow-up after surgery. At baseline, 103 patients had moderately increased ACR (A2: 30–300 mg/g) and 2 patients severely increased ACR (A3: >300 mg/g). Overall, 85% (46% G1-A2 and 39% G2-A2) of patients were classified as being of moderate risk of CKD progression with 15% considered to be at high risk (G1-A3 0.95%, G2-A3 0.95%, and G3a-A2 13.3%). At follow-up, body mass index (BMI) reduced from 38.3 ± 6.1 kg/m2 to 27.0 ± 2.6 kg/m2 and HbA1c reduced from 8.0% ± 0.7% (63.9 ± 7.6 mmol/mol) to 6.0% ± 6% (41.6 ± 6.8 mmol/mol) (both p < 0.001). Median ACR decreased by 80.7% from 88.0 (41.0) mg/g to 14.0 (20.0) mg/g (p < 0.001) and remission of albuminuria (ACR < 30 mg/g) occurred in 82 of 105 (78.1%) patients at follow-up. Reductions in albuminuria were seen across the range of baseline urine ACR values when stratified by tertiles (Figure 1(a)).
Figure 1.
Evidence that remission of albuminuria following RYGB is linked to reductions in podocyte stress and dedifferentiation. (a) Effect of RYGB on ACR stratified by tertiles of baseline albumin–creatinine ratio (ACR) (n = 105). (b) Four weeks after SHAM or RYGB surgery, five animals per group were placed in metabolic cages for determination of urinary ACR. (c) Glomerular expression of WT-1 and desmin was assessed by immunohistochemistry (n = 5 per group) alongside TEM of glomeruli in renal cortical tissue sections obtained at necropsy 8 weeks after surgery (SHAM, n = 5; RYGB, n = 7). Assessment of (d) glomerular tuft size and (e) glomerular volume per podocyte demonstrated that disease-associated alterations in both parameters relative to normal fa/+ rats were completely abrogated by RYGB. Likewise, quantification of (f) desmin staining indicated a reduction in podocyte stress after RYGB which occurred in parallel to a reduced degree of (g) podocyte foot process effacement. (h) GBM thickness was not altered in SHAM-operated rats at 20 weeks of age in this study and no impact of RYGB was noted.
*p < 0.05 versus fa/+ #p < 0.05 versus SHAM. RYGB: Roux-en-Y gastric bypass; ACR: albumin–creatinine ratio; WT-1: Wilm’s Tumour 1; TEM: transmission electron microscopy; GV: glomerular volume; GV/P: glomerular volume per podocyte; PFPF: podocyte foot process frequency; GBM: glomerular basement membrane.
Impact of RYGB on albuminuria in ZDF rats
During the week prior to surgery, body weight (SHAM: 380 ± 8 g vs RYGB: 387 ± 5 g; p = 0.82) and hyperglycaemia (SHAM: 29 ± 2.1 mmol/L vs RYGB 30 ± 0.9 mmol/L; p = 0.8) were comparable between SHAM and RYGB rats. Eight weeks after surgery, mean body weight in RYGB-operated rats was reduced by 31% relative to SHAM-operated rats (SHAM: 410 ± 8 g vs RYGB: 285 ± 7 g; p < 0.01). This was paralleled by a 74% relative reduction in hyperglycaemia in RYGB-operated rats (SHAM: 32.8 ± 0.5 mmol/L vs RYGB: 8.3 ± 1.0 mmol/L, p < 0.01). Improvements in glycaemia and body weight were paralleled by a mean 86% relative reduction in urinary ACR in RYGB-operated rats (SHAM: 495 ± 159 mg/g vs RYGB: 68 ± 21 mg/g; p < 0.01; Figure 1(b)).
Podocyte stress and dedifferentiation in the ZDF rat are attenuated by RYGB
In samples of renal cortex obtained at necropsy 8 weeks after SHAM or RYGB surgery, mean glomerular volume (Figure 1(d)) was 49% lower in RYGB-operated rats (SHAM: 1.3 × 106 ± 6.4 × 104 µM3 vs RYGB: 6.5 × 105 ± 5.7 × 104 µM3; p < 0.01). Mean glomerular volume served per podocyte (Figure 1(e)) was 54% lower in RYGB-operated rats (SHAM: 7.9 × 104 ± 2.0 × 103 µM3 vs RYGB: 3.7 × 104 ± 2.4 × 103 µM3; p < 0.01). Glomerular desmin levels (Figure 1(f)) were reduced by 30% in RYGB relative to SHAM (SHAM: 3.8 ± 0.03 vs RYGB: 2.7 ± 0.4; p = 0.02). Podocyte effacement as assessed by podocyte foot process frequency per unit length of basement membrane was improved after RYGB (SHAM: 11.7 ± 0.6 vs RYGB: 15 ± 0.9; p = 0.01; Figure 1(g)). No significant differences were detected between SHAM and RYGB surgery with regard to GBM thickness (SHAM: 225 ± 7.1 nm vs RYGB: 215 ± 3.6 nm; p = 0.34; Figure 1(h)).
Discussion
Herein, we demonstrate that reductions in albuminuria following RYGB occurred across a spectrum of baseline disease severity with regard to the severity of albuminuria and degree of underlying obesity and hyperglycaemia. We used the ZDF model of DKD to investigate the changes in glomerular architecture that likely explain the marked reductions in albuminuria observed after RYGB in humans. RYGB was associated with reductions in both podocyte stress (de novo desmin expression) and dedifferentiation (foot process effacement). These changes occurred in parallel with normalisation of glomerular tuft size, compatible in turn with a reduction in intra-glomerular pressure. These changes most likely arise as a reflection of the improvements in metabolic control after RYGB, most notably its potent glucose-lowering effect.
This study has important implications for research in the field. First, it highlights that the albuminuria-reducing effects of RYGB are preserved across varying severities of baseline DKD severity in patients at moderate or high risk of progressive renal functional decline. Moreover, the data from the ZDF rat model extend mechanistic insight into the structural and ultrastructural changes in the glomerulus which underlie remission of albuminuria following RYGB. Together these data add to the evidence base for bariatric surgery as a potential therapeutic tool for judicious use in preventing the progression of DKD.
Footnotes
Author contributions: C.W.leR., R.V.C. and N.G.D. conceived and designed the study. R.V.C. conducted the surgery in humans and J.A.E. carried out surgery in the rats. Husbandry and routine longitudinal monitoring in rodents was carried out by N.G.D., J.AE. and A.L.C. C.M.A. collected prospective data on the human RYGB cohort. A.L.C. and N.G.D. analysed and interpreted ZDF rat data; the ZDF rat experiment, W.P.M. analysed and interpreted human RYGB cohort data. N.G.D. drafted the manuscript with critical input from all authors.
Data availability: All animal data including spreadsheets, original digital pathology files and electron micrographs are available from the authors upon written request and following agreement on the intended purpose of the request. Likewise, fully coded anonymised human data are available upon request for secondary data analysis.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Science Foundation Ireland (12/YI/B2480) to C.W.leR; European Foundation for the Study of Diabetes to N.G.D. and C.W.leR (BI 2017_3); and W.P.M.’s contribution was performed within the Irish Clinical Academic Training (ICAT) Programme, supported by the Wellcome Trust and the Health Research Board Ireland (Grant Number 203930/B/16/Z).
ORCID iD: Neil G Docherty  https://orcid.org/0000-0002-0961-2607
https://orcid.org/0000-0002-0961-2607
References
- 1. Gaede P, Tarnow L, Vedel P, et al. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 2004; 19: 2784–2788. [DOI] [PubMed] [Google Scholar]
- 2. Funk J, Ott V, Herrmann A, et al. Semiautomated quantitative image analysis of glomerular immunohistochemistry markers desmin, vimentin, podocin, synaptopodin and WT-1 in acute and chronic rat kidney disease models. Histochem Cell Biol 2016; 145: 315–326. [DOI] [PubMed] [Google Scholar]
- 3. Martin WP, Docherty NG, Le Roux CW. Impact of bariatric surgery on cardiovascular and renal complications of diabetes: a focus on clinical outcomes and putative mechanisms. Expert Rev Endocrinol Metab 2018; 13: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlsson LM, Romeo S, Jacobson P, et al. The incidence of albuminuria after bariatric surgery and usual care in Swedish Obese Subjects (SOS): a prospective controlled intervention trial. Int J Obes 2015; 39: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coimbra TM, Janssen U, Gröne HJ, et al. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int 2000; 57: 167–182. [DOI] [PubMed] [Google Scholar]
- 6. Neff KJ, Elliott JA, Corteville C, et al. Effect of Roux-en-Y gastric bypass and diet-induced weight loss on diabetic kidney disease in the Zucker diabetic fatty rat. Surg Obes Relat Dis 2017; 13: 21–27. [DOI] [PubMed] [Google Scholar]
- 7. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830. [DOI] [PubMed] [Google Scholar]
- 8. Bueter M, Abegg K, Seyfried F, et al. Roux-en-Y gastric bypass operation in rats. J Vis Exp 2012; 64: e3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lane PH, Steffes MW, Mauer SM. Estimation of glomerular volume: a comparison of four methods. Kidney Int 1992; 41: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 10. Haas M. Thin glomerular basement membrane nephropathy: incidence in 3471 consecutive renal biopsies examined by electron microscopy. Arch Pathol Lab Med 2006; 130: 699–706. [DOI] [PubMed] [Google Scholar]
- 11. Deegens JKJ, Dijkman HB, Borm GF, et al. Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int 2008; 74: 1568–1576. [DOI] [PubMed] [Google Scholar]