Abstract
Background and objectives:
The risk of major adverse cardiac and cerebrovascular events following acute coronary syndrome is increased in people with diabetes. Predicting out-of-hospital outcomes upon follow-up remains difficult, and no simple, well-validated tools exist for this population at present. We aim to evaluate several factors in a competing risks model for actionable evaluation of the incidence of major adverse cardiac and cerebrovascular events in diabetic outpatients following acute coronary syndrome.
Methods:
Retrospective analysis of consecutive patients admitted for acute coronary syndrome in two centres. A Fine–Gray competing risks model was adjusted to predict major adverse cardiac and cerebrovascular events and all-cause mortality. A point-based score is presented that is based on this model.
Results:
Out of the 1400 patients, there were 783 (55.9%) with at least one major adverse cardiac and cerebrovascular event (417 deaths). Of them, 143 deaths were due to non-major adverse cardiac and cerebrovascular events. Predictive Fine–Gray models show that the ‘PG-HACKER’ risk factors (gender, age, peripheral arterial disease, left ventricle function, previous congestive heart failure, Killip class and optimal medical therapy) were associated to major adverse cardiac and cerebrovascular events.
Conclusion:
The PG-HACKER score is a simple and effective tool that is freely available and easily accessible to physicians and patients. The PG-HACKER score can predict major adverse cardiac and cerebrovascular events following acute coronary syndrome in patients with diabetes.
Keywords: Diabetes mellitus, myocardial infarction, left ventricle ejection fraction, cardiovascular death, major adverse cardiac and cerebrovascular events, score
Introduction
Diabetes mellitus (DM) is a pro-inflammatory state that promotes and accelerates atherosclerosis.1 It is well known that people with diabetes and a history of acute coronary syndrome (ACS) are at higher risk of death from cardiovascular causes than from non-cardiovascular causes.2,3 In people with diabetes, the risk of death following ACS is increased compared to non-diabetics despite similar infarct size and left ventricular ejection fraction (LVEF).4 In VALsartan In Acute myocardial iNfarcTion (VALIANT) sub-study, for any given LVEF, the presence of DM was associated with a 37% higher risk of all-cause mortality with an adjusted hazard ratio (HR) of 1.37 (95% confidence interval (CI): 1.25–1.51).5 Post-infarction, patients from Germany and Finland with heart failure (HF) and reduced LVEF (HFrEF) demonstrated a significantly higher incidence of cardiac death in the presence of comorbid DM (HR: 3.8, 95% CI: 2.4–5.8; p < 0.001). Moreover, the incidence of sudden cardiac death (SCD) in patients with diabetes with LVEF > 35% was similar to its incidence in non-diabetics with LVEF ⩽ 35% (4.1% vs 4.9%, respectively).6
Upon inpatient admission with ACS, risk stratification is often employed to predict in-hospital mortality risk. The Global Registry of Acute Coronary Events (GRACE) score is commonly used for this purpose and has been shown to offer good predictive power for in-hospital mortality in diabetic patients as well.7 Unfortunately, forecasting out-of-hospital major adverse cardiac and cerebrovascular events (MACCE) upon follow-up remains difficult and no simple, well-validated tool exists for this population at present. The objective of this study was to examine the risk factors that increase the incidence of MACCE in the diabetic population following ACS and facilitate risk stratification of these patients using a simplified, easily accessible risk score.
Methods
Study design
This was a retrospective analysis of all consecutive patients admitted for ACS in two different centres. A total of 1562 patients with diabetes who were admitted for ACS between November 2003 and January 2017 were included. Both centres are tertiary hospitals with coronary units and 24 h availability of coronary interventionism. The study was approved by our Ethical Research Committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Definitions of variables of interest
ACS was defined as the presence of typical clinical symptoms, specifically chest pain and electrocardiographic (ECG) changes indicative of myocardial ischaemia/lesion and elevation of serum markers of myocardial damage.8 ACS was stratified into ST-elevation myocardial infarction (STEMI)- and non-STEMI-based on ECG findings. For the antecedent of previous coronary heart disease, patients needed to have a clinical diagnosis of myocardial infarction (MI), stable or unstable angina or angina-driven coronary revascularization. History of HF was coded if patients had at least one hospitalization with this main diagnosis at discharge in the past. It was also identified in those with typical signs and symptoms of HF who had compatible imaging findings (X-ray or echocardiogram). According to current guidelines9 and previous reports,10 optimal medical therapy (OMT) at discharge included joint implementation of these four treatments: antiplatelets, statins, beta-blockers and angiotensin-converter enzyme inhibitors or mineralocorticoid receptor antagonists (MRAs). LVEF was divided into four categories: LVEF > 51%, LVEF = 41%–51%, LVEF = 30%–40% and LVEF < 30%. A MACCE was defined as cardiovascular mortality, new MI, SCD, cerebrovascular accident (CVA) or HF episode.
Data collection
Data on risk factors, medical history, complementary testing, diagnostics and treatment at discharge were collected from all patients by trained medical staff. The diagnostic and therapeutic ACS protocols in both centres included blood tests in the emergency department and the first fasting state following hospital admission. Glomerular filtration rate was estimated from serum creatinine values with the Modification of Diet in Renal Disease Study equation.11 LVEF was documented by imaging at least 3 calendar months after the most recent MI, percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery.
Follow-up and outcome measures
Post-discharge follow-up conformed to a well-established protocol in each centre. The main endpoints assessed through follow-up were the major cardiovascular events defined above. Follow-up was completed via phone calls, review of electronic medical records and institutional databases. Alive status was confirmed by phone calls in the absence of appropriate medical reports within the institution.
Statistical analysis
Quantitative variables are presented as mean and standard deviation (SD). Categorical variables are presented as frequencies and percentages. Pearson’s chi-square test was used to compare observed frequencies between categorical variables, whereas Kruskal–Wallis test was chosen to compare continuous data grouped by variable with >2 levels. Multiple testing was addressed using a false discovery ratio (FDR) control with the Benjamini–Hochberg procedure. All analyses were performed using R v.3.4 (R Core Team, Vienna, Austria) with the ‘cmprsk’ and ‘riskRegression’ packages.
Competing risks models
A competing risk (CR) is an event whose occurrence either precludes the occurrence of another event under examination or fundamentally alters the probability of occurrence of this other event.12 The cause-specific hazard at time t (Cox CRs model) is defined by the instantaneous risk of failure per time unit due to one risk, as if there were no competing events. However, the HRs obtained are not directly related to the prediction of the cumulative incidence; thus, they are not suitable to predict a given patient’s global risk.
On the other hand, the HR of the subdistribution (Fine & Gray CR model)13 is interpreted as the probability of observing an event of interest in the next time interval, while knowing that either the event of interest did not happen until then or that a competing event was observed. Unfortunately, the absolute values of the regression coefficients in the Fine–Gray model have no direct interpretation. Sub-distribution HR denotes the direction but does not directly provide the magnitude of the effect of the covariate on the cumulative incidence function (CIF). Cause-specific hazard models (Cox-CR) are better suited for studying the aetiology of diseases (the risk independently posed by every covariate), whereas the Fine & Gray model has a use in predicting an individual’s risk (incidence) of an event. Both models were implemented and compared with the GRACE score.
Risk score
Fine–Gray models are suitable to develop risk scores that consider CRs. Accordingly, we developed a simplified, points-based risk-scoring system standardized by age, following Framingham’s score framework and state-of-the art methodology by Austin et al.14 This allows us to determine the incidence of the outcome within a specified duration of time that is associated with each of the possible values of the risk-scoring system. Finally, we studied and compared the concordance (c-index) and calibration properties of the final models and the score at different follow-up times.
Results
Study population and competing events
The mean age of our patients was 69.6 ± 10.8, and 415 (29.6%) were female. The mean LVEF was 53.9 ± 11.7 (Table 1). The mean follow-up was 1636.3 ± 1001.4 days.
Table 1.
Population characteristics.
| Variable | Category | Result | Variable | Category | Result |
|---|---|---|---|---|---|
| Gender – female | 415 (29.6%) | Number of vessels | 0 or 1 vessel | 750 (53.6%) | |
| Age (mean ± SD) | 69.6 ± 10.8 | 2 or 3 vessels | 574 (41%) | ||
| BMI (mean ± SD) | 29.9 ± 11.9 | Left main + 0/1 vessel | 14 (1%) | ||
| Hypertension | 1020 (72.9%) | Left main + 2/3 vessels | 62 (4.4%) | ||
| Smoker | 258 (18.4%) | Complete revascularization | 520 (37.1%) | ||
| LVEF (mean ± SD) | 53.9 ± 11.7 | CABG | 76 (5.4%) | ||
| LVEF category | 1. LVEF > 51% | 934 (66.7%) | Optimal medical therapy | 593 (42.4%) | |
| 2. LVEF = 41%–51% | 213 (15.2%) | MACCE in follow-up | 783 (55.9%) | ||
| 3. LVEF = 30%–40% | 204 (14.6%) | MI in follow-up | 293 (20.9%) | ||
| 4. LVEF ⩽ 30% | 49 (3.5%) | CVA in follow-up | 75 (5.4%) | ||
| Peripheral arterial disease | 209 (14.9%) | CHF in follow-up | 374 (26.7%) | ||
| Previous CHF | 95 (6.8%) | Sudden death | 40 (2.9%) | ||
| CKD | 147 (10.5%) | Days of follow-up (mean ± SD) | 1636.3 ± 1001.4 | ||
| GRACE (mean ± SD) | 148.7 ± 36.5 | Deaths | 508 (36.3%) | ||
| Killip | 1 | 1072 (76.6%) | |||
| 2 | 230 (16.4%) | ||||
| 3 | 77 (5.5%) | ||||
| 4 | 21 (1.5%) |
BMI: body mass index; CABG: coronary artery bypass grafting; CHF: congestive heart failure; CKD: chronic kidney disease; CVA: cerebrovascular accident; LVEF: left ventricle ejection fraction; MACCE: major adverse cardiac and cerebrovascular events; MI: myocardial infarction; SD: standard deviation; GRACE: Global Registry of Acute Coronary Events.
Out of the 1400 patients, there were 783 (55.9%) with at least one MACCE event (417 of them resulting in death). Of them, there were 374 (26.7%) HF episodes during follow-up (8 of them resulting in death), and 75 (5.4%) CVA episodes (none of them resulting in death). A total of 143 deaths were due to non-MACCE causes (Table 1, Online Appendix 5).
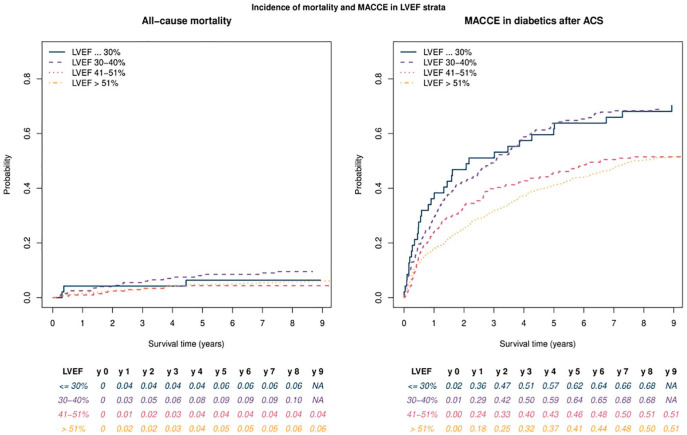
As shown in Online Appendix 1, the proportion of MACCE events increased with lower LVEF (p < 0.0001). There were no significant differences regarding treatment, except for diuretic therapy. Also, lower LVEF strata were associated with older patients (p = 0.0006) and with lower body mass index (BMI).
Risk models of MACCE versus all-cause mortality
For time-to-event models, we considered the risk of MACCE versus all-cause mortality as competing events. Multivariable analyses were adjusted by gender, age, diagnosis of hypertension, categorized LVEF, diagnosis of peripheral artery disease, history of congestive HF, chronic kidney disease (CKD), OMT and Killip class during ACS.
As shown in Table 2, Killip class, lower LVEF, CKD and age were all associated with an increased mortality rate. Predictive Fine–Gray models (CIF) showed that age, lower LVEF, peripheral artery disease and Killip class were associated with higher MACCE incidence (presenting higher sub-distributional hazards) (Figure 1(a)). Female gender and OMT were associated with lower mortality rates as well as longer event-free times. The incidence of cardiovascular outcomes in post-MI patients with diabetes with LVEF 35%–50% is comparable at >3 years to that seen in LVEF < 35%. Moreover, LVEF > 40% had a MACCE incidence that was 17% lower at 5 years [Figure 2(b)]. Conversely, lower LVEF does not increase non-cardiovascular mortality [Figure 2(a)]. The number of affected vessels and the presence of incomplete revascularization or coronary bypass procedure were not independently associated with MACCE incidence.
Table 2.
Competing risks models.
| CSH event-free survival | CSH mortality | CSH MACCE | CIF mortality | CIF MACCE | |
|---|---|---|---|---|---|
| Gender – female | 0.88 [0.75, 1.02] | 0.91 [0.80, 1.03] | 0.95 [0.80, 1.15] | 1.11 [0.69, 1.77] | 0.87 [0.74, 1.03] |
| Age | 1.04 [1.03, 1.05]*** | 1.02 [1.01, 1.02]*** | 1.00 [0.99, 1.01] | 1.03 [1.00, 1.05]* | 1.03 [1.02, 1.04]*** |
| Hypertension | 1.04 [0.88, 1.22] | 1.00 [0.88, 1.13] | 0.90 [0.75, 1.08] | 0.78 [0.47, 1.30] | 1.12 [0.95, 1.33] |
| Smoker | 1.12 [0.91, 1.37] | 1.01 [0.86, 1.18] | 1.00 [0.81, 1.23] | 1.53 [0.82, 2.85] | 0.99 [0.79, 1.23] |
| LVEF category number | 1.11 [1.02, 1.21]* | 1.07 [0.99, 1.15] | 0.98 [0.87, 1.10] | 0.92 [0.72, 1.19] | 1.12 [1.02, 1.23]* |
| Peripheral arterial disease | 1.30 [1.09, 1.56]* | 1.16 [0.99, 1.37] | 1.04 [0.79, 1.37] | 1.30 [0.77, 2.21] | 1.25 [1.02, 1.52]* |
| Previous CHF | 1.47 [1.15, 1.88]* | 1.41 [1.10, 1.80]* | 1.30 [0.80, 2.09] | 1.74 [0.91, 3.33] | 1.26 [0.93, 1.70] |
| CKD | 1.49 [1.21, 1.83]** | 1.27 [1.04, 1.55]* | 1.29 [0.93, 1.81] | 1.88 [1.09, 3.24]* | 1.21 [0.94, 1.55] |
| Killip | 1.30 [1.17, 1.44]*** | 1.19 [1.08, 1.31]** | 1.13 [0.96, 1.33] | 1.27 [0.94, 1.72] | 1.19 [1.06, 1.35]* |
| Optimal medical therapy | 0.81 [0.70, 0.94]* | 0.98 [0.88, 1.10] | 1.16 [0.99, 1.36] | 0.70 [0.43, 1.14] | 0.85 [0.73, 1.00]* |
CABG: coronary artery bypass grafting; CHF: congestive heart failure; CIF: cumulative incidence function (Fine–Gray competing risks models); CKD: chronic kidney disease; CSH: cause-specific hazard (Cox competing risks models); LVEF: left ventricle ejection fraction; MACCE: major adverse cardiac and cerebrovascular events.
Values in the range 0.001 to <0.05; **values in the range 0.0001–0.001; ***values <0.0001.
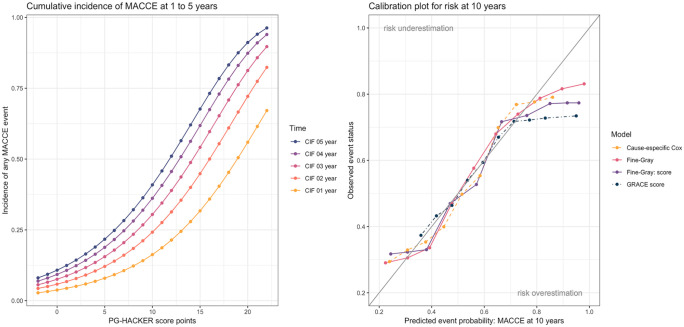
Figure 1.
(a) Cumulative incidence of MACCE. (b) Calibration plot for risk at 10 years of the two models and the scores.
Figure 2.
Cumulative incidence of MACCE and non-cardiac death across different LVEF categories.
Score
A points-based risk-scoring system – PG-HACKER score – was developed with the following variables (Online Appendix 2 and Online Appendix 3): gender (–1 point for female), age [points = (age – 20)/5], previous congestive HF (1 point), peripheral artery disease (1 point) and Killip class during ACS episode (K1 = 0, K2 = 1, K3 = 2 and K4 = 3 points). EF category (>51% = 0, 41%–51% = 1 and <41%= 2) and OMT (–1 point). Figure 1(a) and Online Appendix 3 show the mortality estimates associated with each score by year of follow-up. To facilitate its use in the clinical setting, we wrote a simplified HTML calculator and Android app based on the MACCE risk score for people with diabetes (PG-HACKER), that can be freely online at medicalc.github.io/pghacker (printed in PDF as Supplemental Online material for review).
Model evaluation: concordance and calibration
In the analysis of time-to-event data, a pair of patients is called concordant if the risk of the event predicted by a model is lower for the patient who experiences the event later in time. The concordance probability (C-index) is the frequency of concordant pairs among all pairs of subjects. It can be used to measure and compare the (overall) pre-test discriminative power of risk prediction models.
Estimates of the concordance index were 0.68 for the CR model, 0.67 for the score, 0.63 for the GRACE score and 0.77 for the Cox model. The calibration of the model was assessed comparing the predicted probability of MACCE to the observed probability of MACCE within 5 years, across the 10 deciles of predicted risk. The Fine-Gray risk model (FGR) model and the score displayed a good calibration at 10 years [Figure 1(b)], albeit with risk overprediction at higher deciles of predicted risk (very high-risk patients). GRACE score exhibited the worst calibration of all models, especially at higher risk patients.
Discussion
In this study, we examined, in a real-world cohort, the incidence of MACCE in the diabetic population following ACS. Our data confirm that patients with diabetes with previous ACS are much more likely to have MACCE events than to die of other causes. Moreover, for the first time, we provide a simple and effective score (named PG-HACKER) to predict MACCE following ACS in patients with diabetes. This may help practitioners to monitor changes in modifiable factors to assess the impact of medical interventions. Classical markers used in patients without DM such as the LVEF lose predictive capacities when taken alone. Such markers must be combined with multiple clinical parameters in order to accurately predict outcomes in this population. The recently published EUROASPIRE V study15 highlights the wide margin that still exists for improvement in secondary prevention after ACS. This proposed score aims to step forward towards more personalized medicine. Further investigations will now be needed to address whether mitigation of these conditions associated with worse outcomes may prove beneficial in reducing MACCE incidence.
The utility of risk scores for secondary prevention
Beyond mere risk prediction and stratification, individualized risk scores can be used in clinical practice to monitor changes in modifiable factors and to assess the impact of medical interventions. Currently, the GRACE logistic score is the most widespread in ACS. Although it predicts mortality, it has shown only slightly lower performance compared to the models presented for MACCE prediction in our cohort. However, it carries several disadvantages for outpatient follow-up. First, it relies entirely on factors that must be present at admission and cannot be recalculated unless a new admission due to ACS takes place. Hence, the GRACE score may be of less clinical utility for outpatient visits, where changes in risk factors may dynamically modify MACCE incidence. Moreover, previous studies have focused on cardiovascular mortality without considering death due to other causes as a CR, which may induce bias in predictions. Taking this into account, we evaluated several factors in a CRs model aimed to actionable, dynamic outpatient evaluation. Specifically, OMT as a covariable may enable treatment effect assessment for novel drugs. Our study also shows that intermediate LVEF has similar MACCE outcomes to LVEF ⩽ 30%, opening the door for more aggressive interventions for sudden death prevention in those patients.
MACCE prediction in the diabetic population post-ACS
HF patients with a history of ACS exhibit poorer outcomes with progressive reduction in LVEF.16,17 Savonitto et al. determined that in patients with diabetes with ACS, mortality prediction was dominated by markers of cardiac dysfunction. The most powerful predictor identified was N-terminal prohormone of brain natriuretic peptide (NT-ProBNP).18 Similarly, Evaluation of LIXisenatide in Acute coronary syndrome (ELIXA) trial data support the conclusion that brain natriuretic peptide (BNP) and NT-ProBNP are powerful predictors of HF, MI, stroke and death.19 Other risk factors they identified included age, prior HF and albuminuria. The Multinational MONItoring of trends and determinants in CArdiovascular disease (MONICA) study showed that mortality from acute MI in people with diabetes is four times higher in men and seven times higher in women than their non-diabetic counterparts.20
In our study, worsening outcomes with a progressive reduction in LVEF held in the diabetic population as well. As expressed in the PG-HACKER risk score, left ventricular function is an important predictor of outcomes, but DM is strongly associated with HF and death irrespective of LVEF. As such, the ventricular function must be combined with multiple clinical parameters (gender, age, hypertension, peripheral arterial disease (PAD), previous congestive HF, CKD and Killip class) in order to accurately predict outcomes in this population.
Risk factor modification
We believe that one of the most substantial benefits of the PG-HACKER score is to provide an easily accessible awareness tool for both the provider and the patient alike. It was previously demonstrated that a designated outpatient clinic for high-risk ACS improves long-term prognosis and largely reduces MACCE incidence and hospital readmissions.21 Quantification of risk through the PG-HACKER score may provide a more concrete understanding of the situation as well as the incentive to deliver aggressive prescription and adherence to OMT.
Glucose and insulin resistance are not the only targets available in our attempts to reduce MACCE following ACS in people with diabetes. The PG-HACKER score highlights multiple conditions that appear to contribute independently to MACCE risk in this cohort. Stringent management of all these conditions through special monitoring and implementation of aggressive treatment algorithms may prove beneficial.
As described above, one of the most prominent contributors to MACCE in patients with diabetes post-ACS is the presence and degree of HF. Sacubitril–valsartan is a drug that reduces the risk of cardiovascular death by 20% in patients with HF and LVEF < 40%.22 Furthermore, this treatment has been related to lower ventricular arrhythmias in patients with implantable cardioverter defibrillator (ICD).23,24 The clinical effect of sacubitril–valsartan in post-ACS is currently being investigated in the Prospective ARNI versus ACE Inhibitor Trial to DetermIne Superiority in Reducing Heart Failure Events After MI (PARADISE-MI) trial,25 and outcomes pertaining to the diabetic population may prove particularly interesting. Similarly, an endless number of risk factor modification regimens in optimizing blood pressure control, HF, PAD and CKD may hold research promise in the future.
SCD
Ubiquitously, current data seem to indicate that patients with diabetes are at a significant risk for SCD even if they have moderately reduced/mid-range LVEF.21 As such, this population may be one that benefits from broader indications for ICD implantation than those dictated by current guidelines. The results of the PRE-DETERMINE Study provided contemporary estimates of sudden arrhythmic death (SAD) in patients with coronary heart disease without severe systolic dysfunction. In their cohort of patients with coronary heart disease and LVEF greater than 30%–35%, SAD accounted for approximately one-fifth of total mortality and was the most common mode of cardiovascular death.2 Mid-range left ventricular function (LVEF 40%–49%) was more strongly associated with SAD than non-SAD, whereas age and New York Heart Association (NYHA) class II HF were more strongly associated with non-SAD. In the diabetic population, the already launched Multicenter Automatic Defibrillator Implantation Trial With Subcutaneous Implantable Cardioverter Defibrillator (MADIT S-ICD) study is designed to test the hypothesis that post-MI, patients with diabetes ⩾65 years of age, with an ejection fraction of 36%–49% will attain survival benefit from the implantation of a subcutaneous ICD.26 Shortly, we may see shifting ICD recommendations for people with diabetes with mid-range EF if pending data continue to support such trends. However, we believe that the true impact of ICD therapy must be interpreted in the context of the medical regimen implemented. The score herein proposed could serve to identify those at higher risk and who may stand to benefit from the most aggressive medical therapy following ACS.
Limitations
The present study has several limitations that deserve mention. Echocardiography was done in the setting of routine clinical practice and, for this reason, was limited to a focused assessment of cardiac structure and function. Moreover, evaluation of LVEF could not be standardized and therefore may have been subject to variations among different operators that may have resulted in the misclassification of some patients. Importantly, standardized reassessment of LVEF was not prespecified in the study design, and we cannot comment specifically on the impact of changes in LVEF and interval risk of death. Although all patients underwent a transthoracic echocardiogram, for a minority of them (mostly in order to assess LVEF in dubious cases or to rule out MI complications), a cardiac magnetic resonance (CMR) was also performed (n = 122 patients, 8.7%). From our point of view, due to the small amount of patients with both examinations and the correlation of both techniques, this fact should not have affected, particularly, the conclusion of the study. The diagnosis of HF was not centrally validated, and a central committee did not determine the cause of death. Patients with non-ischemic cardiomyopathy are at risk for cardiovascular mortality, and our findings may not be generalizable to this population. Furthermore, the process of creating a score implies dichotomizing continuous data with subsequent information loss. Risk overprediction of very-high-risk patients could also lead to overinflate the effect of novel interventions aimed at those patients. This is a known issue in routinely used scores27 to which single-number calibration measurements, such as the Hosmer–Lemeshow test, do not offer further information. In this regard, specific cohorts of very high-risk patients could offer novel risk calibration features in this subpopulation.
Conclusion
The PG-HACKER score is a simple and effective tool that is freely available and easily accessible to predict MACCE following ACS in patients with diabetes. Above all, we believe that the utility of this score will come from spreading awareness to the patient and physician alike. In identifying the conditions associated with worse outcomes, future investigation to mitigate these conditions may prove beneficial in reducing MACCE incidence.
Supplemental Material
Supplemental material, Online_appendix_1_Table for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_2_Table for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_3_Table for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_4_calibration_by_year_macce for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_5_flowchart_MACCE for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_6_Web_App_PGHR_score_final_MACCE for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_7_survival_macce_kaplan_meier for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_8_Table_survival_macce for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Aurora Baluja  https://orcid.org/0000-0002-5204-0771
https://orcid.org/0000-0002-5204-0771
Moisés Rodríguez-Mañero  https://orcid.org/0000-0001-7566-9321
https://orcid.org/0000-0001-7566-9321
Supplemental material: Supplemental material for this article is available online.
References
- 1. Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol 2004; 44: 2293–2300. [DOI] [PubMed] [Google Scholar]
- 2. Chatterjee NA, Moorthy MV, Pester JJ, et al. Sudden death in patients with coronary heart disease without severe systolic dysfunction. JAMA Cardiol 2018; 3: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cordero AA, Lopez-Palop R, Carrillo P, et al. Comparison of long-term mortality for cardiac diseases in patients with versus without diabetes mellitus. Am J Cardiol 2016; 117: 1088–1094. [DOI] [PubMed] [Google Scholar]
- 4. Malmberg K, Yusuf SS, Gerstein HC, et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) registry. Circulation 2000; 102: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 5. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349: 1893–1906. [DOI] [PubMed] [Google Scholar]
- 6. Junttila MJ, Barthel P, Myerburg RJ, et al. Sudden cardiac death after myocardial infarction in patients with type 2 diabetes. Heart Rhythm 2010; 7: 1396–1403. [DOI] [PubMed] [Google Scholar]
- 7. Baeza-Roman AA, de Miguel-Balsa E, Latour-Perez J, et al. Predictive power of the grace score in population with diabetes. Int J Cardiol 2017; 248: 73–76. [DOI] [PubMed] [Google Scholar]
- 8. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 9. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64: e139–e228. [DOI] [PubMed] [Google Scholar]
- 10. Iqbal J, Zhang Y-JYJ, Holmes DR, et al. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial at the 5-year follow-up. Circulation 2015; 131: 1269–1277. [DOI] [PubMed] [Google Scholar]
- 11. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 12. Teixeira LL, Rodrigues A, Carvalho MJ, et al. Modelling competing risks in nephrology research: an example in peritoneal dialysis. BMC Nephrol 2013; 14: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 14. Austin PC, Lee DS, D’Agostino RB, et al. Developing points-based risk-scoring systems in the presence of competing risks. Stat Med 2016; 35: 4056–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kotseva K, De Backer G, De Bacquer D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol 2019; 26: 824–835. [DOI] [PubMed] [Google Scholar]
- 16. Stecker EC, Vickers CC, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 2006; 47: 1161–1166. [DOI] [PubMed] [Google Scholar]
- 17. Vaduganathan M, Patel RB, Michel A, et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 2017; 69: 556–569. [DOI] [PubMed] [Google Scholar]
- 18. Savonitto S, Morici N, Nozza A, et al. Predictors of mortality in hospital survivors with type 2 diabetes mellitus and acute coronary syndromes. Diab Vasc Dis Res 2018; 15: 14–23. [DOI] [PubMed] [Google Scholar]
- 19. Wolsk E, Claggett B, Pfeffer MA, et al. Role of B-Type natriuretic peptide and N-terminal prohormone BNP as predictors of cardiovascular morbidity and mortality in patients with a recent coronary event and Type 2 diabetes mellitus. J Am Heart Assoc 2017; 6: e004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lundberg VV, Stegmayr B, Asplund K, et al. Diabetes as a risk factor for myocardial infarction: population and gender perspectives. J Intern Med 1997; 241: 485–492. [DOI] [PubMed] [Google Scholar]
- 21. Cordero AA, Bertomeu-Gonzalez V, Moreno-Arribas J, et al. Prognosis and lipid profile improvement by a specialized outpatient clinic for acute coronary syndrome patients. Atherosclerosis 2018; 275: 28–34. [DOI] [PubMed] [Google Scholar]
- 22. McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 23. De Diego CC, Gonzalez-Torres L, Nunez JM, et al. Effects of angiotensin-neprilysin inhibition compared to angiotensin inhibition on ventricular arrhythmias in reduced ejection fraction patients under continuous remote monitoring of implantable defibrillator devices. Heart Rhythm 2018; 15: 395–402. [DOI] [PubMed] [Google Scholar]
- 24. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2009; 352: 225–237. [DOI] [PubMed] [Google Scholar]
- 25. Prospective ARNI vs ACE inhibitor trial to determine superiority in reducing heart failure events after MI. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02924727 (accessed 7 March 2019). [DOI] [PubMed]
- 26. Kutyifa V, Beck CC, Brown MW, et al. Multicenter Automatic Defibrillator Implantation Trial-Subcutaneous Implantable Cardioverter Defibrillator (MADIT S-ICD): design and clinical protocol. Am Heart J 2017; 189: 158–166. [DOI] [PubMed] [Google Scholar]
- 27. DeFilippis AP, Young RR, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015; 162: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Online_appendix_1_Table for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_2_Table for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_3_Table for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_4_calibration_by_year_macce for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_5_flowchart_MACCE for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_6_Web_App_PGHR_score_final_MACCE for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_7_survival_macce_kaplan_meier for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research
Supplemental material, Online_appendix_8_Table_survival_macce for Prediction of major adverse cardiac, cerebrovascular events in patients with diabetes after acute coronary syndrome by Aurora Baluja, Moisés Rodríguez-Mañero, Alberto Cordero, Bahij Kreidieh, Diego Iglesias-Alvarez, Jose M García-Acuña, Alvaro Martínez-Gómez, Rosa Agra-Bermejo, Leyre Alvarez-Rodríguez, Charigan Abou-Jokh, Mónica López-Ratón, Francisco Gude-Sampedro, Julián Alvarez-Escudero and Jose R González-Juanatey in Diabetes & Vascular Disease Research