Abstract
Empagliflozin reduces the risk of cardiovascular mortality in subjects with type 2 diabetes. We demonstrated that empagliflozin increases blood viscosity and carotid shear stress and decreases carotid wall thickness. Shear stress is the force acting on the endothelial surface and modulates arterial function. The current study evaluates the influence of empagliflozin on brachial artery shear stress and endothelial function compared to incretin-based therapy. The study is a nonrandomized, open, prospective cohort study including 35 subjects with type 2 diabetes administered empagliflozin or incretin-based therapy. Shear stress was calculated with a validated formula, and endothelial function was evaluated using the flow-mediated dilation technique. Both treatments resulted in comparable reductions in blood glucose and glycated haemoglobin. Brachial artery shear stress significantly increased exclusively in the empagliflozin group (61 ± 20 vs 68 ± 25 dynes/cm2, p = 0.04), whereas no significant difference was detected in the incretin-based therapy group (60 ± 20 vs 55 ± 12 dynes/cm2, p = not significant). Flow-mediated dilation significantly increased in the empagliflozin group (4.8 ± 4.5% vs 8.5 ± 5.6%, p = 0.03). Again, no change was detected in the incretin-based therapy group (5.1 ± 4.5% vs 4.7 ± 4.7%, p = not significant). The present findings demonstrate the beneficial effect of empagliflozin on shear stress and endothelial function in subjects with type 2 diabetes independent of the hypoglycaemic effect.
Keywords: Empagliflozin, blood viscosity, rheology, shear stress, endothelial function
Introduction
Atherosclerosis is a complex disease involving the function and morphology of small and large arteries.1 The function of the arteries through the endothelium rests on a delicate balance that can be disturbed by major cardiovascular risk factors, such as diabetes mellitus.2 The major challenge in the prevention of atherosclerosis is to detect early arterial abnormalities to implement all of the procedures limiting overt cardiovascular diseases.3
In recent years, the new antidiabetic medication empagliflozin has been reported to reduce cardiovascular mortality and morbidity through mechanisms not yet fully understood.4 Empagliflozin is a sodium–glucose cotransporter protein 2 (SGLT2) inhibitor whose mechanism of action is mainly to limit the absorption of glucose by the kidney. It has been hypothesized that urinary glucose loss could lead to increased hematocrit, with possible consequences for the function of the endothelium. Indeed, significant increases in hematocrit and haemoglobin were described in a recent post hoc analysis of the Empagliflozin Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG Outcomes) study.5 Changes in hematocrit and haemoglobin appear to be the variables with the largest impact on the rates of cardiovascular diseases. Consistent with this result, we have recently demonstrated that empagliflozin influences blood rheology and reduces intimal-plus-media thickening of carotid arteries after only 3 months of treatment. Through osmotic diuresis, empagliflozin increases blood viscosity and subsequently increases shear stress (SS), which is the major hemodynamic force acting on the endothelial surface promoting the synthesis of vasoactive and atheroprotective substances.6 SS physiologically modulates endothelial function in both acute and chronic conditions. In detail, acute transient increases in SS, which occurs during reactive hyperaemia following ischaemia or exercise, and induces arterial vasodilation to meet metabolic demand. In other words, SS might be defined as the mechanical regulator of peripheral blood flow.7 In the long term, SS influences vessel wall morphology and the development of atherosclerotic plaque.8–10 The lower the SS is, the greater the wall thickness and plaque formation risk. SS can be calculated if blood viscosity, blood velocity and arterial diameter are known.11
We designed the current research with the aim to evaluate whether empagliflozin influences short-term SS and endothelial function of the brachial artery evaluated by the ultrasound-based flow-mediated dilation (FMD) technique. The FMD technique estimates the capacity of SS and endothelium to cause vasodilation after local transient ischaemia. Vasodilation is usually estimated to occur 50–60 s after ischaemia. However, the timing of maximal dilation changes among subjects. In most cases, and mainly in healthy young subjects, it occurs at 50–60 s; however, in subjects with cardiovascular diseases or cardiovascular risk factors, it might be delayed. Interestingly, subjects with late dilation have a higher atherosclerotic burden despite an acceptable dilation than subjects with maximal dilation at 50–60 s.12–14 Thus, we also evaluated whether empagliflozin influences the kinetics of dilation.
Methods
Study design and subjects
This is a nonrandomized, open, prospective and exploratory study including subjects with type 2 diabetes attending our metabolic clinic administered empagliflozin or incretin-based therapy in combination with insulin ± metformin to improve metabolic control. Drug assignment was suggested by the physician not involved in the research. The study was approved by the independent local Ethical Committee ‘Calabria Centro’, protocol number 105-2016, and was conducted according to the ethical guidelines of the Declaration of Helsinki. All subjects signed an informed consent before enrolment in the study, the nature of which was clearly explained. Inclusion criteria were age ⩾18 and <75 years, glycated haemoglobin >7% and <9.5% and current therapy with insulin ± metformin. Exclusion criteria were type 1 diabetes and previous treatment with or contraindication to gliflozin or incretin-based therapy. The study included three visits as follows: baseline, and 1- and 3-month visits. At baseline, before starting the new treatment, and at the 3-month visit, all patients underwent clinical examination and venous blood withdrawal for blood viscosity, HbA1c, fasting blood glucose (FBG) and lipids measurement. Blood viscosity was also measured at the 1-month visit. A vascular study was performed at each visit. At the 1-month visit, self-monitoring blood glucose (SMBG) was used to uptitrate medications based on mean fasting and postprandial glucose.15 Cardiovascular risk factors were defined as follows: hypertension, use of anti-hypertensive drugs or blood pressure >140/90 mmHg; hyperlipidaemia, use of lipid-lowering agents or total-cholesterol and/or triglycerides above the targets suggested for diabetes; and smoking habit, regular smoking during the previous 12 months. Furthermore, subjects were defined as obese if the body mass index (BMI), calculated as kg/m2, was ⩾30. We also enrolled 25 nondiabetic healthy subjects with no other cardiovascular risk factors as control subjects to compare baseline SS and FMD. Healthy subjects were studied on only one occasion.
Blood analyses
Blood was withdrawn after an 8-h fast and soon after the vascular study. The subjects were asked to inject basal insulin the evening before the study and not to take metformin the morning of the study. Fasting plasma glucose (FPG), HbA1c and lipids [total-cholesterol (chol), high-density lipoprotein (HDL-chol), low-density lipoprotein (LDL-chol) and triglycerides] were measured with commercially available kits. HbA1c was measured with high-performance liquid chromatography aligned with Diabetes Control and Complications Trial (DCCT). Blood and plasma viscosity were measured within 2 h following blood withdrawal and after adding heparin (35 U/ml). Viscosity was measured at 37 °C with a cone-plate viscometer (Well-Brookfield DVIII, Middleboro, MA, United States) equipped with a cp-40 spindle. Blood viscosity was evaluated at shear rate 225/s, which is the shear rate that develops in the mid-sized and large arteries.11
Echo-Doppler
All subjects underwent evaluation of brachial arteries and FMD tests at baseline and at the 1- and 3-month visits. The study was performed using an echo-Doppler Philips HD 11XE (Royal Philips Electronics, the Netherlands) equipped with a 12- to 3-MHz linear array, steerable pulsed wave Doppler and simultaneous electrocardiogram (ECG) recording before blood withdrawal in a temperature-controlled (24 °C) room. The single expert sonographer performing the study was blinded to hypoglycemic treatment. On the day of the study, subjects were asked to consume a light dinner and to refrain from exercise and smoking. The sonographer visualized the brachial artery of the nondominant arm approximately 10 cm above the elbow. The probe adapted the frequency automatically based on the depth of artery. The gain setting and the position were adjusted until the lumen–intima interface was clearly displayed on the screen. Thereafter, the skin was marked, and the probe was clamped to maintain the same position throughout the study. Brachial artery diameter was recorded at baseline and at 1, 2 and 3 min after forearm ischaemia. Ischaemia was created by inflating a pneumatic cuff, which was placed around the forearm immediately below the elbow, at supra systolic pressure for 5 min. Brachial artery internal diameter (ID), which is defined as the distance from the intima–lumen interface of the near wall and the lumen–intima interface of the far wall, was measured offline using dedicated software (AUTODESK Design Review, BSA, Italy).12 The diameter was measured in 1-cm lengths at three different locations of the vessel. The mean of the three measurements was used for statistical analyses. FMD was expressed as the percentage of dilation from baseline to each observation time and was calculated as follows: {[postdeflation (1, 2, 3 min) ID − baseline ID/baseline ID]}. The highest value detected during the study was defined as the peak FMD. Finally, based on the time of maximal dilation, subjects were divided into three different groups: early dilators, peak dilation at 1 min; late dilators, peak dilation after 1 min; no dilators, absence of dilation. Blood flow velocities, systolic peak velocity (SPV), end diastolic (EDV) and mean velocity (MV) of the brachial artery were automatically detected by the instrument.12
SS measurement
Peak wall SS (τP) and mean wall SS (τM) were calculated based on blood viscosity at shear rate 225/s, blood flow velocity (SPV, MV) and ID using the following formulas:
where η is the blood viscosity measured in poise, SPV and MV in cm/s and ID in cm. The coefficients of variation of τP and τM in our laboratory were 3% and 2%, respectively.11
Statistical analyses
Analyses were performed using SPSS 23 for Macintosh. Subjects with diabetes were divided in two groups based on additional treatment to ongoing therapy: the empagliflozin and incretin groups. Nondiabetic healthy subjects were defined as the control group. Based on the time to peak dilation at the FMD test, subjects in the empagliflozin and incretin groups were divided into three different categories: early, late and no dilators. The incretin group was further divided into those who were administered sitagliptin or liraglutide to evaluate drug-related differences. Triglycerides, blood viscosity and SS were not normally distributed; therefore, they were log-transformed before statistical analyses. Continuous variables were compared using the t-test for paired and unpaired tests where applicable. The chi-square test was used to compare percentages between groups. Analysis of variance (ANOVA) and Bonferroni post hoc tests as well as the general linear model for repeated measures were used to evaluate differences among subjects divided in three groups.
Results
Subjects with type 2 diabetes who met the inclusion and exclusion criteria were enrolled after signing the informed consent. The characteristics of the subjects in the empagliflozin (n = 20) and incretin (n = 15) groups are reported in Table 1. No statistically significant differences were detected between the two groups.
Table 1.
Age, gender, disease duration, smoking habit and clinical characteristics of subjects divided according to suggested therapy.
| Empagliflozin group | Incretin group | |
|---|---|---|
| Number | 20 | 15 |
| Age (years) | 58 ± 9 | 60 ± 7 |
| Males (%) | 75 | 80 |
| Disease duration (years) | 15 ± 9 | 17 ± 10 |
| Obesity (%) | 50 | 40 |
| Hypertension (%) | 93 | 100 |
| Hyperlipidaemia (%) | 90 | 93 |
| Smoking habit (%) | 30 | 33 |
SD: standard deviation.
The data are expressed as the means ± SDs or percentages.
The mean age of the 25 healthy control subjects was 55 ± 2 years. Moreover, 76% were male, and 28% were smokers. Again, no differences were detected between all subjects with diabetes and controls. All subjects with diabetes were taking basal insulin ± metformin at the maximal tolerated dose. In all, 20 were administered empagliflozin, and 15 were administered incretin-based (7 liraglutide and 8 sitagliptin) therapy as an add-on therapy. The starting empagliflozin dose was 10 mg once daily, which was uptitrated at 25 mg once daily in 13 subjects (65%). Sitagliptin was given at the dose of 100 mg once daily, while liraglutide was started at the dose of 0.6 mg once daily and uptitrated to 1.2 after 1 week in all subjects. In four subjects with unsatisfactory glycaemic control based on FPG and SMBG, liraglutide was further increased to 1.8 mg once daily. The mean daily insulin injected at baseline was 43 ± 20 U in the empagliflozin group and 39 ± 26 U in the incretin group. At the end of the study, the insulin dosage was slightly but not significantly reduced in both groups (39 ± 17 U and 36 ± 23 U in the empagliflozin and the incretin groups, respectively). The results did not change when subjects in the incretin group were divided according to specific drug (liraglutide and sitagliptin), and the data were compared with those of the subjects in the empagliflozin group (data not shown). All hypertensive (n = 34) and hyperlipidaemic (n = 33) subjects were taking specific treatments [62% renin–angiotensin–aldosterone system (RAAS) inhibitors, 38% RAAS inhibitors plus diuretic and 100% statins].
Table 2 shows biochemical and clinical variations during the 3-month treatment period. At baseline, the only difference between the two groups was the higher heart rate in the empagliflozin group. Body weight, BMI, waist circumference, FPG and HbA1c significantly decreased, and HDL-cholesterol significantly increased in the empagliflozin group. Incretin subjects had significant reductions in FPG, HbA1c and total-cholesterol at the follow-up visit, with no significant change in body weight. In particular, no changes were observed in patients taking liraglutide, while FPG and total-cholesterol significantly decreased (FPG baseline 9.1 ± 0.4 and 3 month 7.4 ± 0.6 mmol/L, p < 0.01; total-cholesterol baseline 4.7 ± 1.8 and 3 month 4.1 ± 1.4 mmol/L, p < 0.01) in those taking sitagliptin.
Table 2.
Biochemical and clinical variables measured at baseline and after 3 months in subjects according to therapy.
| Empagliflozin group |
Incretin group |
|||
|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |
| Number | 20 | 20 | 15 | 15 |
| Body weight (kg) | 89 ± 14 | 85 ± 13* | 80 ± 8 | 79 ± 10 |
| BMI (kg/m2) | 30 ± 4 | 29 ± 4* | 27 ± 3 | 26 ± 3 |
| Waist (cm) | 107 ± 10 | 105 ± 10* | 102 ± 7 | 100 ± 8 |
| SBP (mmHg) | 141 ± 14 | 136 ± 16 | 136 ± 23 | 135 ± 12 |
| DBP (mmHg) | 86 ± 11 | 84 ± 10 | 78 ± 8 | 79 ± 9 |
| HR (bpm) | 75 ± 11# | 69 ± 17 | 62 ± 6 | 69 ± 9 |
| FPG (mmol/L) | 10.3 ± 3.1 | 7.9 ± 1.1* | 9.7 ± 1.7 | 8.1 ± 0.9* |
| HbA1c (%) | 8.4 ± 0.7 | 7.6 ± 0.9* | 8.3 ± 0.7 | 7.7 ± 0.9^ |
| Total-chol (mmol/L) | 4.09 ± 0.91 | 4.12 ± 0.98 | 4.51 ± 1.40 | 4.17 ± 1.17* |
| HDL-chol (mmol/L) | 1.11 ± 0.34 | 1.19 ± 0.31* | 0.98 ± 0.31 | 1.04 ± 0.28 |
| LDL-chol (mmol/L) | 2.20 ± 0.78 | 2.28 ± 0.91 | 2.31 ± 1.17 | 2.25 ± 0.88 |
| Triglycerides (mmol/L) | 1.76 ± 1.18 | 1.45 ± 0.67 | 2.13 ± 1.55 | 1.88 ± 1.29 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; FPG: fasting plasma glucose; HbA1c: glycated haemoglobin; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SD: standard deviation.
The data are expressed as the means ± SDs; t-test for paired data *p < 0.002, ^p < 0.02 versus baseline; t-test for unpaired data #p = 0.01 versus the incretin group.
Blood viscosity, which was measured at shear rate 225/s, significantly changed in the empagliflozin group, while no change was detected in the incretin group. In detail, empagliflozin: baseline 4.87 ± 0.57 cP; 1 month 5.19 ± 0.75 cP; 3 month 5.32 ± 0.66 cP; p < 0.0001; and incretin: baseline 4.66 ± 0.56 cP; 1 month 4.53 ± 0.50 cP; 3 month 4.98 ± 0.73 cP; p = not significant (NS). Blood viscosity measured at shear rate 225/s in control healthy subjects was 4.80 ± 0.42 cP, which did not differ from the baseline blood viscosity measured in subjects with diabetes (4.78 ± 0.57 cP).
Brachial artery parameters collected during the study in subjects taking empagliflozin or incretin therapy are reported in Table 3. FMD measured at 1 min significantly increased in the empagliflozin group, while it was unchanged in the incretin group and in subjects taking liraglutide or sitagliptin. Peak FMD increased in the empagliflozin group without reaching a statistically significant difference. When we compared variables measured in the two groups at baseline and the 1- and 3-month visits, we found significantly higher FMD at 1 min and peak FMD values in the empagliflozin group than in the incretin group. Brachial artery diameter and blood flow velocity did not change during the study in either group.
Table 3.
Brachial artery diameter and blood velocity in empagliflozin and control group measured at baseline and at the 1- and 3-month visits.
| Empagliflozin group |
Incretin group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 month | 3 months | p | Baseline | 1 month | 3 months | p | |
| Number | 20 | 20 | 20 | – | 15 | 15 | 15 | – |
| BA ID T (mm) | 3.58 ± 0.69 | 3.50 ± 0.57 | 3.51 ± 0.52 | NS | 3.51 ± 0.55 | 3.55 ± 0.51 | 3.66 ± 0.59 | NS |
| SPV (cm/s) | 108 ± 20 | 107 ± 21 | 109 ± 26 | NS | 108 ± 21 | 109 ± 26 | 119 ± 22 | NS |
| EDV (cm/s) | 25 ± 6 | 25 ± 6 | 24 ± 4 | NS | 24 ± 6 | 27 ± 5 | 24 ± 8 | NS |
| MV (cm/s) | 41 ± 8 | 41 ± 4 | 39 ± 6 | NS | 38 ± 7 | 41 ± 2 | 40 ± 10 | NS |
| FMD 1 min (% dilation) | 4.8 ± 4.5 | 7.7 ± 5.3^ | 8.5 ± 5.6*^ | 0.03 | 5.1 ± 4.5 | 4.5 ± 3.8 | 4.7 ± 4.7 | NS |
| Peak FMD (% dilation) | 7.1 ± 5.3 | 9.3 ± 5.8 | 9.7 ± 5.8^ | NS | 6.8 ± 3.0 | 6.5 ± 5.0 | 5.9 ± 4.0 | NS |
BA: brachial artery (rest condition); ID: internal diameter; NS: not significant; SPV: systolic peak velocity; EDV: end diastolic velocity; MV: mean velocity; FMD: flow-mediated dilation; SD: standard deviation.
The data are expressed as the means ± SDs; p column shows the general linear model for repeated measures; Bonferroni post hoc test: *p = 0.04 versus baseline; ^t-test for unpaired data p = 0.04 versus the Incretin group.
The mean and peak brachial artery wall SS values at baseline and after treatment are reported in Table 4. Peak SS significantly increased in the empagliflozin group, while no variation was detected in the incretin group. The mean wall SS was significantly higher in the empagliflozin group than in the incretin group after the 1- and 3-month treatment.
Table 4.
Mean and peak brachial artery wall shear stress measured at baseline and after treatment.
| Empagliflozin group |
Incretin group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 month | 3 months | p | Baseline | 1 month | 3 months | p | |
| Number | 20 | 20 | 20 | – | 15 | 15 | 15 | – |
| Mean wall SS (dynes/cm2) | 29 ± 14 | 36 ± 20^ | 38 ± 20^ | NS | 24 ± 13 | 24 ± 17 | 23 ± 15 | NS |
| Peak wall SS (dynes/cm2) | 61 ± 20 | 65 ± 23* | 68 ± 25* | 0.04 | 60 ± 20 | 54 ± 19 | 55 ± 12 | NS |
SS: shear stress; NS: not significant; SD: standard deviation.
The data are expressed as the means ± SDs; p column shows the general linear model for repeated measures; Bonferroni post hoc test: *p < 0.01 versus baseline; t-test for unpaired data ^p < 0.05 versus the incretin group.
We further compared SS and FMD in subjects with diabetes and control subjects. Peak wall SS as well as FMD 1 min were significantly higher in controls: peak wall SS, control subjects 107 ± 42 dynes/cm2 versus subjects with diabetes 61 ± 17 dynes/cm2, p < 0.0001; FMD 1 min, control subjects 7.1 ± 2.8 versus subjects with diabetes 4.9% ± 4.4%, p = 0.03.
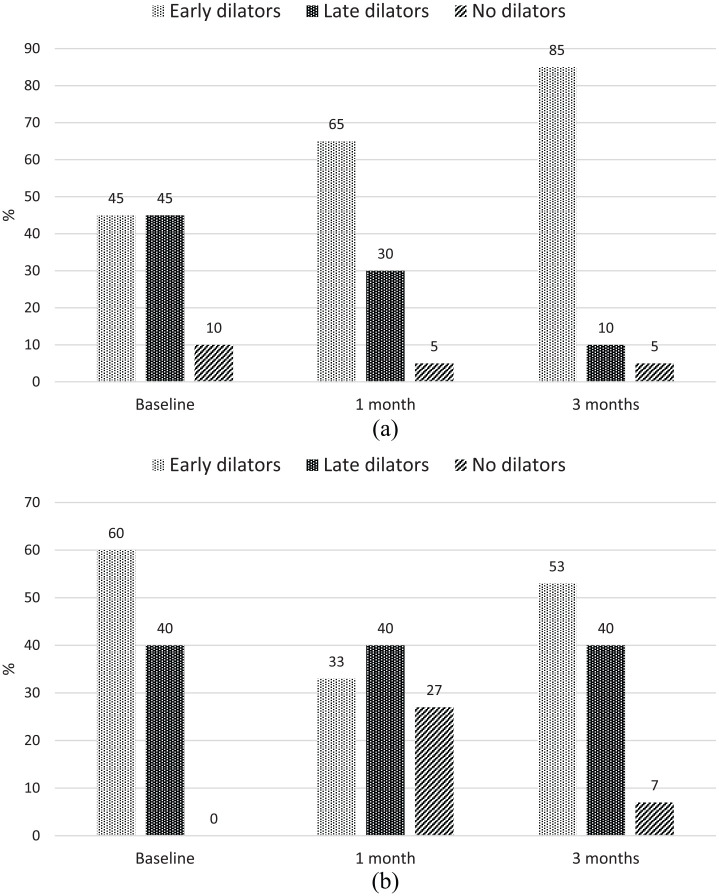
Finally, subjects with diabetes were further divided according to the time of maximal dilation into three different FMD categories: early, late and no dilators. The prevalence of each category at different observation times is illustrated in Figure 1. There were significant increases in early dilators after 1 and 3 months in the empagliflozin group (p = 0.01), while no differences were detected in the incretin group.
Figure 1.
Prevalence of early, late and no dilators in (a) the empagliflozin group and (b) the incretin group.
Discussion
The present findings show that empagliflozin, but not incretins, significantly increases brachial artery wall SS and flow-mediated vasodilation. Wall SS is the ‘controller’ of vascular tone. Each variation of SS causes, through the stimulation of appropriate receptors, the release of vasoactive substances that modify the vascular tone to restore the initial value of SS. As a result, the blood flow changes and meets the exact metabolic needs of the tissue.16 In the long term, the wall SS level influences the morphology of the arteries, modifying the thickness of the vessel wall and predisposing to the formation of atherosclerotic plaques. Within certain limits, higher levels of wall SS exert atheroprotective effects and favour the release of nitric oxide (NO). Cardiovascular risk factors, including diabetes, alter the endothelial response to SS variations, which can be assessed through flow-mediated (or SS-mediated) vasodilatation of the brachial artery.12–14,17
In the present study, we hypothesized that empagliflozin might influence SS and endothelial function by influencing blood viscosity. Therefore, we accurately measured blood flow velocity, arterial diameter and blood viscosity. The results demonstrate that treatment with empagliflozin increases blood viscosity and consequently increases wall SS. The increase in the latter is certainly independent of the improved glycaemic compensation, as the patients treated with incretin show a comparable improvement of blood glucose without any effect on the SS values. We believe that higher levels of SS are responsible for the improved FMD according to the physiological mechanism that involves higher shear, higher stimulus and higher arterial dilation.
To our knowledge, this is the first study demonstrating the beneficial effect of empagliflozin on vascular function. A similar study, named the Dapagliflozin Effectiveness on Vascular Endothelial Function and Glycemic Control (DEFENCE) study, which aimed to evaluate the effect of dapagliflozin on endothelial function and glycaemic control, was recently published.18 However, in that study, the authors did not report any improvement of vascular function mediated by the SGLT2 inhibitor. The technique used to evaluate endothelial function was roughly the same, but the study design, patients and metabolic characteristics were different. Indeed, subjects enrolled in the DEFENCE study had quite good glycaemic control at baseline that remained unchanged during the study and had shorter disease durations. Furthermore, the LDL-cholesterol value was significantly increased at the follow-up visit, and the prevalence of hypertensive subjects taking anti-hypertensive drugs was definitely lower in the dapagliflozin group than in the incretin group. Subjects enrolled in our study had longer disease durations, suboptimal HbA1c levels at baseline and other cardiovascular risk factors that were well controlled. Indeed, in our study, LDL-cholesterol was in the target range throughout the study, and greater than 90% of hypertensive subjects were taking anti-hypertensive drugs at the time of the enrolment. These differences might account for the different outcomes in the DEFENCE study and our study. It is noteworthy that baseline FMD was comparable in our study and the DEFENCE study. In the DEFENCE study, no information about blood flow or arterial diameter was provided. By comparing these two studies, we can state that advanced diabetes should not be considered a limitation. On the contrary, this information should stimulate the intensification of therapy to achieve benefits that go beyond glycaemic control. Empagliflozin and likely other molecules belonging to the same class are not only effective in terms of vascular protection but are also safe as demonstrated in these pivotal trials. Based on our current and previous results and results from the post hoc analysis by Inzucchi et al.,5 the hypothesis that the extraglycaemic effect of empagliflozin on blood rheology might represent a benefit in the management of cardiovascular diseases is strongly supported. Treatment with empagliflozin also changes the vasodilating pattern. Brachial artery SS and blood viscosity are crucial not only for the magnitude but also for the timing of vasodilation.19,20 Very often, the vasodilatory response is evaluated only in the first 50–60 s after cuff deflation. This approach is limiting because some subjects dilate after 1 min.21 The delayed vasodilation seems to be important as a cardiovascular risk factor as a damped dilation after 1 min. We demonstrated that late dilation is frequent in subjects with diabetes and is associated with carotid and coronary atherosclerosis and higher Framingham risk scores.13,14 The reasons for delayed vasodilation are unknown and are likely not mediated by NO release.19 Microcirculation downstream of the brachial artery might influence the timing of maximal dilation.22 The opinion is that additional late-operating mechanisms mediate the dilation of the artery but are not indicative of good health in the artery. The absence of dilation or even vasoconstriction represents the worst condition from a cardiovascular risk perspective.23 In our study, we observed a significant increase in early dilators and decreases in late and no dilators in the empagliflozin group after 3 months, reinforcing the additional benefit of empagliflozin on vascular reactivity based on the time of maximal dilation. No significant change in the kinetics of vasodilation was detected among subjects taking incretin-based therapy.
Improvements in endothelial function and more general vessel functions have been described in two other studies. One study describes reductions in in vivo arterial stiffness and afterload, and the other describes an attenuation of endothelial dysfunction evaluated by acetylcholine in vivo in aortic rings with empagliflozin.24,25 A recent meta-analysis conducted to investigate the effects of newer antidiabetic drugs demonstrated that the SGLT2 inhibitor dapagliflozin might influence arterial stiffness.26
Beyond these results, the rapidity with which the effect is obtained in our study is interesting and worthy of comment. Indeed, endothelial function improves after 1 month of treatment. The data are consistent with the results of the large cardiovascular outcome trial EMPA-REG. In this trial, subjects who were given empagliflozin showed early and significant reductions in the rates of major adverse cardiac events, mainly cardiovascular mortality and heart failure. Possible mechanisms hypothesized in the slowing of the progression of heart failure are increased diuresis leading to reduced extracellular liquid volume, improved cardiac metabolism, alleviation of cardiac stress by reduction of cardiac pre- and post-load and systolic pressure, suppression of adverse neuro-hormonal systems and reduction in cardiac fibrosis and hypertrophy.27 We believe that the present results might support the hypothesis of improved cardiac function as a consequence of improved vascular function. As previously noted, haemodynamic, that is, SS, plays an important role in the modulation of endothelial function and vascular tone. A target SS value has not been identified. However, when we compare subjects with higher SS to subjects with lower SS, the capacity of the vessel to dilate in response to ischaemia is higher and the presence of atherosclerotic plaques is lower in subjects with higher SS.8,9 With regard to the present findings, the mechanism inducing the increase in SS is precisely the modification of blood viscosity. The present study first demonstrates that the SGLT2 inhibitor empagliflozin causes significant increases in brachial artery SS and, consequently, a significant increase in FMD.
The study has some limitations. It is not randomized, and the number of subjects enrolled is low. In the attempt to reduce the impact of the first limit, the operator performing the vascular study was totally blinded to the treatment suggested to each patient. As far as the second point is concerned, the study was conceived as a pilot study to verify whether, even in a small sample, the initial hypothesis would be confirmed by preliminary results. However, we also performed a post hoc power calculation to determine whether our study was adequately powered to detect the treatment effect in 35 subjects divided into two treatment arms.28 Based on the current evidence, the mean FMD in subjects with diabetes ranges from 5% to 6%. An increase in 5% of the mean FMD value can be considered clinically relevant. Therefore, based on this assumption, considering that the standard deviation (SD) of the FMD technique at our laboratory is 4.5% and including α = 0.05 and β = 0.20, the total number of subjects to be included into the study should be 30.
In summary, for the first time, this study demonstrates the beneficial in vivo effect of empagliflozin on hemodynamic forces regulating endothelial function. The results are consistent with other recent published experimental animal and human studies describing the effect of this new drug on vascular function evaluated using different techniques. All of these results make empagliflozin and SGLT2 inhibition in general attractive antidiabetic therapeutic targets given the additional cardiovascular benefits.
Acknowledgments
We thank the Department of Health Science, University Magna Graecia Catanzaro, for the logistic support.
Footnotes
Authors’ contribution: C.I. participated in the planning and write-up of the study. A.C. and M.P. performed the US study. M.F. performed the blood viscosity measurements. R.F. was the specialized nurse who participated in the clinical study. C.G. collected and organized data. F.C. participated in the design of the study and organized visits. A.G. was responsible for the interpretation of the data and the editing of the article. All authors read and approved the final article.
Consent for publication: On behalf of all authors, I give consent for publication.
Data availability: The datasets generated during and/or analysed during the current study are available on reasonable request.
Ethical approval and consent to participate: The study was approved by the independent local Ethical Committee ‘Calabria Area Centro’ (protocol number 105-2016) and was conducted according to the ethical guidelines of the Declaration of Helsinki. All participants provided informed consent to be included in the study.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Concetta Irace  https://orcid.org/0000-0001-5182-5473
https://orcid.org/0000-0001-5182-5473
References
- 1. Ross R, Glomset JA. The pathogenesis of atherosclerosis. N Engl J Med 1976; 295: 420–425. [DOI] [PubMed] [Google Scholar]
- 2. Rajendran P, Rengarajan T, Thangavel J, et al. The vascular endothelium and human disease. Int J Biol Sci 2013; 9: 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greenland P, Alpert JS, Beller GA, et al. ; American College of Cardiology Foundation; American Heart Association. ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56: e50–e103. [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, et al. ; for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 5. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insight from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2018; 41: 356–363. [DOI] [PubMed] [Google Scholar]
- 6. Irace C, Casciaro F, Scavelli FB, et al. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc Diabetol 2018; 17: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris RA, Nishiyama SK, Wray DW, et al. Ultrasound assessment of flow mediated dilation. Hypertension 2010; 55: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irace C, Carallo C, De Franceschi MS, et al. Human common carotid wall shear stress as a function of age and gender: a 12-year follow-up study. Age 2012; 34: 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Irace C, Cortese C, Fiaschi E, et al. Wall shear stress is associated with intima-media thickness and carotid atherosclerosis in subjects at low coronary heart disease. Stroke 2004; 35(2): 464–468. [DOI] [PubMed] [Google Scholar]
- 10. Gnasso A, Irace C, Carallo C, et al. In vivo association between low wall shear stress and plaque in subjects with asymmetrical carotid atherosclerosis. Stroke 1997; 28: 993–998. [DOI] [PubMed] [Google Scholar]
- 11. Gnasso A, Carallo C, Irace C, et al. Association between intima-media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation 1996; 94: 3257–3262. [DOI] [PubMed] [Google Scholar]
- 12. Irace C, Tschakovsky ME, Carallo C, et al. ; Endothelial dysfunction or dysfunctions? Identification of three different FMD responses in males with type 2 diabetes. Atherosclerosis 2008; 2: 439–445. [DOI] [PubMed] [Google Scholar]
- 13. Irace C, Padilla J, Carallo C, et al. Delayed vasodilation associates with elevated cardiovascular Risk. Eur J Clin Invest 2014; 44: 549–556. [DOI] [PubMed] [Google Scholar]
- 14. Irace C, De Rosa S, Tripolino C, et al. Delayed flow-mediated vasodilation and critical coronary stenosis. J Investig Med 2018; 66: 905–911. [DOI] [PubMed] [Google Scholar]
- 15. Standard Italiani per la cura del Diabete Mellito, 2016, www.standarditaliani.it
- 16. Chatterjee S. Endothelial mechanotransduction, redox signaling and the regulation of vascular inflammatory pathways. Front Physiol 2018; 9: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doshi SN, Naka KK, Payne N, et al. Flow-mediated dilation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci 2001; 101: 629–635. [PubMed] [Google Scholar]
- 18. Shigiyama F, Kumashiro N, Miyagi M, et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type2 diabetes: DEFENCE study. Cardiovasc Diabetol 2017; 16: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow mediated dilation: implications for the assessment of endothelial function. J Physiol 2005; 568(2): 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Irace C, Tripolino C, Scavelli F, et al. Blood viscosity but not shear stress associates with delayed flow mediated dilation. Eur J Appl Physiol 2015; 115: 747–753. [DOI] [PubMed] [Google Scholar]
- 21. Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guidelines. Am J Physiol Heart Circ Physiol 2010; 300: H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Irace C, Messiniti V, Tassone B, et al. Evidence for congruent impairment in micro and macrovascular function in type 1 diabetes. PLoS ONE 2017; 12: e0187525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Irace C, Tripolino C, Scavelli FB, et al. Brachial low-flow mediated constriction is associated with delayed brachial flow-mediated dilation. J Atheroscler Thromb 2016; 23: 355–363. [DOI] [PubMed] [Google Scholar]
- 24. Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 2015; 17: 1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steven S, Oelze M, Hanf A, et al. The SGLT-2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol 2017; 13: 370–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Batzias K, Antonopoulos AS, Oikonomou GS, et al. Effect of newer antidiabetic drugs on endothelial function and arterial stiffness: a systematic review and meta-analysis. J Diabetes Res 2018; 2018: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verma S, Juni P, Mazer CD. Pump, pipes, and filter: do SGLT2 inhibitors cover it all? Lancet 2018; 393: 3–5. [DOI] [PubMed] [Google Scholar]
- 28. Walters SJ. Consultants’ forum: should post hoc sample size calculation be done? Pharmaceut Statist 2009; 8: 163–169. [DOI] [PubMed] [Google Scholar]