Abstract
Objectives
The case fatality rate (CFR) of coronavirus disease 2019 (COVID-19) varies significantly between countries. We aimed to describe the associations between health indicators and the national CFRs of COVID-19.
Methods
We identified for each country health indicators potentially associated with the national CFRs of COVID-19. We extracted data for 18 variables from international administrative data sources for 34 member countries of the Organization for Economic Cooperation and Development (OECD). We excluded the collinear variables and examined the 16 variables in multivariable analysis. A dynamic web-based model was developed to analyse and display the associations for the CFRs of COVID-19. We followed the Guideline for Accurate and Transparent Health Estimates Reporting (GATHER).
Results
In multivariable analysis, the variables significantly associated with the increased CFRs were percentage of obesity in ages >18 years (β = 3.26; 95%CI = 1.20, 5.33; p 0.003), tuberculosis incidence (β = 3.15; 95%CI = 1.09, 5.22; p 0.004), duration (days) since first death due to COVID-19 (β = 2.89; 95%CI = 0.83, 4.96; p 0.008), and median age (β = 2.83; 95%CI = 0.76, 4.89; p 0.009). The COVID-19 test rate (β = –3.54; 95%CI = –5.60, –1.47; p 0.002), hospital bed density (β = –2.47; 95%CI = –4.54, –0.41; p 0.021), and rural population ratio (β = –2.19; 95%CI = –4.25, –0.13; p 0.039) decreased the CFR.
Conclusions
The pandemic hits population-dense cities. Available hospital beds should be increased. Test capacity should be increased to enable more effective diagnostic tests. Older patients and patients with obesity and their caregivers should be warned about a potentially increased risk.
Keywords: Case fatality rate, COVID-19, Epidemiologic, Health indicators, SARS-CoV-2
Introduction
A novel coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in December 2019 in Wuhan, the capital of Hubei province in China [1]. The virus is responsible for coronavirus disease (COVID-19), a disease ranging from a mild respiratory illness to a serious condition that can cause considerable mortality [1,2]. COVID-19 has a much lower case fatality rate (CFR) than severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), which had CFRs of 9.5% and 34.4%, respectively [3]. However, SARS-CoV-2 is more transmissible, with an average Ro of 3, and has caused a higher total number of deaths [[3], [4], [5]].
According to descriptive data sources, the CFR of COVID-19 varies greatly between countries. Analysis of this variation might shed light on the bottlenecks of the pandemic. The models that can project mortality based on health indicators and covariates could be beneficial for the development of preventive measures.
We aimed to investigate the potential health indicators and covariates influencing national CFRs of COVID-19 among the countries of the Organization for Economic Cooperation and Development (OECD). These indicators were relevant for preparedness but not for responsive actions that were taken during the pandemic. Although the pandemic is still ongoing with fluctuating numbers, we aimed to contribute to the increasing rapid response using the current available data. Our findings provide a global perspective based on the health and demographic factors of each country adjusted by pandemic duration.
Methods
Our study investigated the association between 18 health indicators and covariates and the national CFRs of COVID-19 adjusted by pandemic duration in 34 OECD member countries. We obtained the input data from various international databases according to our inclusion and exclusion criteria. We followed the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) to collect and report our data (Supplementary Material Table S1).
Selection and definition of the variables
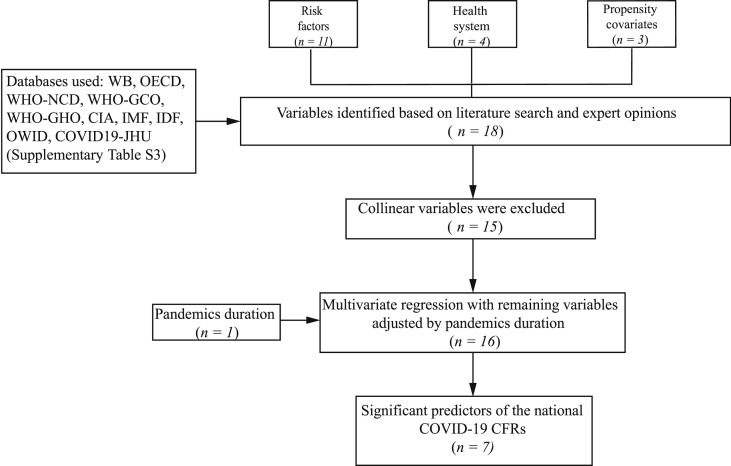
We included variables of 34 OECD member countries. Variables were selected if accessible in standardized databases providing data and statistics for health indicators and covariates, searchable by year and by country. Health indicators defined as prevalence or incidence were included, whereas variables based on hospital admission or mortality data were excluded. We also excluded gender-specific variables. Our study design is presented in Fig. 1 . We selected 18 variables according to published literature and expert opinions, and classified them into three major categories: population health risk factors (n = 11), health system (n = 4) and propensity covariates (n = 3). We adjusted our model by pandemic duration in each country (n = 1) (Supplementary Material Table S2).
Fig. 1.
Study flow for data extraction.
The risk factors included variables for major non-communicable and communicable diseases, which were diabetes prevalence in ages 20–79 (%) (diabetes prevalence) [6], all cancers prevalence in ages 15+ (%) (all cancers prevalence) [7], all cancers incidence in ages 15+ per 1000 people (all cancers incidence) [7], obesity in ages 18+ (%) (obesity prevalence) [8], raised blood pressure in ages 18+ (%) (hypertension prevalence) [8], tuberculosis incidence per 1000 people (tuberculosis incidence) [9], HIV/AIDS prevalence in ages 15–49 (%) (HIV/AIDS prevalence) [10], as well as other potential COVID-19 risk factors including median age (years) (median age) [11], population in ages 65+ (%) (percentage of age 65+) [12], male population (%) (percentage of males) [13] and tobacco smoking in ages 15+ (%) (tobacco smoking prevalence) [14].
The variables under the ‘health system’ category were: number of hospital beds per 1000 people (hospital bed density) [15], number of nurses and midwives per 1000 people (nurse and midwife density) [16], number of doctors per 1000 people (doctor density) [17,18], and number of tests per 1000 people (test rate) [19]. The propensity covariates were selected as gross domestic product (US dollars per capita) (GDP) [20], health spending (US dollars per capita) (health spending) [21] and rural population (%) (rural population ratio) [22]. We adjusted our model by time elapsed since first death (days) (duration since first death) [4] caused by COVID-19 in each country.
We used population-based international administrative data sets based on our inclusion and exclusion criteria. We included 34 OECD member countries with complete information in the most up-to-date sources. Health indicators and covariates were accessible from a variety of international sources. In our study we obtained these data from the World Bank (WB), the Organization for Economic Cooperation and Development (OECD), the World Health Organization Noncommunicable Diseases Country Profile (WHO-NCD), the World Health Organization Global Cancer Observatory (WHO-GCO), the World Health Organization Global Health Observatory (WHO-GHO), the Central Intelligence Agency (CIA), the International Monetary Fund (IMF), the International Diabetes Federation Diabetes Atlas (IDF), Our World in Data (OWID) databases, and COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University (COVID19-JHU) (Supplementary Material Table S3).
We used mainly the OECD, WB, and WHO databases, which provided easily accessible, reproducible, and standardized data. If necessary data were not available, we used additional international sources for all countries. Among the initial 18 variables, 16 were obtained directly from the main sources without any modification. We calculated cancer prevalence and test rate by dividing the total numbers of all cancers and tests, respectively, by the total population of that country. WHO-GCO cancer prevalence data were from 2018, therefore we used the 2018 population data from the WB database [23] in our calculation. The test rate was calculated according to the 2020 population, which is the latest available census for all countries. For this purpose, we used the USCB database [24] which provides accurate and up-to-date demographic data.
Statistical analysis
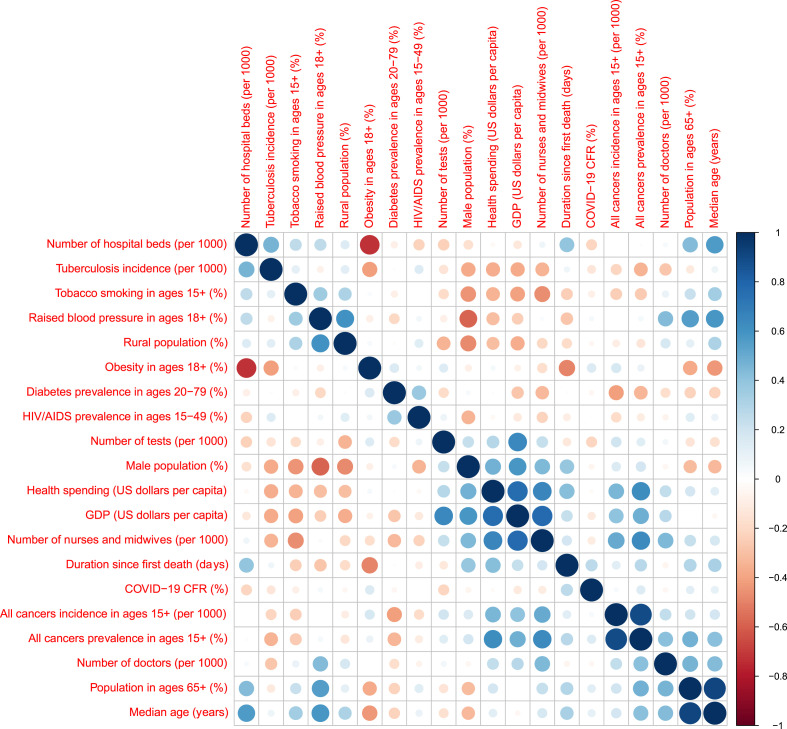
We first checked the correlation between variables to screen for collinear variables (Fig. 2 ). We started with 18 variables and excluded three of those with Pearson's correlation >0.75 and at least one other variable from further analysis. We considered the number of days after the first recorded death due to COVID-19 to represent the stage of the pandemic in each country. Thus, we added this information as an adjustment variable (duration since the first death) to take into account differences between countries in terms of their pandemic durations, leading to 16 variables in total. We performed linear regression for each variable adjusted by duration since the first death, with national CFR values as the outcome. We then performed multivariable linear regression with backward selection to identify a minimal set of predictors from our variable list. In both adjusted and multivariable linear regression analyses, the predictors with a fixed range (percentages and incidence rates) were transformed using logit transformation. All statistical analyses were performed using R version 3.6.3.
Fig. 2.
Collinearity analysis showing the correlation between input variables as of 18th August 2020.
Results
In total, 18 variables representing risk factors (n = 11), health system indicators (n = 4) and propensity covariates (n = 3) were analysed for 34 OECD member countries (Fig. 1). After collinearity check, the number of variables was reduced to 15. We decided not to include three variables—cancer incidence in ages 15+ (per 1000 people), population aged >65 (%), and gross domestic product (GDP, US dollars per capita)—in our further analyses since the correlation with another variable exceeded 0.75: incidence of cancers in ages ≥15 (per 1000 people) was highly correlated with cancer prevalence in ages ≥15 (%) ( = 0.89), population aged ≥65 (%) is highly correlated with median age (years) ( = 0.91), and GDP (US dollars per capita) is highly correlated with health spending (US dollars per capita) ( = 0.76) and number of nurses and midwives per 1000 people ( = 0.77). After exclusion of these three variables, the correlation values between the pairs of remaining 15 variables vary between –0.72 and + 0.65.
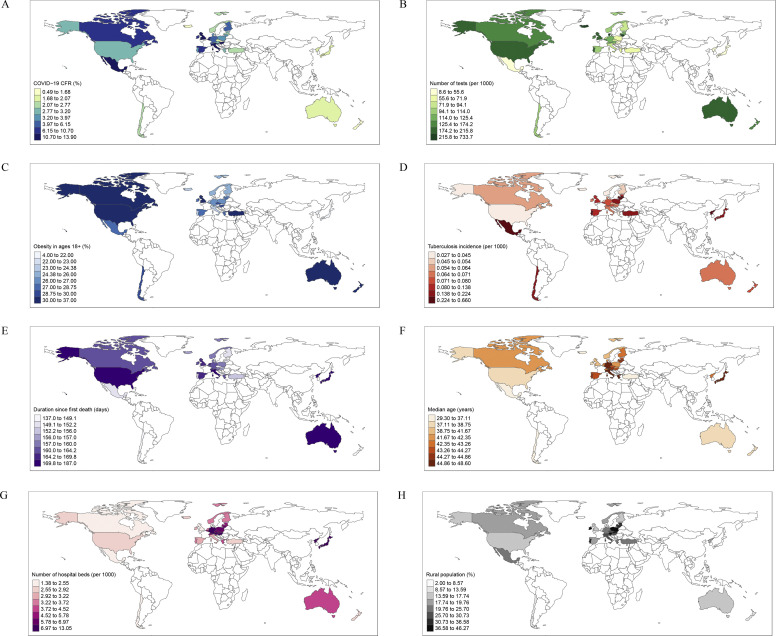
We included the ‘duration since first death’ variable to adjust our model since the pandemic started at different times in different countries. We first analysed each of the 15 variables adjusted by duration since first death by linear regression model (Table 1 ). As of 18th August 2020, in multivariable backward regression analysis the variables significantly associated with the increased CFRs were obesity in ages >18 years (β = 3.26; 95%CI = 1.20, 5.33; p 0.003), tuberculosis incidence (β = 3.15; 95%CI = 1.09, 5.22; p 0.004), duration since first death due to COVID-19 (β = 2.89; 95%CI = 0.83, 4.96; p 0.008), and median age (β = 2.83; 95%CI = 0.76, 4.89; p 0.009). The COVID-19 test rate (β = –3.54; 95%CI = –5.60, –1.47; p 0.002), hospital bed density (β = –2.47; 95%CI = –4.54, –0.41; p 0.021), and rural population ratio (β = –2.19; 95%CI = –4.25, –0.13; p 0.039) decreased the CFR (Table 2 ). Nationwide distribution of the CFR and its significant predictors are presented in Fig. 3 .
Table 1.
Analysis for the health indicators and covariates of national case fatality rates (CFRs) adjusted by duration since first death (as of 18th August 2020)
| Adjusted standardized coefficient | Adjusted 95% confidence interval | Adjusted p value | |
|---|---|---|---|
| Risk factors: | |||
| Obesity in ages 18+ (%) | 2.42 | [0.38, 4.46] | 0.021 |
| Male population (%) | –1.37 | [–3.41, 0.67] | 0.180 |
| HIV/AIDS prevalence in ages 15–49 (%) | 1.20 | [–0.84, 3.24] | 0.239 |
| Raised blood pressure in ages 18+ (%) | 0.98 | [–1.06, 3.02] | 0.333 |
| Median age (years) | 0.96 | [–1.08, 3.00] | 0.345 |
| Tobacco smoking in ages 15+ (%) | 0.51 | [–1.53, 2.55] | 0.616 |
| Tuberculosis incidence (per 1000 people) | 0.44 | [–1.60, 2.48] | 0.660 |
| All-cancer prevalence in ages 15+ (%) | 0.41 | [–1.63, 2.45] | 0.682 |
| Diabetes prevalence in ages 20–79 (%) | –0.31 | [–2.35, 1.73] | 0.760 |
| Health system | |||
| Number of hospital beds (per 1000 people) | –1.78 | [–3.82, 0.26] | 0.085 |
| Number of tests (per 1000 people) | –1.11 | [–3.15, 0.92] | 0.274 |
| Number of nurses and midwives (per 1000 people) | –0.94 | [–2.98, 1.10] | 0.356 |
| Number of doctors (per 1000 people) | –0.03 | [–2.07, 2.01] | 0.978 |
| Propensity covariates: | |||
| Rural population (%) | 0.71 | [–1.33, 2.75] | 0.485 |
| Health spending (US dollars per capita) | –0.44 | [–2.48, 1.60] | 0.662 |
Table 2.
Multivariable analysis for the prediction of national case fatality rates (CFRs) (as of 18th August 2020)
| Standardized coefficient | 95% confidence interval | p value | |
|---|---|---|---|
| Number of tests (per 1000) | –3.54 | [–5.60, –1.47] | 0.002 |
| Obesity in ages 18+ (%) | 3.26 | [1.20, 5.33] | 0.003 |
| Tuberculosis incidence (per 1000 people) | 3.15 | [1.09, 5.22] | 0.004 |
| Duration since first death (days) | 2.89 | [0.83, 4.96] | 0.008 |
| Median age (years) | 2.83 | [0.76, 4.89] | 0.009 |
| Number of hospital beds (per 1000 people) | –2.47 | [–4.54, –0.41] | 0.021 |
| Rural population (%) | –2.19 | [–4.25, –0.13] | 0.039 |
| Raised blood pressure in ages 18+ (%) | 1.50 | [–0.57, 3.56] | 0.148 |
| Male population (%) | 1.35 | [–0.71, 3.42] | 0.189 |
Fig. 3.
National case fatality rates of COVID-19 and their significant predictors in 34 OECD member countries as of 18th August 2020. (A) National case fatality rates of COVID-19. (B) Number of tests (per 1000 people). (C) Obesity in ages 18+ (%). (D) Tuberculosis incidence (per 1000 people). (E) Duration since first death (days). (F) Median age (years). (G) Number of hospital beds (per 1000 people). (H) Rural population (%).
Discussion
In this study we investigated the potential predictors of the national CFRs of COVID-19 in 34 OECD member countries. Increased percentage of obesity in ages >18 years, higher tuberculosis incidence, longer duration since first death due to COVID-19, and older age were found to be significantly associated with higher CFR, whereas increased COVID-19 test rate, higher hospital bed density, and higher rural population were found to be significantly associated with lower CFR in the SARS-CoV-2 pandemic by 18th August 2020 (Table 2). We developed a web-based tool to continuously update the calculations for the ongoing pandemic (http://midas.ku.edu.tr/COVID19CFR).
Our findings suggest that obesity is significantly associated with higher CFR. Obesity is a main risk factor for comorbidities such as hypertension, diabetes mellitus and cardiovascular diseases, and is also linked to an increased risk of pneumonia [25]. In a study from New York, hypertension and obesity were reported as the most common comorbidities among the hospitalized COVID-19 patients [26]. In multivariable analysis we detected that increased hypertension was associated with higher CFR, although this was not statistically significant. Recent retrospective studies and national reports on COVID-19 have identified hypertension as the most prevalent comorbidity in patients who have had a more severe disease course [27,28] and those who have died due to COVID-19 [29,30].
Median age was detected as an independent significant variable in our multivariate analysis, in parallel with the current clinical studies on mortality from COVID-19 [[29], [30], [31]].
We found that the duration since first death due to COVID-19 was significantly associated with higher national CFR at this moment of the pandemic. During the course of the pandemic, the effect of duration variable on national CFRs may change, and comparing countries at different stages of the pandemic could lead to biased results. By inclusion of the duration, we are more confident that our model was less affected by temporality, and hence we limited the temporal bias. Many of the 34 OECD member countries have not yet reached the peak level of the pandemic, therefore as time progresses since the first death, the CFR still increases. Most of the countries relaxed the strict rules of lockdown for economic and social reasons, and therefore could not limit the spread of COVID-19 to the population at risk.
We detected that higher test rate decreased the national CFR significantly. The test rate was calculated as dividing the total test number by the population of each country. The WHO recommends the testing of all those suspected for COVID-19 for the control of the pandemic [32]. Higher numbers of tests result in more confirmed cases, and therefore an increase in the denominator of the CFR, which yields more realistic estimations of the mortality profile, whereas countries with lower test rates may have biased CFRs.
According to our findings, increased number of hospital beds has a significant effect on decreasing the national CFR. Hospital bed density is an important indicator of the overall healthcare system [33]. It reflects the potential of the healthcare system to cope with the burden of a pandemic. As the number of infected people increases, both the hospital admissions and the number of occupied beds increase. The average respiratory support need of a severe COVID-19 patient is reported as approximately 13 days [34]. A high number of patients requiring such long periods of hospital stay places pressure on the healthcare system. As there are currently no treatment options available for COVID-19, and the treatment is mainly supportive, it is of utmost importance that the patients have hospital access to benefit from the supportive treatment, which could be life-saving. As an indicator of the capacity of the healthcare system in the pandemic, the intensive care unit (ICU) bed density could have been studied; however, standardized data for ICU bed density were not available for all 34 countries.
We found that the increasing percentage of rural population has a decreasing effect on the national CFR. This could be directly related to population density, which is much lower in rural areas compared to the crowded cities that are the epicentres of the pandemic [35]. Our finding supports the significance of social distancing in the control of the pandemic.
Increased tuberculosis incidence was also significantly associated with CFR. In countries where tuberculosis is highly prevalent there might be other risk factors for mortality. For instance, in resource-limited settings, poverty and malnutrition might play an important role in increasing morbidity and mortality [36].
This study has several limitations. We had to limit our study to 34 OECD member countries because of the lack of the availability of data in many other countries. If it were possible, inclusion of resource-poor and highly populated countries might highlight the impact of economic variables on CFR. We extracted our data from the most relevant and reliable databases, but not all the variables were effectively updated. Some variables could not be added into the study as they did not have data for all countries. We reviewed risk factors such as ischaemic heart disease, heart failure, chronic kidney disease, chronic obstructive pulmonary disease and asthma, but we could not include them in our study because either they did not match our inclusion criteria or data for all 34 countries were lacking. Diagnosis of the disease is one of the bottlenecks of the pandemic, and there is no current consensus on diagnosing COVID-19. Some countries report only the cases based on confirmed diagnosis, whereas others are based on clinical and radiological examinations. The CFR calculated per total cases seems to remain the best tool to express the fatality of the disease, even though it might underestimate this figure in the initial phase of an outbreak [37]. In our study, using CFR after 6 months from the beginning of the outbreak increased the stability and the confidence. We did not include the interventions at the community level—such as school closures, social distancing, etc.—into our analysis. These measures are taken to minimize the spread of the infection, and the CFR values can safely be assumed to be relatively less affected by these measures that impact disease spread.
Conclusion
The results of our analysis for the case fatality rate of COVID-19 describe possible bottlenecks of the pandemic. Our results showed that the pandemic hits population-dense cities. Available hospital beds should be increased. Diagnostic capacity should be increased by increasing the number of effective diagnostic tests. Older patients and patients with obesity and related comorbidities and their caregivers should be warned of the significantly increased risk of mortality.
Author contributions
MA and CT: data collection, interpretation of the findings, manuscript writing. OE: interpretation of the findings, manuscript writing. MG: data analysis, interpretation of the findings, manuscript writing. EP, NP, HT: interpretation of the findings.
Transparency declaration
The authors declare that they have no conflicts of interest. No funding was received for this work.
Access to data
The input covariates and our computational results reported in this study are accessible at http://midas.ku.edu.tr/COVID19CFR/.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.09.024.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, Coronavirus Overview, World Health Organization 2020. https://www.who.int/health-topics/coronavirus#tab=tab_1
- 3.Rajgor D.D., Lee M.H., Archuleta S., Bagdasarian N., Quek S.C. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20:776–777. doi: 10.1016/S1473-3099(20)30244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–553. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IDF. Diabetes . International Diabetes Federation; 2019. Atlas.https://www.diabetesatlas.org [Google Scholar]
- 7.WHO. World Health Organization Global Cancer Observatory 2020. https://gco.iarc.fr/today/home
- 8.WHO. World Health Organization noncommunicable disease country profiles. 2018. https://www.who.int/nmh/countries/en/ [DOI] [PubMed]
- 9.WB . World Bank; 2020. Incidence of tuberculosis (per 100,000 people)https://data.worldbank.org/indicator/SH.TBS.INCD [Google Scholar]
- 10.Roser M., Ritchie H. HIV/AIDS, our World in data. 2020. https://ourworldindata.org/hiv-aids
- 11.CIA . Central Intelligence Agency; 2020. World factbook 2020, median age.https://www.cia.gov/library/publications/the-world-factbook/fields/343rank.html [Google Scholar]
- 12.WB . World Bank; 2020. Population ages 65 and above (% of total population)https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS [Google Scholar]
- 13.WB . World Bank; 2020. Population, male (% of total population)https://data.worldbank.org/indicator/SP.POP.TOTL.MA.ZS [Google Scholar]
- 14.WHO World health organization global health observatory. 2020. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/age-standardized-prevalence-of-current-tobacco-smoking-among-persons-aged-15-years-and-older
- 15.OECD . Organization for Economic Co-operation and Development; 2020. Hospital beds Total, Per 1000 inhabitants, 2018 or latest available.https://data.oecd.org/healtheqt/hospital-beds.htm [Google Scholar]
- 16.WB . World Bank; 2020. Nurses and midwives (per 1,000 people)https://data.worldbank.org/indicator/SH.MED.NUMW.P3 [Google Scholar]
- 17.WB . World Bank; 2020. Physicians (per 1,000 people)https://data.worldbank.org/indicator/SH.MED.PHYS.ZS [Google Scholar]
- 18.OECD Doctors Total, Per 1000 inhabitants, 2018 or latest available. Organization for Economic Cooperation and Development; 2020. https://data.oecd.org/healthres/doctors.htm [Google Scholar]
- 19.Roser M., Ritchie H., Ortiz-Ospina E., Hasell J. Coronavirus disease (COVID-19), our World in data. 2020. https://ourworldindata.org/grapher/full-list-total-tests-for-covid-19
- 20.World Economic Outlook . Global manufacturing downturn, rising trade barriers. In: Dept I.M.F.R., editor. vol. 2019. 2019. p. 208. (World economic and financial surveys, international monetary Fund). October 15. [Google Scholar]
- 21.OECD . Organization for Economic Cooperation and Development; 2020. Health spending Total/Government/compulsory/Voluntary.https://data.oecd.org/healthres/health-spending.htm US dollars/capita, 2018 or latest available. [Google Scholar]
- 22.WB . World Bank; 2020. Rural population (% of total population)https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS [Google Scholar]
- 23.Population W.B. 2018. World Bank.https://databank.worldbank.org/data/download/POP.pdf [Google Scholar]
- 24.USCB . United States Census Bureau; 2020. U.S. And World population clock.https://www.census.gov/popclock/ [Google Scholar]
- 25.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. China medical treatment expert group for clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ISS Characteristics of SARS-CoV-2 patients dying in Italy. Report based on available data on April 16th, 2020, Istituto Superiore di Sanita. Clin Microbiol Infec. 2020;2020 doi: 10.1016/j.cmi.2020.09.024. [DOI] [Google Scholar]
- 31.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO . World Health Organization; 2020. Laboratory testing strategy recommendations for COVID-19. [Google Scholar]
- 33.Pan J., Shallcross D. Geographic distribution of hospital beds throughout China: a county-level econometric analysis. Int J Equity Health. 2016;15:179. doi: 10.1186/s12939-016-0467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavallo J.J., Donoho D.A., Forman H.P. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the nth patient. JAMA Netw March. 2020;17 doi: 10.1001/jamahealthforum.2020.0345. [DOI] [PubMed] [Google Scholar]
- 35.Kaneda T., Greenbaum C. 2020. How demographic changes make us more vulnerable to pandemics like the coronavirus. April 13. [Google Scholar]
- 36.Tadolini M., Garcia-Garcia J.M., Blanc F.X., Borisov S., Goletti D., Motta I. On tuberculosis and COVID-19 co-infection. Eur Respir J. 2020;56 doi: 10.1183/13993003.02328-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spychalski P., Blazynska-Spychalska A., Kobiela J. Estimating case fatality rates of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.