Abstract
Coronavirus disease 2019 (COVID-19) has become a major threat to public health since the outbreak in Wuhan in 2019. Chest computed tomography is recommended for COVID-19 cases for evaluation and follow up of pneumonia and related complication. We report the case of a 66-year-old man with underlying hypertension and a history of smoking 76 packs a year; he was frequently monitored by computed tomography for pulmonary changes during the period from early symptom onset to death. Furthermore, he developed a pneumothorax during the course. The occurrence of pneumothorax in COVID-19 patients is not common, and there have been only a few previous reports. This is a valuable case of pneumothorax in a patient with COVID-19 treated with a ventilator and extracorporeal membrane oxygenation. This case and previous reports suggest that pneumothorax occurs in COVID-19 with a relatively late onset (3–8 weeks). Long-term pneumonia morbidity, steroid therapy, positive pressure ventilation, and extracorporeal membrane oxygenation can cause pneumothorax, leading to capillary and alveolar damage.
Keywords: Chest imaging, Computed tomography, COVID-19, Pneumothorax, SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19) in Wuhan in 2019 quickly spread around the world and has become a threat to public health, economy, and other areas [1]. Chest computed tomography (CT) is valuable in the evaluation and follow up of pneumonia in patients with COVID-19. The most common characteristics of CT manifestations are isolated or patchy ground glass opacification (GGO) within 1-14 days of symptom onset. In the advanced phase, consolidative opacities with air bronchograms in multiple lobes, especially a peripheral and/or posterior distribution of the lower lobes, appear and often spread throughout the lungs. Some patients with these image characteristics develop acute respiratory distress syndrome (ARDS) and require a ventilator and/or extracorporeal membrane oxygenation (ECMO) [2]. The condition followed this course in our patient; moreover, he developed a bilateral pneumothorax, and death eventually occurred in the patient.
Case report
In April, 2020, a 66-year-old male patient with underlying hypertension and a history of smoking (76 packs a year) complained of fever and fatigue and presented to our outpatient clinic. CT showed GGO in the right lower lobe (Fig. 1). Four days after the onset of symptoms, his real-time fluorescence polymerase chain reaction assay of pharyngeal swabs was positive for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Because his symptoms got worse, on the sixth day of illness, the patient was hospitalized and started on empirically intravenous antibiotics and favipiravir. His respiratory status progressively deteriorated and he required intensive care, with repeat CT revealing that the lesions had increased in extent and had progressed to consolidative opacities with air bronchograms in both lungs (Fig. 2). On the ninth day of illness, he was intubated and mechanically ventilated and started on hydroxychloroquine and tocilizumab. However, his condition continued to deteriorate and he was diagnosed with ARDS on the 16th day of illness. ECMO and prone position therapy were started. On the 50th day of illness, the CT scan showed in the bilateral lungs worsening consolidations, diffuse GGO, and atelectasis, and pleural effusion in both thoraxes (Fig. 3). His respiratory status deteriorated further, and a CT scan was obtained on the 56th day of illness (Fig. 4), which showed the appearance of pneumothorax and giant bulla in bilateral lungs and an increase in plural effusion. At the onset of the pneumothorax, the ventilator was set up in airway pressure release ventilation mode with FiO2 of 40%, inspiratory phase pressure of 20 cmH2O, and expiratory phase pressure of 0 cmH2O, and total breathing frequency was set at 10 breaths/min. The respiratory frequency was controlled at 25 breaths/min, and the tidal volume was controlled at approximately 200-300 mL. A chest drain was placed, and the pneumothorax improved temporarily. However, the patient continued to experience remission and exacerbation of pneumothorax, and his lungs showed widespread consolidative opacities and decreased volume, and he eventually died of multiple organ failure on the 97th day of illness, despite aggressive treatment attempts (Fig. 5).
Fig. 1.
The initial chest plain computed tomography (CT) scan showed a small isolated ground glass opacification (GGO) in the lower lobe of the right lung.
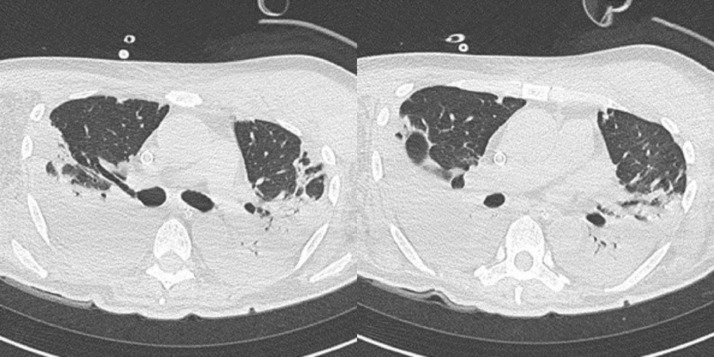
Fig. 2.
The CT scan on the 7th day of illness showed an increase in GGO and changes to consolidative opacities with air bronchograms in both lungs.
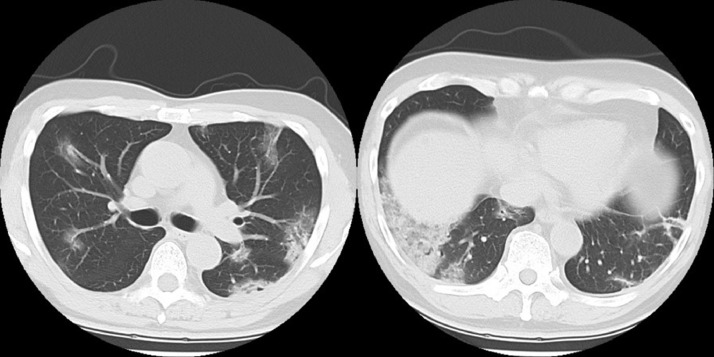
Fig. 3.
On the 50th day of illness, the CT scan showed progression of consolidations and GGO and appearance of atelectasis in bilateral lungs and pleural effusion in both thoraxes.
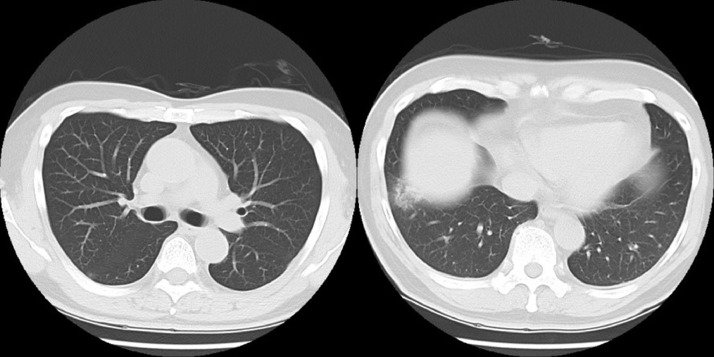
Fig. 4.
(A) On the 56th day of illness, the CT scan showed the appearance of pneumothorax and giant bulla in bilateral lungs and an increase in plural effusion.
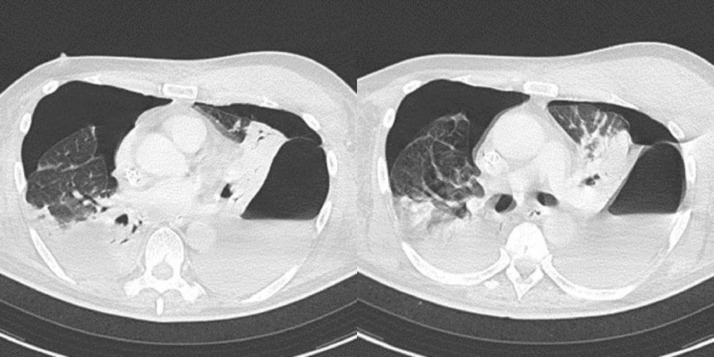
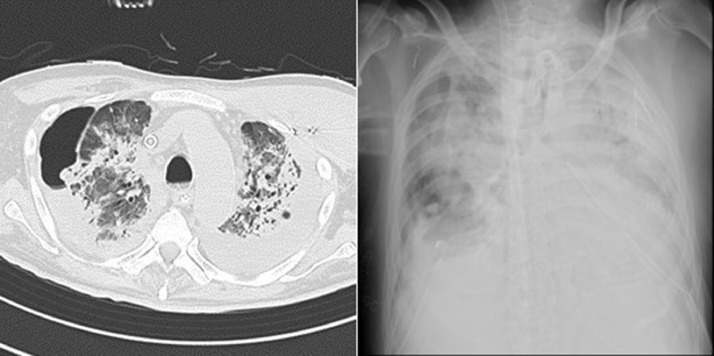
Fig. 5.
On the 87th day of illness, (A) the CT scan showed bilateral pneumothorax, GGO, and consolidative opacities with contraction and tractional bronchiectasis throughout the lungs. (B) The chest radiograph revealed a pneumothorax in the right lung and consolidation in the whole of bilateral lungs.
Discussion
In COVID-19 practice, chest CT is useful for initial evaluation and follow up of respiratory status [3]. CT findings may also be present prior to symptom onset ,. Common features of COVID-19 on initial CT are isolated or patchy GGO in peripheral or posterior distribution. Despite various treatments, in some cases, GGO are multiple and progress to consolidative opacities throughout the lungs, which is diagnosed as ARDS [4]. This case followed such a course and progressed to ARDS, requiring treatment with a ventilator and ECMO, and he developed incurable pneumothorax with giant bulla. The patient was a former smoker (76 packs a year) but had no history of bulla, emphysema, or pneumothorax. Our patient had a chest drain placed and improved temporarily but did not recover and eventually died.
We discuss the risks and mechanisms of developing pneumothorax with COVID-19 with reference to previous literature. Where positive pressure ventilation, prone position therapy, and steroid pulse therapy may also have affected damage to the lungs and bronchi, previous reports have shown that SARS-CoV-2 damages alveoli and the vascular endothelium [5]. These mechanisms may be associated with the development of pneumothorax. The ventilator was in airway pressure release ventilation mode, and keeping the patient under high airway pressure may well be a risk for pneumothorax. However, there are many other patients in our hospital who have been managed in a similar condition, but pneumothorax occurred in only 2 cases. This suggests that a combination of factors may have contributed to the pneumothorax.
To our knowledge, 5 previous case reports of pneumothorax in COVID-19 patients have been published to date [6], [7], [8], [9], [10]. In addition, 2 cases, including this case, of COVID-19 patients with pneumothorax have developed in our hospital. The clinical characteristics of COVID-19 patients with pneumothorax in our case and in previous reports are that all patients were males over the age of 50, and all patients had different backgrounds and received different treatments. However, all cases developed pneumothorax at a relatively late stage (3-8 weeks after onset). Five patients had bullas at the time of pneumothorax onset, and 4 had no abnormalities in the lungs prior to the onset of COVID-19. Therefore, even in patients without respiratory comorbidities, it is necessary to keep in mind the occurrence of pneumothorax in the late period of worsening respiratory conditions such as ARDS.
The incidence of pneumothorax in COVID-19 is not common, and a previous study reported that only 1 of 99 patients developed pneumothorax [11]. At our hospital, 86 patients with COVID-19 have been admitted between March and June 2020. Of these, pneumothorax was found in two patients (2.3%). Aside from the onset of pneumothorax being later in our study than in other reports, the incidence rate of pneumothorax in our report also being higher than that of previous studies may be due to the fact that our hospital deals with relatively severe COVID-19 patients who require intensive care and long hospital stays.
Since the direct trigger of the development of pneumothorax is unknown, more cases need to be obtained to elucidate the cause.
In conclusion, we report a late-onset pneumothorax in severe COVID-19. This is the first case of a pneumothorax in which both ventilator and ECMO have been used and it is a valuable and important case that follows the course of the patient from the early stages of pneumonia to the later stages of pneumothorax development. This case and previous reports suggest that COVID-19 pneumothorax occurs with a relatively late onset (3-8 weeks). Long-term pneumonia morbidity, steroid therapy, positive pressure ventilation, and ECMO can cause pneumothorax, leading to capillary and alveolar damage.
Patient consent statement
The Tokyo Medical and Dental University Hospital Ethics Committee approved this retrospective study (approval ID: M2020-054, approval date: 15 June 2020) and waived the requirement for written informed consent.
Footnotes
Competing Interest: The authors have no competing interests to report.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujioka T, Takahashi M, Mori M, Tsuchiya J, Yamaga E, Horii T. Evaluation of the usefulness of CO-RADS for chest CT in patients suspected of having COVID-19. Diagnostics. 2020;10:608. doi: 10.3390/diagnostics10090608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sana S, Aidin A, Sudheer B, Ali G. Coronavirus Disease 2019 (COVID-19): A systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 5.Sardu Celestino, Gambardella Jessica, Morelli Marco Bruno, Wang X, Marfella R, Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: Is COVID-19 an endothelial disease? a comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9(5):1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caitlyn H, Jennifer H. Spontaneous pneumothorax following COVID-19 pneumonia. IDCases. 2020;21:e00868. doi: 10.1016/j.idcr.2020.e00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Gao R, Zheng Y, Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020 doi: 10.1093/jtm/taaa062. taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luke F, Carter John-Paul L, Lopez Juan Rosales, Henry Alun Marc. Tension pneumothorax in a patient with COVID-19. BMJ Case Rep. 2020;13(5) doi: 10.1136/bcr-2020-235861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruihong S, Hongyuan L, Xiang W. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020;21(5):541–544. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang C, Wu G. SARS-CoV-2 pneumonia with subcutaneous emphysema, mediastinal emphysema, and pneumothorax: a case report. Medicine. 2020;99(20):e20208. doi: 10.1097/MD.0000000000020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]