Abstract
As of May 2020, an emerging immune-mediated syndrome mainly affecting children has been detected primarily in Europe and the United States. The incidence of this syndrome appears to mirror the initial infectious assault, with a delay of several weeks. This syndrome has been termed “multisystem inflammatory syndrome in children” (MIS-C) and is observed in association with the coronavirus disease 2019 (COVID-19). The phenotypes of presentation include several characteristic features, including prolonged fever, skin eruption, neck stiffness, and gastrointestinal manifestations with pronounced abdominal pain. Shock and organ dysfunction on presentation are frequent but inconsistent, whereas respiratory distress is typically and notably absent. We have reviewed recently published data aiming to better understand MIS-C, with a focus on its mucocutaneous manifestations.
Introduction
SARS-CoV-2 is a novel coronavirus that originated in Wuhan, China, in December 2019.1 This virus causes a severe respiratory distress syndrome among several other severe manifestations.1 Starting in early 2020, the virus spread rapidly worldwide, compelling the World Health Organization (WHO) to declare a global pandemic on March 11, 2020. To date, there are more than 15 million cases with more than half a million deaths due to COVID-19, globally. Children make up a small percentage of these cases. According to the Centers for Disease Control (CDC) Morbidity and Mortality Weekly Report, published April 6, 2020, in the United States 2572 (1.7%) of roughly 150,000 known cases of COVID-19 infection were among children <18 years of age.2 This is consistent with a review of more than 72,000 COVID-19 cases performed by the Chinese Center for Disease Control and Prevention, which revealed that <1% of infected patients were children under the age of 10 years3; furthermore, infected children tend to be completely asymptomatic or exhibit mild clinical manifestations.[4], [5], [6], [7], [8] Although the majority of children with COVID-19 are asymptomatic or have mild clinical manifestations, a small percentage require hospitalization and intensive care for shock and multiorgan failure due to the emerging multisystem inflammatory syndrome.[7], [8], [9], [10] On May 14, 2020, 2 months after the WHO declaration of a global pandemic, the CDC released a health advisory about multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19.
Defining multisystem inflammatory syndrome in children
MIS-C describes an emergent childhood inflammatory disorder, with similarities to Kawasaki disease (KD), Kawasaki disease shock syndrome (KDSS), toxic shock syndrome (TSS), and macrophage activating syndrome (MAS). Due to its recent nascence, the constellations of clinical manifestations defining MIS-C can be found under a number of names such as “pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS),” “Kawasaki-like disease,” or “toxic-shock–like syndrome,” among others.11 , 12 Altogether, there are now approximately 1,000 cases of MIS-C documented worldwide.13 As the spotlight shines on this new inflammatory disease, we are beginning to get more clarification of its clinical presentation and pathogenesis.
Both the CDC and the WHO have released their own MIS-C diagnostic criteria to help clinicians in making such a diagnosis. Table 1, Table 2 review these criteria in detail. The main clinical manifestations that both groups focus on include fever for more than 24 hours, laboratory evidence of inflammation, involvement of two or more organs (commonly gastrointestinal, followed by cardiac and renal), mucocutaneous findings, and either a positive test or exposure within 4 weeks of clinical manifestations. A negative COVID polymerase chain reaction (PCR) does not rule out this diagnosis. Because MIS-C is thought to be an immune-mediated secondary response to the virus, COVID PCRs are usually negative at the time of the illness, and antibodies are positive in the majority of cases.11 , [14], [15], [16], [17], [18], [19], [20], [21] Because diagnostic criteria for this condition are generally very broad, there is a significant challenge in identifying which patients falling under these diagnostic criteria are true MIS-C.22
Table 1.
The CDC case definition for MIS-C
| All four diagnostic criteria must be met: |
|---|
| 1. Age <21 years |
| 2. Clinical presentation should include all of the following: |
| a. Fever ≥38.0°C for ≥24 hours or report of subjective fever lasting ≥24 hours |
| b. Laboratory evidence of inflammation, including, but not limited to, one or more of the following: |
| • Elevated C-reactive protein (CRP) |
| • Elevated erythrocyte sedimentation rate (ESR) |
| • Elevated fibrinogen |
| • Elevated procalcitonin |
| • Elevated D-dimer |
| • Elevated ferritin |
| • Elevated lactic acid dehydrogenase (LDH) |
| • Elevated interleukin 6 (IL-6) |
| • Elevated neutrophils |
| • Reduced lymphocytes |
| • Low albumin |
| c. Evidence of clinically severe illness requiring hospitalization |
| d. Multisystem (≥2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurological) |
| 3. No alternative plausible diagnoses |
| 4. Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test, or COVID-19 exposure within the 4 weeks before the onset of clinical manifestations |
CDC, Centers for Disease Control; MIS-C, multisystem inflammatory syndrome in children; RT-PCR, reverse transcription polymerase chain reaction.
Source: United States Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp.
Table 2.
The WHO case definition of MIS-C
| All six diagnostic criteria must be met: |
|---|
| 1. Children and adolescents 0–19 years of age |
| 2. Fever ≥3 days |
| 3. At least two of the following clinical signs of multisystem involvement: |
| • Eruption or bilateral nonpurulent conjunctivitis or mucocutaneous inflammation signs (oral, hands, or feet) |
| • Hypotension or shock |
| • Features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including ECHO findings or elevated Troponin/NT-proBNP |
| • Evidence of coagulopathy (by PT, PTT, elevated D-dimers) |
| • Acute gastrointestinal problems (diarrhea, vomiting, or abdominal pain) |
| 4. Elevated markers of inflammation such as ESR, C-reactive protein, or procalcitonin |
| 5. No other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal, or streptococcal shock syndromes |
| 6. Evidence of COVID-19 (RT-PCR, antigen test or serology positive), or likely contact with patients with COVID-19 |
ECHO, echocardiogram; ESR, erythrocyte sedimentation rate; MIS-C, multisystem inflammatory syndrome in children; NT-proBNP, n-terminal pro b-type natriuretic peptide; PT, prothrombin time; PTT, partial thromboplastin time; RT-PCR, reverse transcription polymerase chain reaction; WHO, World Health Organization
Source: World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. Available at: https://www.who.int/publicationsdetail/multisystem-inflammatory-syndrome-in-children-and-adolescents-withcovid-19.
Demographic data of children with MIS-C from 13 recent large case series are summarized in Table 3 . The size of these studies ranges from 8 to 186 children. We recap patient mean/median age, sex, cutaneous signs/clinical manifestations, SARS-CoV-2 testing method, treatments, and outcome. Fever and gastrointestinal clinical manifestations were the top two most common systemic signs/clinical manifestations seen in children who met criteria for MIS-C. The majority of patients were previously healthy. The two most common comorbidities were asthma and obesity. The median age of children with MIS-C was between 8 and 12 years. Boys were equal or more prevalent in all but two publications. In the U.S. studies, the majority of the children affected were black non-Hispanic, Hispanic or Latino, or Ashkenazi Jewish.15 , 19 , 23 , 24 Most patients were treated with intravenous immunoglobulin ± systemic steroids. The majority of patients had a negative COVID PCR at the time of diagnosis, likely because the disease tends to present 4 to 6 weeks after the viral infection. With the exception of one report,20 all other case series found that patients were more likely to have positive antibodies compared with PCR. COVID PCR positivity ranged from 13% to 50%, whereas antibody positivity ranged from 75% to 100%.[10], [11], [12] , [14], [15], [16], [17], [18], [19], [20], [21] , 23 , 24
Table 3.
Recently published descriptive studies on MIS-C from the United States and Europe
| Study | No. of cases | Mean/median age, y | Sex, % boys or men | Cutaneous signs and clinical manifestations | Reactive PCR or serology | Treatment | Deaths |
|---|---|---|---|---|---|---|---|
| Riphagen et al.12 May 2020 UK |
8 | 9 | 63 | Variable eruption 50% Conjunctivitis 63% |
25% PCR or Ab | IVIG 100% Steroids 63% |
1 |
| Belhadjer et al.16 May 2020 France |
35 | 10 | 51 | Eruption 57% Conjunctivitis 80% Red/cracked lips 54% |
34% PCR 86% Ab |
IVIG 100% Steroids 33% |
0 |
| Verdoni et al.10 June 2020 Italy |
10 | 7.5 | 70 | Polymorphic eruption Conjunctivitis |
20% PCR 80% Ab |
IVIG 100% Steroids 80% |
0 |
| Toubiana et al.18 June 2020 France |
21 | 7.9 | 43 | Polymorphous eruption 76% Conjunctivitis 81% Lips/oral mucosa 76% |
38% PCR 90% Ab |
IVIG 100% Steroids 48% |
0 |
| Dufort et al.19 June 2020 USA |
99 | 6-12 | 54 | Eruption 60% Conjunctivitis 56% Oral mucosa 27% |
20% PCR 99% Ab |
IVIG 70% Steroids 64% |
2 |
| Feldstein et al.24 June 2020 USA |
186 | 8.3 | 62 | Eruption 59% Conjunctivitis 55% Oral mucosa 42% Extremity changes 37% |
70% PCR or Ab |
IVIG 77% Steroids 49% IL-6 or IL-1RA 20% |
4 |
| Riollano-Cruz et al.17 June 2020 USA |
15 | 12 | 73 | Eruption 47% Conjunctivitis 27% Hand/feet edema 27% |
47% PCR 100% Ab |
IVIG 80% IL-6 80% Steroids 20% IL-1RA 13% Remdesivir 13% |
1 |
| Pouletty et al.20 June 2020 France |
16 | 10 | 50 | Diffuse eruption 81% Conjunctivitis 93% Dry/cracked lips 87% Hands/feet eruption/edema 68% |
75% PCR 50% Ab |
IVIG 94% Steroids 20% IL-1RA 6% IL-6 6% |
0 |
| Grimaud et al.21 June 2020 France |
20 | 10 | 50 | Eruption 50% Conjunctivitis 30% Cheilitis 25% |
50% PCR 75% Ab |
IVIG 100% Steroids 5% IL-6 5% |
0 |
| Kaushik et al.15 June 2020 USA |
33 | 10 | 61 | Authors did not comment | 33% PCR 81% Ab |
IVIG 54% Steroids 50% IL-6 3% Remdesivir 3% |
1 |
| Whittaker et al.11 June 2020 UK |
58 | 9 | 53 | Eruption 52% Conjunctivitis 45% Mucus membrane and cracked red lips 29% |
26% PCR 87% Ab |
IVIG 71% Steroids 64% Infliximab 14% IL-1RA 5% |
0 |
| Ramcharan et al.14 June 2020 UK |
15 | 8.8 | 73 | Eruption Conjunctivitis Swollen hands/feet Oral mucosa |
13% PCR 100% Ab |
IVIG 67% Steroids 33% |
0 |
| Cheung et al.23 July 2020 USA |
17 | 8 | 47 | Eruption 71% Conjunctivitis 65% Lip redness/swelling 53% Skin desquamation 18% |
47% PCR 53% Ab |
IVIG 76% Methylpred 71% Hydrocortisone 21% |
0 |
Ab, antibody; IL-1RA, interleukin 1 receptor antagonist; IL-6, interleukin 6; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children; PCR, polymerase chain reaction
Mucocutaneous manifestations of MIS-C
Although mucocutaneous manifestations are not very common among children with COVID-19 at large, they are among the top clinical manifestations in children with MIS-C, making them important to identify and recognize in an attempt to understand the disease. (See Fig. 1, Fig. 2, Fig. 3 ). Most of the clinical information to date comes from small descriptive studies, such as case series and case reports. Documented cutaneous findings reported in children with COVID-19 include nonspecific maculopapular eruptions, followed by chilblainlike or perniolike acral lesions, urticarial lesions, livedo reticularis, papulovesicular or varicellalike lesions, petechiae- or denguelike lesions, and erythema-multiforme–like lesions.[25], [26], [27], [28], [29], [30], [31], [32], [33]
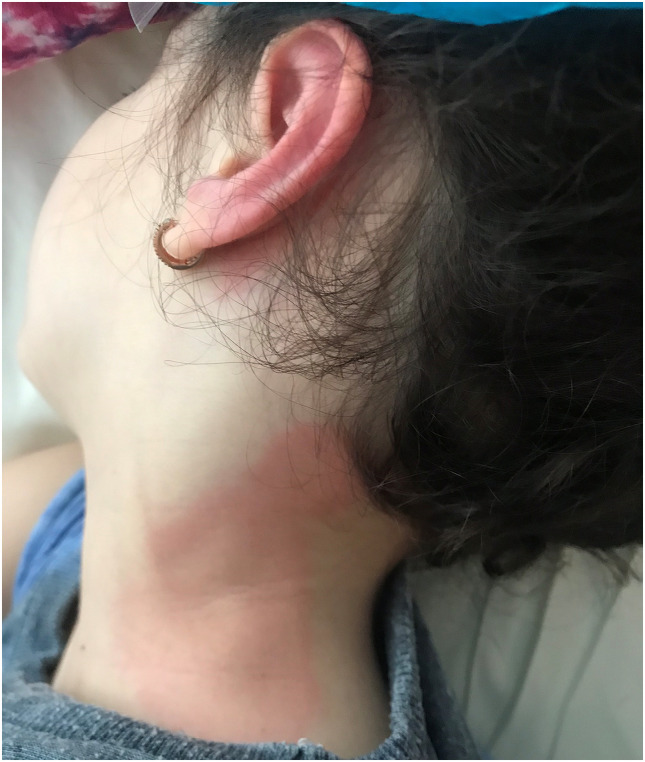
Fig. 1.
7-year-old girl, diagnosed with MIS-C, presented with erythematous urticaria-like plaques on the neck and overlying lymphadenopathy.
Fig. 2.
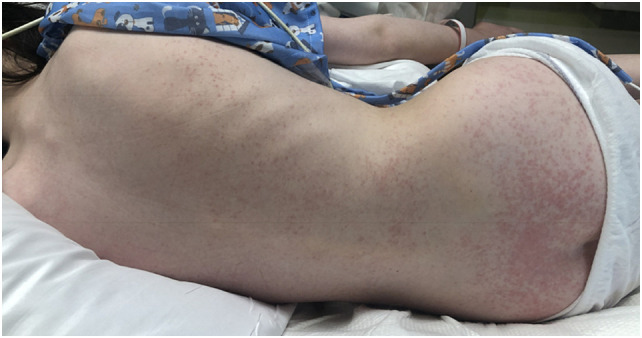
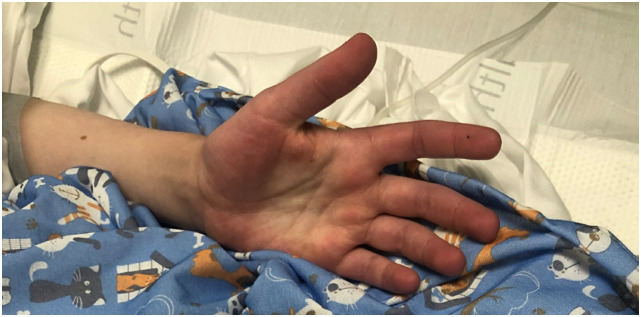
12-year-old boy, diagnosed with MIS-C, presented with erythematous macules and papules coalescing into patches and plaques, respectively, on the trunk and extremities. This patient also hadpalmar erythema as in Fig. 3.
Fig. 3.
12-year-old boy, diagnosed with MIS-C, presented with bilateral palmar erythema (the same patient as in Fig. 2).
Multiple larger case series published in the recent months[10], [11], [12] , [14], [15], [16], [17], [18], [19], [20], [21] , 23 , 24 focus on characterizing and understanding MIS-C. Each case series has identified children who met criteria for MIS-C and described the cutaneous findings with which they presented. The following descriptive terminology was used to describe the skin findings: “conjunctivitis,” “eruption,” “red/cracked lips,” lips/oral cavity changes,” “cheilitis,” “extremity changes,” or “hand/feet edema.” Most of the studies did not provide photos of the eruption and did not attempt to describe it. Others that did used the following nondescriptive terminology: “polymorphous,” “general,” “variable,” “skin desquamation,” “diffuse,” “nonspecific,” or simply “eruption.” Refer to Table 3.
The investigators of one of the major series conducted targeted surveillance for MIS-C at multiple pediatric health centers across the United States and identified 126 children who met criteria for MIS-C. Mucocutaneous findings were identified in 74% of children who met criteria for MIS-C. Of these, 59% had nonspecific eruption, 55% bilateral conjunctivitis, 42% oral mucosal changes, and 37% peripheral extremity changes.24 In a review of more than 191 potential MIS-C cases of hospitalized children reported to the New York State Department of Health, 95 met criteria for MIS-C. They found that 60% of children who met criteria for MIS-C had a diffuse nonspecific eruption, whereas 56% had conjunctivitis and 27% oral mucosal changes. 19 Looking at all the 13 case series presented in Table 3, the percentage of children diagnosed with MIS-C who developed mucocutaneous findings included conjunctivitis 27% to 93%, oral mucosal changes 25% to 87%, eruption 47% to 81%, and hand/feet erythema and edema 27% to 68%. Table 4 summarizes the top mucocutaneous manifestations of children with MIS-C.[10], [11], [12] , [14], [15], [16], [17], [18], [19], [20], [21] , 23 , 24 The skin findings associated with MIS-C tend to be more common in younger children and decrease with age; 87% of children between 0 and 5 years of age had mucocutaneous findings, compared with only 61.5% of those 13 and 20 years of age.19
Table 4.
Top four mucocutaneous presentations of MIS-C
| Conjunctivitis |
|---|
| Diffuse, nonspecific eruption |
| Dry and red lips and/or other mucosal changes |
| Hand and feet erythema and edema |
MIS-C, multisystem inflammatory syndrome in children.
Similarities and differences with Kawasaki disease
As we discuss the various mucocutaneous manifestations associated with MIS-C, many sound similar to other diseases, specifically KD (both typical and atypical); however, a wide differential diagnosis consideration is needed when seeing a child with a mucocutaneous eruption. The differential diagnosis also includes the following: Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), drug reaction with eosinophilia and systemic clinical manifestations (DRESS), mycoplasma-induced eruption and mucositis/reactive infectious mucocutaneous eruption (MIRM/RIME), toxic shock syndrome (TSS), and other viral or postviral etiologies. Identifying the similarities and differences between these entities is crucial in distinguishing them and making the correct diagnosis.
From a clinical perspective, a subset of patients with MIS-C show features that are similar to KD, including mucocutaneous findings. The question arises as to whether these two syndromes represent a single entity, rather than completely separate conditions. Kawasaki shock syndrome, which is a known variant of KD, as well as severe KD, both recapitulate many of the more common features found in MIS-C, such as shock, proinflammatory state and pathology, and some of the hematologic disturbances such as thrombocytopenia, which at first glance appear unique to MIS-C. A reasonable argument can be made that MIS-C represents a severe Kawasaki spectrum.
Many of the demographic features of the disease differ, such as age (mean age 8-12 years in MIS-C versus the rarity of Kawasaki cases in children older than 5 years). The absence of predilection for Asian populations being affected in MIS-C is also contrary to what one might see in KD.34 For instance, only 1 of 17 children diagnosed with MIS-C in New York City was of Asian descent.23 The most common cardiac abnormality associated with MIS-C is left ventricular dysfunction/myocarditis, unlike coronary artery abnormalities in KD.16 Thirty-five percent of children who met criteria for MIS-C at Columbia University Irving Medical Center/New York-Presbyterian Morgan Stanley Children’s Hospital in New York had moderate left ventricular dysfunction, whereas only one child developed a medium-size aneurysm.23 Coronary aneurysms themselves are observed in other vasculitis syndromes, such as Takayasu arteritis and polyarteritis nodosa35 but rarely are they seen in association with other viral and infectious conditions.36 The presence of an aneurysm itself is not a defining feature that would necessitate relating the two syndromes. Table 5 further compares and contrasts clinical manifestations of MIS-C and KD.
Table 5.
Comparing and contrasting MIS-C with Kawasaki disease
| MIS-C | Kawasaki disease |
|---|---|
| • Mean age 8-12 years | • Mean age <5 years |
| • Non-Hispanic blacks at higher risk | • Asians at higher risk |
| • Fever >24 hours | • Fever >5 days |
| • Gastrointestinal clinical manifestations common (severe abdominal pain) | • Gastrointestinal complaints are not common |
| • Myocarditis/myocardial dysfunction (left ventricular dysfunction) | • Myocardial function normal, mildly reduced |
| • Coronary artery aneurysms unusual | • Coronary artery abnormalities such as aneurysms more common |
| • Renal involvement more common | • Renal involvement rare |
| • Proinflammatory state common | • Proinflammatory state typically less common and less severe |
| • Lymphopenia common | • Lymphopenia not common |
| • Thrombocytopenia | • Thrombocytosis |
MIS-C, multisystem inflammatory syndrome in children.
Conclusions
MIS-C is a recently recognized childhood inflammatory disorder seen in patients with confirmed or suspected COVID-19. The increasing incidence 3 to 4 weeks after COVID-19 infection suggests a postinfectious phenomenon occurring in susceptible individuals. We have recently published a large case series on MIS-C in an effort to identify and characterize the mucocutaneous manifestations of MIS-C. We concluded that the vast majority of MIS-C cases present with mucocutaneous manifestations, including conjunctivitis, a diffuse eruption, and oral mucosal changes, as the three most common. The eruption of MIS-C is typically diffuse and nonspecific. Mucocutaneous manifestations of MIS-C are more common in younger children, and their prevalence decreases with age. Finally, although the mucocutaneous findings of MIS-C may resemble those of KD, the two conditions differ widely in terms of mean age of onset, race predilection, and the associated clinical manifestations. This syndrome requires intensive supportive care and therapy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC COVID-19 Response Team Coronavirus disease 2019 in children - United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19 [Scientific brief]. 15 May 2020. Available at: https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed July 25, 2020.
- 5.Lu X., Zhang L., Du H. SARSCoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castagnoli R., Votto M., Licari A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y., Mo X., Hu Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Information for health care providers about multisystem inflammatory syndrome in children (MIS-C): case definition for MIS-C. Available at: https://www.cdc.gov/mis-c/hcp/. Accessed June 25, 2020.
- 9.Royal College of Pediatrics and Child Health. Guidance: pediatric multisystem inflammatory syndrome temporally associated with COVID-19. Available at: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims. Accessed July 25, 2020.
- 10.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicenter of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittaker E., Bamford A., Kenny J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riphagen S., Gomez X., Gonzalez-Martinez C. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin M. Childhood multisystem inflammatory syndrome - a new challenge in the pandemic. N Engl J Med. 2020;383:393–395. doi: 10.1056/NEJMe2023158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramcharan T., Nolan O., Lai C.Y. Pediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary pediatric hospital. Pediatr Cardiol. 2020;12:1–11. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushik S., Aydin S.I., Derespina K.R. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection: a multi-institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belhadjer Z., Méot M., Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 17.Riollano-Cruz M., Akkoyun E., Briceno-Brito E. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York City experience. J Med Virol. 2020 doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toubiana J., Poirault C., Corsia A. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dufort E.M., Koumans E.H., Chow E.J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouletty M., Borocco C., Ouldali N. Pediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicenter cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimaud M., Starck J., Levy M. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10 doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakra N.A., Blumberg D.A., Herrera-Guerra A. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. 2020;7:69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung E.W., Zachariah P., Gorelik M. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldstein L.R., Rose E.B., Horwitz S.M. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galván-Casas C., Català A., Carretero-Hernández G. Classification of the cutaneous manifestations of COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genovese G., Colonna C., Marzano A.V. Varicella-like exanthem associated with COVID-19 in an 8-year-old girl: a diagnostic clue? Pediatr Dermatol. 2020;37:435–436. doi: 10.1111/pde.14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez-Cauhe J., Ortega-Quijano D., Prieto-Barrios M. Reply to COVID-19 can present with a rash and be mistaken for Dengue: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol. 2020;83:141–142. doi: 10.1016/j.jaad.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colonna C., Monzani N.A., Rocchi A. Chilblain-like lesions in children following suspected COVID-19 infection. Pediatr Dermatol. 2020;37:437–440. doi: 10.1111/pde.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cordoro K.M., Reynolds S.D., Wattier R. Clustered cases of acral perniosis: clinical features, histopathology, and relationship to COVID-19. Pediatr Dermatol. 2020;37:419–423. doi: 10.1111/pde.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andina D., Noguera-Morel L., Bascuas-Arribas M. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406–411. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joob B., Wiwanitkit V. Comment on “Chilblains-like lesions in children following suspected COVID-19 infection.”. Pediatr Dermatol. 2020;37:441. doi: 10.1111/pde.14238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torrelo A., Andina D., Santonja C. Erythema multiforme-like lesions in children and COVID-19. Pediatr Dermatol. 2020;37:442–446. doi: 10.1111/pde.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shulman S.T. Pediatric coronavirus disease-2019–associated multisystem inflammatory syndrome. J Pediatric Infect Dis Soc. 2020;9:285–286. doi: 10.1093/jpids/piaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sajeev C.G., Krishnan M.N., Venugopal K. Aneurysm of the left main coronary artery in Takayasu arteritis. Heart. 2004;90:660. doi: 10.1136/hrt.2003.023655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wi J., Choi H.H., Lee C.J. Acute myocardial infarction due to polyarteritis nodosa in a young female patient. Korean Circ J. 2010;40:197–200. doi: 10.4070/kcj.2010.40.4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]