Abstract
Background
Coronavirus disease 2019 (COVID-19) pneumonia tends to affect cardiovascular system and cause cardiovascular damage. This study aimed to explore the prevalence of myocardial injury and risk factors for mortality in patients with COVID-19 pneumonia.
Method
Two hundred and twenty-four consecutive patients with confirmed diagnosis of SARS-CoV-2 infection and definite outcomes (discharge or death) were retrospectively analyzed. Laboratory results including myocardial biomarkers, oxygen saturation, inflammatory indicators and coagulation function were compared between survivors and non-survivors. Univariate and multivariate logistic regression model were used to explore risk factors for in-hospital mortality, and a chart with different combinations of risk factors was constructed to predict mortality.
Results
Two hundred and three patients were included in the final analysis, consisting of 145 patients who recovered and 58 patients who died. Compared with survivors, non-survivors were older, with more comorbidities, more severe inflammation and active coagulation function, higher levels of myocardial biomarkers and lower SaO2. 28 (50%) non-survivors and 9 (6%) survivors developed myocardial injury, which was associated with disease severity at admission. Elevated d-dimer (OR = 9.51, 95% CI [3.61–25.0], P < 0.001), creatinine kinase-myocardial band (OR = 6.93, 95% CI [1.83–26.2], P = 0.004), Troponin I (OR = 10.1, 95% CI [3.1–32.8], P < 0.001) and C-reactive protein (OR = 15.1, 95% CI [1.7–129.3], P = 0.013) were risk factors for mortality. Patients with abnormal levels of d-dimer, Troponin I and CRP were predicted to have significantly higher probability of death.
Conclusions
Our results suggest that SARS-CoV-2 infection may induce myocardial injury and consequently exacerbate the clinical course and worsen prognosis. Abnormal d-dimer, CK-MB, Troponin I and CRP are risk factors for short-term mortality.
Keywords: Coronavirus disease 2019, Myocardial injury, Pneumonia, Mortality, Biomarkers
Abbreviations: ACS, acute coronary syndrome; CAD, cardiovascular disease; COVID-19, coronavirus disease 2019; IQR, interquartile range; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. Introduction
Since early December 2019, an outbreak of pneumonia of unknow aetiology has been reported in Wuhan, China [[1], [2], [3]]. The pathogen was then named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by WHO, and the illness caused by it was termed as the coronavirus disease 2019 (COVID-19) [[4], [5], [6]]. Currently, the disease has rapidly spread to the whole world and become an international public health emergency [7].
Although most infected patients can completely recover from this disease, severe or critically ill patients were reported to have higher risk of experiencing multiorgan failure and death [8,9]. Thus, early identification of patients with poor prognosis is essential. Previous researches mainly focused on pulmonary damage but underestimated other organ dysfunction, such as heart, liver and kidney. Pneumonia has been proved to have an important effect on the cardiovascular system, leading to a quarter of patients experiencing cardiovascular complications and resulting in increased short-term mortality [10,11]. In patients with COVID-19 pneumonia either admitted to ICU or dead, the incidence of developing heart failure, arrhythmia and myocardial injury was 52%, 44% and 56% respectively [12]. These evidences demonstrate that pneumonia-related cardiovascular damage was not rare in COVID-19 patients, and might be an independent risk factor associated with poor prognosis.
Recent studies have reported that cardiovascular diseases (CAD) may increase in-hospital mortality in patients with COVID-19 pneumonia [[13], [14], [15]]. But whether these events are induced by SARS-CoV-2 is uncertain. And to date, available evidences on relation between cardiac involvement and adverse clinical outcomes remain elusive. In order to identify high risk patients and to adapt customized treatments, we designed this retrospective study aiming to analyze laboratory parameters related to cardiovascular involvement and investigate the prevalence of myocardial injury, and to explore risk factors for short-term mortality in patients with COVID-19 pneumonia.
2. Materials and methods
2.1. Study design
The present multicenter, retrospective observational study was conducted at Wuhan Jinyintan Hospital and Wuhan Central Hospital, both of which were designated hospital treating COVID-19 patients. The study protocol was complied with the principles of the Declaration of Helsinki and was approved by the institutional ethics committee of both hospitals. Informed consent was waived given the retrospective nature of this study.
2.2. Study population and data collection
From January 10, 2020 to February 29, 2020, all consecutive patients with laboratory-confirmed COVID-19 and admitted to the above two hospitals were screened retrospectively. Exclusion criteria were as follows: (1) age older than 80; (2) without definite outcomes (transferred to other hospital or still hospitalized); (3) with other severe co-existing diseases; (4) laboratory results could not be obtained from medical records. Diagnosis of SARS-CoV-2 infection was based on World Health Organization interim guidance and was performed using the reverse transcription polymerase chain reaction (RT-PCR) or gene sequencing at admission or during hospitalization [16]. Details of laboratory confirmation processes have been described previously [3]. Patients without exclusion criteria were divided into survivor group and non-survivor group according to their outcomes. Baseline laboratory parameters reflecting cardiac injury, coagulation function and inflammation and their temporal change were compared between two groups.
We obtained data from electronic medical records in the context of standard practice. These data have been collected in our database for different researches on the prognosis of COVID-19. Data regarding epidemiological and demographical characteristics, clinical symptoms, laboratory tests, chest CT images, treatment and outcomes were recorded with data collection form at admission and during hospitalization. Laboratory parameters mainly included complete blood count, blood biochemistry, coagulation function, inflammatory indicators and blood gas analysis. Patients were followed until discharged from hospital, death or at the end of this study (March 20, 2020). Data were censored at the end of follow-up.
2.3. Definitions
The severity of COVID-19 pneumonia was defined according to the guideline of Diagnosis and Treatment of pneumonia Caused by SARS-CoV-2 (trial seven version) [17], which divided patients into four degrees (mild, moderate, severe or critically ill) and the classification criteria were: a. mild: asymptomatic patients without abnormality on chest CT; b. moderate: patients with respiratory symptoms and pneumonia on CT; c. severe: severe respiratory distress (respiratory rate > 30 breaths/min), oxygenation index (PaO2/FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa) or oxygen saturation (SaO2) ≤ 93% at rest; d. critically ill: conditions aggravate and develop respiratory failure, shock or other organ failure. SaO2 was monitored by fingertip or by blood gas analysis to reflect patient's condition and clinical course. Myocardial injury was diagnosed by the detection of a rise of Troponin I above the 99th-percentile upper reference limit. The date of illness onset was defined as the date initial symptoms occurred. Discharge criteria according to aforementioned guideline were: a. temperature returned to normal for at least 3 days; b. substantial improvement of respiratory symptoms and CT findings; c. at least two times of negative results of RT-PCR for SARS-CoV-2 from respiratory tract specimens with an interval ≥ 24 h.
2.4. Statistical analysis
Quantitative variables were expressed as median (interquartile range [IQR]) and compared using Student's t-test or Mann-Whitney U test. Qualitative variables were presented as frequency (percentages) and compared by means of Chi-squared or two-tailed Fisher's exact test.
To identify patients at high risk of poor outcomes, we used univariate and multivariate logistic analysis to explore risk factors for in-hospital death. The choice of candidate variables was based on potential mechanisms of developing pneumonia-related myocardial injury. Previous researches suggested that pneumonia could affect cardiovascular system through multiple mechanisms including relative hypoxemic state, uncontrolled inflammation, procoagulant state and direct pathogen-mediated damage. Therefore, we included four types of corresponding laboratory parameters in the univariate analysis: myocardial enzymes and protein, SaO2, inflammatory indicators and coagulation function. Besides, the incidence of myocardial injury increased with age, and recent studies found gender difference in mortality among COVID-19 patients, so we included age and sex either. Variables with univariate P value less than 0.10 were considered candidate for multivariate analysis. A two-sided α of less than 0.05 was considered statistically significant. All statistical analyses were processed using IBM SPSS (version 25.0).
3. Results
3.1. Participants
Between January 10 and February 29, 2020, 224 consecutive patients (197 from Wuhan Jinyintan Hospital and 27 from Wuhan Central Hospital) with laboratory-confirmed SARS-CoV-2 infection and with definite outcomes (discharged from hospital or death) were retrospectively analyzed. Twenty-one patients who met the exclusion criteria were excluded from the study and the final cohort included 203 patients (Fig. 1 ). Baseline characteristics of the study population are summarized in Table 1 . As seen, 58 patients died during hospitalization and the remaining 145 patients recovered and met the discharge criteria, with a median age of 67.0 (IQR, 60.0–75.0) and 57.0 (IQR, 45.0–67.0), respectively. Co-existing diseases were more prevalent in non-survivors, but the difference of CAD and hypertension between two groups was not obvious, with only COPD had statistical significance. Sex proportion was similar between two groups. Fever, cough and dyspnea were the most common symptoms at admission, which were presented in more than half of patients. Most survivors (46.8%) were defined as moderate at admission, while severe (51.7%) and critically ill (43.1%) were more commonly seen in non-survivors.
Fig. 1.
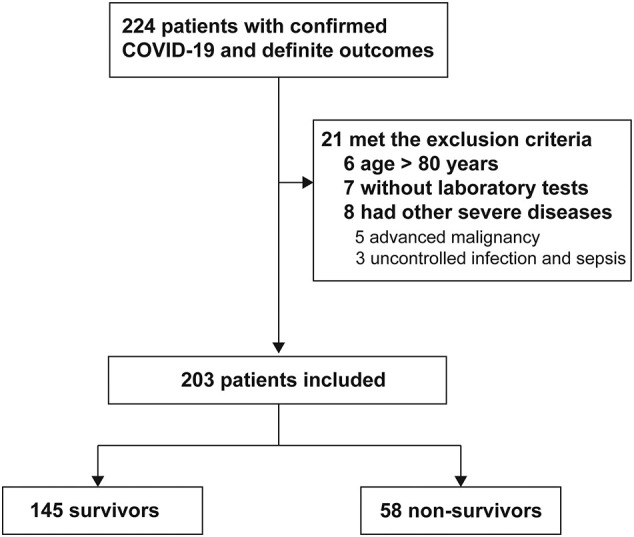
Flowchart of patients admitted with confirmed COVID-19 and with definite outcomes from January 10 to February 29, 2020 and selection of study cohort.
Table 1.
Comparison of baseline characteristics between survivors and non-survivors.
| Total |
Survivor |
Non-survivor |
P value | |
|---|---|---|---|---|
| (n = 203) | (n = 145) | (n = 58) | ||
| Age- yearsa | 62.0 (49.0–69.0) | 57.0 (45.0–67.0) | 67.0 (60.0–75.0) | <0.001 |
| Sex | 0.11 | |||
| Male | 115 (56.7%) | 77 (53.1%) | 38 (65.5%) | |
| Female | 88 (43.3%) | 68 (46.9%) | 20 (34.5%) | |
| Smoking history (never smoke) | 171 (84.2%) | 119 (82.1%) | 52 (89.7%) | 0.18 |
| Co-existing disease | ||||
| COPD | 6 (3%) | 2 (1.4%) | 4 (6.9%) | 0.04 |
| Hypertension | 80 (39.4%) | 51 (35.2%) | 29 (50%) | 0.05 |
| Diabetes | 29 (14.3%) | 18 (12.4%) | 11 (19.0%) | 0.23 |
| Cardiovascular disease | 9 (4.4%) | 4 (2.8%) | 5 (8.6%) | 0.06 |
| Cerebrovascular disease | 16 (7.9%) | 11 (7.6%) | 5 (8.6%) | 0.80 |
| Signs and symptoms | ||||
| Fever | 177 (87.2%) | 125 (86.2%) | 52 (89.7%) | 0.50 |
| Cough | 142 (70.0%) | 106 (73.1%) | 36 (62.1%) | 0.12 |
| Dyspnea | 120 (59.1%) | 74 (51.0%) | 46 (79.3%) | <0.001 |
| Sputum | 31 (15.3%) | 24 (16.6%) | 7 (12.1%) | 0.42 |
| Myalgia | 14 (6.9%) | 10 (6.9%) | 4 (6.9%) | 1 |
| Diarrhea | 30 (14.8%) | 21 (14.5%) | 9 (15.5%) | 0.85 |
| Type of disease at admission | <0. 001 | |||
| Mild | – | – | – | |
| Moderate | 95 (46.8%) | 92 (63.4%) | 3 (5.2%) | |
| Severe | 75 (36.9%) | 45 (31.0%) | 30 (51.7%) | |
| Critically ill | 33 (16.3%) | 8 (5.5%) | 25 (43.1%) | |
| Time from disease onset toa | ||||
| Hospital admission – days | 8.0 (6.0–11.0) | 6.0 (5.0–8.0) | 10.0 (7.0–12.0) | |
| Discharged from hospital – days | – | 29.0 (24.0–35.0) | – | |
| Death – days | – | – | 21.0 (19.0–27.0) |
Other categorical data were expressed as number (percentage).
Abbreviations: COPD chronic obstructive pulmonary disease.
Nonparametric continuous variables were expressed as median (interquartile range [IQR]).
3.2. Baseline laboratory findings between two groups
We compared laboratory results between two groups at the time of admission and found that most parameters including cardiac biomarkers, inflammatory indicators and coagulation function were higher in non-survivors, and the difference between two groups was statistically significant (except activated partial thromboplastin time, creatine kinase and Interleukin-6). Details of laboratory findings between two groups are summarized in Table 2 .
Table 2.
Comparison of laboratory parameters at admission between survivors and non-survivors.
| Total |
Survivor |
Non-survivor |
P value | |
|---|---|---|---|---|
| (n = 203) | (n = 145) | (n = 58) | ||
| Blood routine | ||||
| Leucocyte count (×109/L) | 6.2 (4.2–9.3) | 5.5 (3.9–7.5) | 8.9 (5.1–13.1) | <0.001 |
| Neutrophil count (×109/L) | 4.7 (2.9–7.9) | 3.7 (2.6–5.8) | 8.1 (4.4–11.8) | <0.001 |
| Lymphocyte count (×109/L) | 0.8 (0.5–1.2) | 1.0 (0.7–1.3) | 0.5 (0.4–0.7) | <0.001 |
| Coagulation function | ||||
| D-dimer (μg/ml) | 0.9 (0.5–2.7) | 0.6 (0.4–1.3) | 4.2 (1.1–27.0) | <0.001 |
| APTT (s) | 27.7 (24.7–32.2) | 27.6 (24.3–31.8) | 28.0 (24.8–33.7) | 0.50 |
| Prothrombin time (s) | 11.3 (10.4–12.2) | 11.1 (10.4–12.0) | 12.0 (10.3–13.0) | 0.007 |
| International normalized ratio | 1.0 (0.9–1.1) | 0.9 (0.8–1.0) | 1.0 (0.9–1.1) | <0.001 |
| Blood biochemistry | ||||
| Total bilirubin (mmol/L) | 11.8 (9.3–15.8) | 11.1 (8.7–14.5) | 14.2 (10.5–20.4) | 0.001 |
| Alanine aminotransferase (U/L) | 31.0 (18.5–52.5) | 29.0 (17.0–52.0) | 33.0 (22.5–55.2) | 0.178 |
| Aspartate aminotransferase (U/L) | 36.0 (25.9–55.0) | 31.0 (23.0–49.0) | 50.0 (33.7–67.0) | <0.001 |
| Albumin (g/L) | 31.6 (28.3–35.5) | 32.5 (29.7–36.2) | 28.9 (25.6–32.3) | <0.001 |
| Creatinine (μmol/L) | 70.8 (60.0–87.4) | 69.0 (56.9–82.0) | 76.7 (65.2–101.9) | 0.003 |
| Blood urea nitrogen (mmol/L) | 5.1 (3.7–7.0) | 4.6 (3.3–5.9) | 7.0 (5.2–10.5) | <0.001 |
| Lactate dehydrogenase (U/L) | 320 (229–448) | 276 (216–377) | 457 (372–669) | <0.001 |
| Creatine kinase (U/L) | 91 (57–147) | 89 (55–135) | 109 (58–239) | 0.149 |
| Creatine kinase-MB (U/L) | 14 (9–19) | 11.8 (9.0–17.0) | 18.0 (15.0–25.0) | <0.001 |
| Myoglobin (ng/ml) | 61.8 (37.9–125) | 50.1 (31.4–90.0) | 114.2 (59.2–179) | <0.001 |
| Troponin I (pg/ml) | 7.0 (2.0–18.4) | 4.0 (1.2–9.4) | 20.7 (9.6–115.4) | <0.001 |
| Infection-related biomarkers | ||||
| C-reactive protein (mg/L) | 42.2 (10.6–122) | 24.2 (6.0–66.5) | 126 (57.7–160) | <0.001 |
| Interleukin-6 (pg/mL) | 9.5 (6.5–13.4) | 8.9 (6.3–13.4) | 10.7 (7.9–13.6) | 0.063 |
| B-type natriuretic peptide (pg/ml) | 61.9 (26.0–170) | 34.8 (17.0–74.6) | 109 (50–299) | 0.001 |
| Blood gas analysis | ||||
| PaO2 (mm Hg) | 10.3 (8.4–16.4) | 11.1 (9.1–19.7) | 9.0 (6.8–13.5) | 0.014 |
| PaCO2 (mm Hg) | 5.1 (4.3–6.8) | 5.1 (4.5–5.8) | 4.7 (3.8–8.2) | 0.447 |
| SaO2 (%) | 96 (91–98) | 96 (95–99) | 91 (88–96) | 0.001 |
Nonparametric continuous variables were expressed as median (interquartile range [IQR]) and compared using Student's t-test or Mann-Whitney U test. P value <0.05 was considered statistically significant.
Abbreviations: APTT activated partial thromboplastin time; PaO2 partial pressure of oxygen in artery; PaCO2 partial pressure of carbon dioxide in artery; SaO2 oxygen saturation.
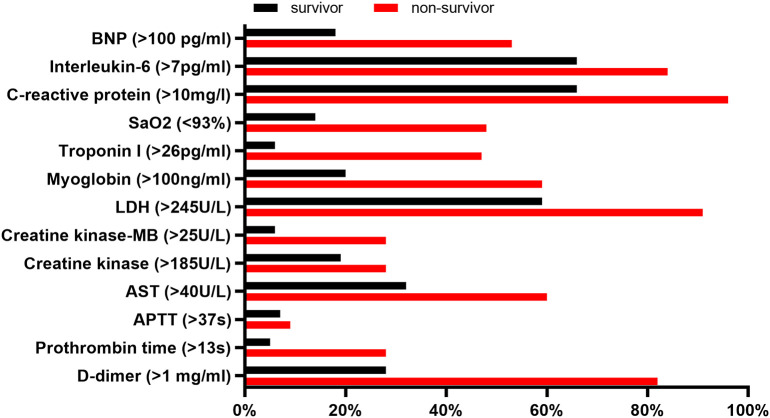
We also compared the percentage of patients with abnormal results between two groups (Fig. 2 ). The frequency of myocardial injury was higher in non-survivors than survivors (50% vs 6%, P < 0.001). Among patients experiencing myocardial injury, 47% were diagnosed as critically ill, 41% as severe and only 12% as moderate at admission. While the percentage of patients without myocardial injury were 10%, 36% and 54%, respectively (P < 0.001). Interleukin-6 (IL-6), C-reactive protein (CRP), and lactate dehydrogenase (LDH) were the most commonly elevated indicators, with percentages of 84%, 94% and 91% in non-survivors and 66%, 66% and 59% in survivors. But the specificity seemed low since their abnormality were prevalent in both non-survivors and survivors. D-dimer, Troponin I and N-terminal pro-B-type natriuretic peptide (Nt-proBNP) had both higher sensitivity and specificity, with more than half of non-survivors (82%, 50%, 53%, respectively) had abnormal elevation but relatively lower incidence of abnormality in survivors (28%, 6%, 18%, respectively).
Fig. 2.
Percentage of patients with abnormal laboratory results between survivors and non-survivors.
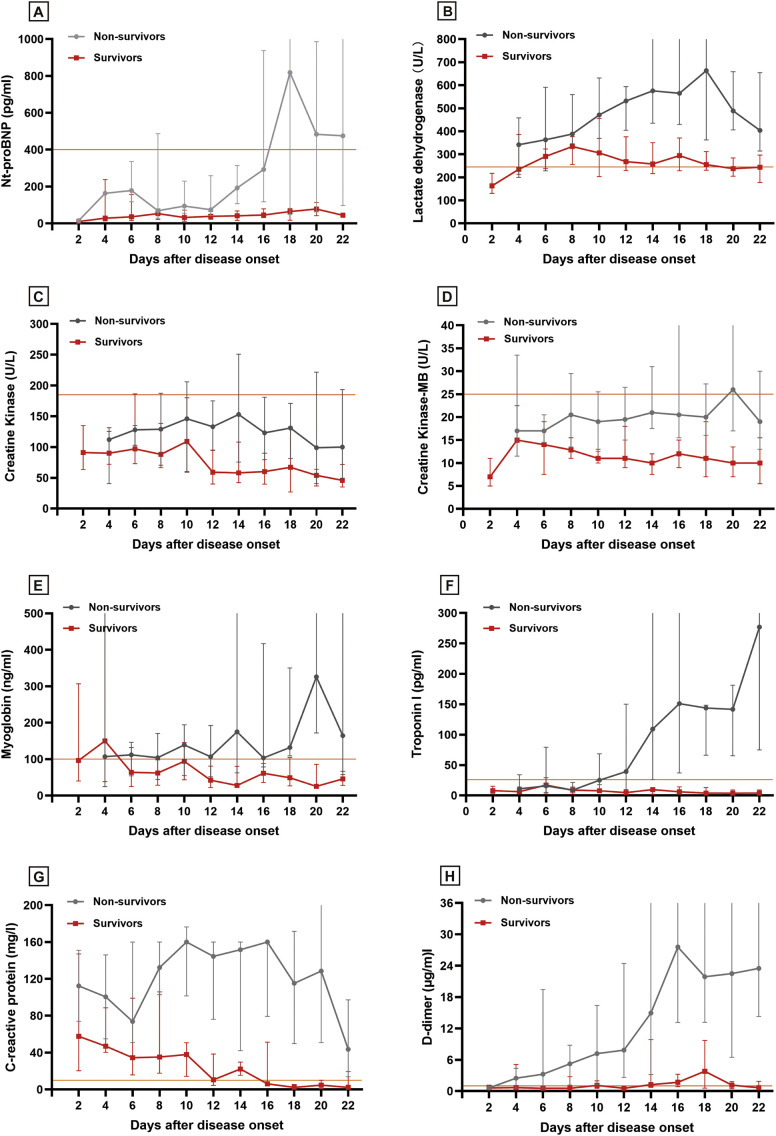
3.3. Temporal change of related biomarkers
To better appreciate pathophysiological change of cardiovascular system during the clinical course of COVID-19 pneumonia, we recorded serial biomarker measurements of each patient for dynamic change analysis (Fig. 3 ). Nt-proBNP, LDH, myoglobin and Troponin I began to elevate at day 8 after disease onset, and continued to increase in non-survivors but decreased or remained stable in survivors. Nt-proBNP, LDH and Troponin I were elevated markedly in non-survivors and the difference between two groups was significant. CRP increased substantially at the time of disease onset and stabilized at a high level in non-survivors. But in survivors, CRP level continued to decrease and returned to normal at day 16. D-dimer raised rapidly from day 12 in non-survivors and its level was significantly higher than in survivors. The change of creatine kinase (CK) and creatine kinase-myocardial band (CK-MB) were not obvious and they were almost within the normal range either in survivors or in non-survivors.
Fig. 3.
Temporal change of myocardial biomarkers between survivors and non-survivors. The solid line in orange represents the upper limit of the reference range. (A) N-terminal pro-B-type natriuretic peptide; (B) Lactate dehydrogenase; (C) Creatine kinase; (D) Creatine kinase-myocardial band; (E) Myoglobin; (F) Troponin I; (G) C-reactive protein; (H) D-dimer.
3.4. Cardiac-related risk factors for mortality
In univariate logistic analysis, age, d-dimer, prothrombin time (PT), Aspartate aminotransferase (AST), CK-MB, LDH, Troponin I, CRP, Nt-proBNP and SaO2 were associated with mortality. Limited by the sample size and given that not all variables had clinical and research value, we excluded variables from univariate analysis if the number of events were so small that would affect the stability of the final model, or indicators with low accuracy and specificity in reflecting the change of organ function. Finally, age, d-dimer, CK-MB, LDH, Troponin I and CRP were included in the final model. Complete data of the above six variables from 187 patients were analyzed in multivariate analysis. The factors associated with mortality were elevated d-dimer (OR = 9.51, 95% CI [3.61–25.0], P < 0.001), CK-MB (OR = 6.93, 95% CI [1.83–26.2], P = 0.004), Troponin I (OR = 10.1, 95% CI [3.1–32.8], P < 0.001) and CRP (OR = 15.1, 95% CI [1.7–129.3], P = 0.013) from the final model. Details of univariate and multivariate analysis were shown in Table 3 .
Table 3.
Univariate and multivariate analysis of risk factors for in-hospital mortality.
| Variables | Univariate analysis |
Multivariate analysis |
|---|---|---|
| OR (95% CI) P value | OR (95% CI) P value | |
| Age (per year increase) | 1.05 (1.03–1.08) <0.001 | 1.03 (0.99–1.06) 0.152 |
| Sex (Male vs Female) | 1.68 (0.89–3.15) 0.109 | – |
| D-dimer (>1 μg/ml) | 11.7 (5.4–25.5) <0.001 | 9.51 (3.61–25.0) <0.001 |
| APTT (>37 s) | 1.41 (0.45–4.41) 0.554 | – |
| Prothrombin time (>13 s) | 7.2 (2.7–18.7) <0.001 | – |
| AST (>40 U/L) | 3.24 (1.72–6.10) <0.001 | – |
| Creatine kinase (>185 U/L) | 1.6 (0.78–3.26) 0.19 | – |
| Creatine kinase-MB (>25 U/L) | 6.58 (2.63–16.48) <0.001 | 6.93 (1.83–26.2) 0.004 |
| Lactate dehydrogenase (>245 U/L) | 7.23 (2.72–19.19) <0.001 | 2.24 (0.63–7.97) 0.213 |
| Myoglobin (>100 ng/ml) | 5.85 (2.94–11.64) <0.001 | – |
| Troponin I (>26 pg/ml) | 14.7 (6.0–35.6) <0.001 | 10.1 (3.1–32.8) <0.001 |
| C-reactive protein (>10 mg/l) | 28.0 (3.7–208.5) <0.001 | 15.1 (1.7–129.3) 0.013 |
| Interleukin-6 (per pg/ml increase) | 2.75 (1.18–6.42) 0.019 | – |
| B-type natriuretic peptide | – | |
| ≥100 pg/ml | 4.61 (1.85–11.48) 0.001 | |
| ≥400 pg/ml | 6.84 (1.66–28.06) 0.008 | |
| SaO2 (<93%) | 6.25 (2.14–18.16) 0.001 | – |
Abbreviations: APTT activated partial thromboplastin time; AST Aspartate aminotransferase; SaO2 oxygen saturation; OR odds ratio.
To facilitate clinical interpretation of the four risk factors from the final model, we constructed a simple chart with the expected probabilities of mortality determined by different combinations of variables (Table 4 ). Considering CK-MB and Troponin I were both used to evaluate myocardial injury, we selected the latter given its higher sensitivity and OR. As seen, the subgroup of patients with baseline d-dimer <1 μg/ml, Troponin I < 26 pg/ml and CRP <10 mg/l had the lowest mortality (2.9%). As the three indicators all raised above the upper limit of reference range, mortality could reach to approximately 90%. It was noteworthy that the cases in some subgroups were <10, which might affect the accuracy of this combination.
Table 4.
Expected mortality of different combinations of D-dimer, Troponin I and CRP.
| D-dimer (μg/ml) | Troponin I (pg/ml) | C-reactive protein (mg/l) | Mortality (%) |
|---|---|---|---|
| <1 | <26 | <10 | 2.9 |
| >10 | 9.9 | ||
| Total | 7.6 | ||
| >26 | <10 | 25.0a | |
| >10 | 50.0 | ||
| Total | 40.0 | ||
| >1 | <26 | <10 | 8.3 |
| >10 | 44.9 | ||
| Total | 37.7 | ||
| >26 | <10 | 50.0* | |
| >10 | 92.0 | ||
| Total | 88.9 |
The number of events was less than 10.
4. Discussion
In this multicenter observational study, we found 50% of non-survivors and 6% of survivors experienced myocardial injury, and abnormal d-dimer, CK-MB, Troponin I and C-reactive protein were risk factors for short-term mortality. Our results demonstrate that myocardial injury is a common complication during COVID-19 pneumonia, which incidence is associated with disease severity at admission. Abnormal elevation of several cardiovascular-related biomarkers are predictors of high probability of mortality.
Although exact mechanism remains uncertain, pneumonia has been proved to affect cardiovascular system through complicated interactions between procoagulant state, relative hypoxemia and ischemia, intense inflammation and direct virus-mediated damage [[18], [19], [20]]. In our research, non-survivors presented with marked and persistent abnormalities in parameters reflecting coagulation function, inflammation severity and myocardial injury, indicating the above mechanism may contribute to cardiovascular damage and consequently worsen outcomes in patients with SARS-CoV-2 infection.
Pneumonia is a pro-inflammatory disease which can be controlled by activating immune system and up-regulating circulating levels of cytokines and chemokines. But uncontrolled infection will trigger cytokine storm as a consequence of overactive immune response, which can result in acute coronary syndrome (ACS) and induce Troponin I and CK-MB increasement [21,22]. Potential pathogenesis might be related to the instability and rupture of atherosclerotic plaque induced by severe endothelial inflammation, especially in the presence of pre-existing coronary heart disease. In addition, exaggerate inflammation and specific virus-meditated mechanism can increase coagulation activation and compromise endothelial anti-coagulant feature, and subsequently cause thrombus formation, which could block coronary flow and contribute further to ACS either [[23], [24], [25]]. Apart from ACS, heart failure, characterized by left ventricular malfunction and elevated Nt-proBNP, may be another important complication induced by uncontrolled inflammation [11]. Nt-proBNP, Troponin I and CK-MB were elevated in 53%, 50% and 28% of non-survivors in our research, which coincided with previous studies [22,26]. Non-survivors presented with higher levels of CRP, IL-6, d-dimer, and both CRP and d-dimer were associated with in-hospital mortality. These evidences indicate that a cytokine storm may occur and coagulation function is activated during COVID-19 pneumonia, leading to an elevated risk of development of myocardial injury and worse prognosis.
Another potential mechanism of myocardial injury may be the myocardial ischemia caused by relative hypoxemia. Infection can induce increased metabolism and decreased SaO2, this imbalance between supply and demand will result in myocardial ischemia. We defined disease severity of each patient at admission mainly based on their blood gas results and pulmonary function. Our analysis showed that 88% of patients with myocardial injury were defined as severe or critically ill, while this percentage was only 46% in ptients without it. In addition, about 95% of non-survivors were diagnosed as severe or critically ill, while 63% survivors were diagnosed as moderate. Although we excluded SaO2 from multivariate logistic model due to the small number of recorded events, it showed a marked difference between two groups and an association with mortality in univariate analysis. These results coincide with previous studies and suggest that relative hypoxemia secondary to alveolar consolidation caused by COVID-19 pneumonia may be another risk factor for adverse clinical outcomes [9,19].
Despite these laboratory indices, other factors were also suggested to be associated with pneumonia-related myocardial injury, such as an older age and pre-existing CAD. Patients with older age are often accompanied by declined immunity, which make them more likely to develop respiratory and heart diseases and thus result in worse outcomes [22]. In addition, previous studies reported that patients with pre-existing chronic CAD had threefold risk of developing pneumonia and had higher mortality, indicating that the cause-effect relation between cardiovascular damage and pneumonia may be bidirectional [11,15]. Our results found no statistical difference of pre-existing CAD between two groups, which might be interpreted as these comorbidities in some cases were neglected by physicians in the context of emergency situation, or the ability of viral transmission was so strong that all populations were susceptible to infection.
SARS-CoV-2, which has a phylogenetic similarity to SARS-CoV, could bind to vascular endothelial cells and cardiomyocytes. This was due to the angiotensin-converting enzyme 2 (ACE2), expressed not only in lung but also in heart, could be identified by the spike protein of SARS-CoV-2, and thus provided an entry to virus. This direct viral infection might provoke heart damage as a result of endothelial dysfunction [[27], [28], [29]]. However, a recent postmortem study of a COVID-19 patient found no substantial cardiac damage and a few inflammatory infiltrates [30]. In view of limited pathological evidence, further biopsies and autopsies are needed to confirm this theory.
Our study has several limitations that should be considered. First, some variables we included, such as age, inflammatory indices and coagulation function, are not specified in reflecting change of cardiovascular system, which means these variables can affect prognosis through other mechanisms. Second, although some variables may be predictive of mortality (SaO2, IL-6. etc.), they were excluded from the final logistic model because of absence of events. Therefore, their role in predicting prognosis might be underestimated. Third, electrocardiography and echocardiography were not performed in most cases, so the incidence of other cardiovascular complications in our cohort besides myocardial injury, such as heart failure and arrythmia were unclear. Fourth, our study mainly focused on the temporal effects of this disease on cardiovascular system. Given that systemic inflammation and pro-coagulant state caused by pneumonia can exist for a long time [19]. Thus, further studies with longer follow-up period on survivors are needed to investigate the long-term effects of viral infection on cardiovascular system and clinical prognosis.
In conclusion, myocardial injury is not rare in patients with SARS-CoV-2 infection, which incidence is associated with the severity of pneumonia at admission. This complication caused by pneumonia may exacerbate the clinical course and worsen prognosis. Regarding this association, active surveillance and early identification of patients at high risk of developing myocardial injury is essential in the setting of COVID-19 pneumonia.
Author contributions
Dr. Bin Xiong had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Project administration: The institutional ethics committee of Wuhan Union Hospital, Wuhan Jinyintan Hospital and Wuhan Central Hospital.
Funding acquisition: National Natural Science Foundation of China (81873917).
Declaration of competing interest
None.
Acknowledgments
Acknowledgements
We thank all the patients involved in this study. We want to express our gratitude to Chuang Sun for statistical analysis, and the front-line medical staff fighting for the virus.
Founding source
This work was supported by the National Natural Science Foundation of China (81873917).
References
- 1.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X., Chen P., Wang J. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Situation Report - 65. 2020.3.25. https://wwwwhoint/docs/default-source/coronaviruse/situation-reports/20200325-sitrep-65-covid-19pdf?sfvrsn=ce13061b_2
- 8.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Violi F., Cangemi R., Falcone M. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clin. Infect. Dis. 2017;64:1486–1493. doi: 10.1093/cid/cix164. [DOI] [PubMed] [Google Scholar]
- 11.Corrales-Medina V.F., Musher D.M., Shachkina S., Chirinos J.A. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari R., Di Pasquale G., Rapezzi C. Commentary: what is the relationship between Covid-19 and cardiovascular disease? Int. J. Cardiol. 2020 doi: 10.1016/j.ijcard.2020.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance. 2020.3.8. https://wwwwhoint/docs/default-source/coronaviruse/clinical-management-of-novel-covpdf?sfvrsn=bc7da517_2
- 17.General Office of National Health Committee Notice on the Issuance of a Program for the Diagnosis and Treatment of Novel Coronavirus Infected Pneumonia (Trial Seven Edition) 2020.2.19. http://wwwnhcgovcn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989shtml
- 18.Madjid M.S.S., Vardeny O. ACC Clinical Bulletin: Cardiac Implications of Novel Wuhan Coronavirus (2019-nCoV) https://wwwaccorg/latest-in-cardiology/articles/2020/02/13/12/42/acc-clinical-bulletin-focuses-on-cardiacimplications-of-coronavirus-2019-ncov
- 19.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong M.D., Simmons C.P., Thanh T.T. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Restrepo M.I., Reyes L.F. Pneumonia as a cardiovascular disease. Respirology. 2018;23:250–259. doi: 10.1111/resp.13233. [DOI] [PubMed] [Google Scholar]
- 23.Warren-Gash C., Smeeth L., Hayward A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect. Dis. 2009;9:601–610. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 24.Corrales-Medina V.F., Madjid M., Musher D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect. Dis. 2010;10:83–92. doi: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 25.Cuervo G., Viasus D., Carratala J. Acute myocardial infarction after laboratory-confirmed influenza infection. N. Engl. J. Med. 2018;378:2540. doi: 10.1056/NEJMc1805679. [DOI] [PubMed] [Google Scholar]
- 26.Christ-Crain M., Breidthardt T., Stolz D. Use of B-type natriuretic peptide in the risk stratification of community-acquired pneumonia. J. Intern. Med. 2008;264:166–176. doi: 10.1111/j.1365-2796.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher P.E., Ferrario C.M., Tallant E.A. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020 doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]