Fatty liver disease (FLD) arouses increasingly more attention in research and clinical practice, due to the increasing prevalence of the disease and the fact that nowadays it represents a leading cause of liver-related morbidity and mortality (1).
The need of a change in the definition from a “non-condition” into a clearly defined disease has been suggested since the early 2000s (2). Recently, a consensus of international experts proposed to overcome the current nomenclature “Non-Alcoholic Fatty Liver Disease” (NAFLD) and adopt the acronym MAFLD, or “Metabolic-Associated Fatty Liver Disease”, giving relevance to the underlying condition of systemic metabolic dysfunction (3). Although a broader consensus by all stakeholders in the field is still needed before a definite change in FLD definition can be implemented, the publication of the first statement signed by a large international panel of experts represents an initial step in this process.
Briefly, according to the aforementioned proposal, MAFLD diagnosis would be based on the detection of hepatic steatosis (diagnosed by imaging, biomarkers, or histology) and at least one feature among overweight/obesity, type 2 diabetes, and metabolic dysregulation. The last criterium is met when at least two features are present among: increased waist circumference, arterial hypertension, hypertriglyceridemia, low HDL-C, prediabetes, insulin resistance, and subclinical inflammation. These criteria will identify a more homogenous condition than NAFLD, overcoming the difficulties and controversies in the definition of at-risk alcohol intake, thereby hopefully fostering new pathophysiological developments and facilitating clinical studies (as brilliantly reviewed by Fouad et al. in this journal (4)). However, the impact of the new FLD classification in clinical practice is not yet known. Indeed, this does not represent a simple change in the nomenclature: differently from NAFLD, MAFLD will be diagnosed in individuals with fatty liver and dysmetabolism, even when at-risk alcohol intake is reported, but not in lean individuals with fatty liver without metabolic comorbidities (such as a fraction of those with lean NAFLD) (5).
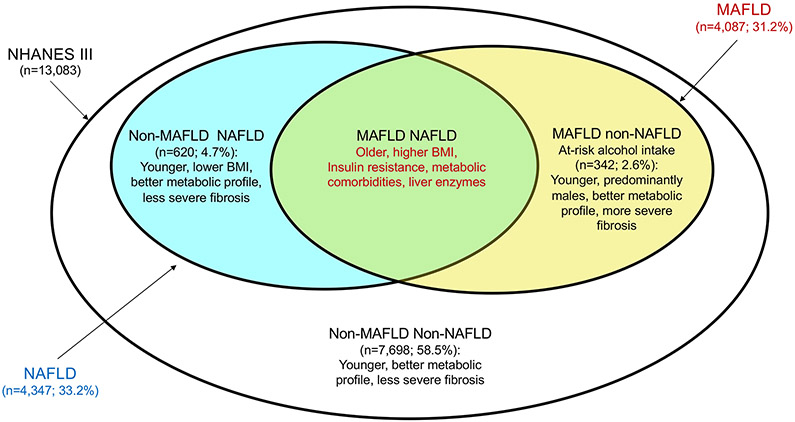
In this issue of Liver International, Lin et al. (6) compared the characteristics of individuals with MAFLD vs. NAFLD in 13,083 subjects from the general population enrolled in the third National Health and Nutrition Examination Survey of the United States (NHANES III). The prevalence of MAFLD, as defined by the new criteria, was comparable to that of NAFLD (31.2% vs. 33.2%) and only 4.7% of participants met the diagnostic criteria of NAFLD, but not those of MAFLD (Fig. 1). Remarkably, MAFLD criteria were able to identify participants at higher risk of progressive liver and cardiovascular diseases. Indeed, individuals with MAFLD had higher body mass index (BMI), proportion of metabolic comorbidities, and ALT levels than those with NAFLD (Fig. 1). On the other hand, individuals with NAFLD without MAFLD had less frequently metabolic comorbidities and non-invasively assessed hepatic fibrosis, whereas MAFLD individuals reporting at-risk alcohol consumption were younger than the others, and had a more favourable metabolic profile, but more severe liver fibrosis.
Figure 1. MAFLD vs. NAFLD in the general population (NHANES III; n=13,083).
Prevalence of patients with the diagnosis of MAFLD (Metabolic associated fatty liver disease) was comparable to that of NAFLD (nonalcoholic fatty liver disease). MAFLD improved the detection of patients at higher risk of liver and cardiovascular comorbidities. New criteria of MAFLD include also at-risk alcohol users, who were younger males with a better metabolic profile but more severe liver fibrosis respect to non-alcohol users. NHANES III: third National Health and Nutrition Examination Survey of the United States. BMI: body mass index.
The proposed nomenclature change can benefit FLD awareness campaigns. The implications of co-existing metabolic dysfunction are neglected by the NAFLD definition, whereas interventions that improve the diet and life-style are key to reduce not only the hepatic, but also the cardiovascular, metabolic, and neoplastic complications of fatty liver disease. Therefore, changing the name to MAFLD and giving clear diagnostic criteria would be helpful to focus on the underlying trigger. Secondly, but not of secondary importance, one should consider the role of alcohol in FLD. Accepted NAFLD criteria may suggest the misleading idea that a certain amount of alcohol consumption is acceptable. However, there is currently no robust demonstration of the existence of a safe threshold for alcohol, especially in persons suffering from a liver condition (8). Once more, the definition of a disease based on the exclusion of just one risk factor is at very least simplistic. The new MAFLD criteria focus on the role of dysmetabolism on hepatic fat accumulation, that is the most frequent driver of FLD progression (9-10). However, alcohol, together with dietary fructose, inherited factors and so on, represent other triggers of liver disease progression (11), and even modest alcohol consumption contributes to FLD development (12). Therefore, alcohol and dysmetabolism should rather be considered as co-risk factors than as opposites when defining and classifying FLD.
Recently however, Younossi et al. on behalf of the American Association for the Study of Liver Disease (AASLD) pointed out that renaming NAFLD may be premature: indeed, most physicians who are not hepatologists still have difficulties recognizing the importance of FLD screening in their practice, and regulatory agencies and patient organizations should be included in the decision process (7). Furthermore, it was suggested that increasing the public and general awareness on FLD has a greater priority than to improve the diagnostic algorithm. These important and shared concerns should be carefully weighed against the potential benefits of this change. Therefore, it seems reasonable to retain the nomenclature nonalcoholic steatohepatitis (NASH) for awareness campaigns (e.g. “International NASH day”) to define the most severe form of the disease as opposed to that caused by alcohol excess and at least, until a consensus is reached, in clinical trials.
For these reasons, and in order to contribute with more high-quality data to inform the current debate without supporting a priori either position, Liver International will keep publishing pathophysiological, genetic, clinical (e.g. comparing the natural history between MAFLD/NAFLD concerning both the hepatic and extra-hepatic complications), and public health research aimed at comparing MAFLD vs. NAFLD. Let the contest begin then, and the best win!
Acknowledgments
LV was supported by MyFirst Grant AIRC n.16888, Ricerca Finalizzata Ministero della Salute RF-2016-02364358, Ricerca corrente Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, the European Union (EU) Programme Horizon 2020 (under grant agreement No. 777377) for the project LITMUS- “Liver Investigation: Testing Marker Utility in Steatohepatitis”, Fondazione IRCCS Ca’ Granda “Liver BIBLE” PR-0391, Fondazione IRCCS Ca’ Granda core COVID-19 Biobank (RC100017A). LV reports having received speaking fees from MSD, Gilead, AlfaSigma, AbbVie, having served as a consultant for: Gilead, Pfizer, Astra Zeneca, Novo Nordisk, Intercept, Diatech Pharmacogenetics, Ionis Pharmaceuticals, and received research grants from: Gilead.
SR reports having served as consultant for: Astra Zeneca, AMGEN, Sanofi-Aventis, CAMP4, Celgene, MEDACORP, Foresite labs. He received research grants from Astra-Zeneca, Sanofi-Aventis, AMGEN.
SP acted as speaker and/or consultant for MSD, Gilead, AbbVie, Pfizer, Intercept.
MTL is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases K23 DK113252, the Doris Duke Charitable Foundation, Gilead Sciences, Echosens Corporation, the Boston University School of Medicine Department of Medicine Career Investment Award and the Boston University Clinical Translational Science Institute UL1 TR001430. MTL is on the speaking bureau for Intercept and serves as a consultant for Iterative Scopes and Ionis Pharmaceuticals.
Footnotes
Conflict of interest: Authors declare that they do not have any conflict of interest relevant to this manuscript.
REFERENCES
- (1).Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896–904. doi: 10.1016/j.jhep.2018.05.036 [DOI] [PubMed] [Google Scholar]
- (2).Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference [published correction appears in Hepatology. 2003 Aug;38(2):536]. Hepatology. 2003;37(5):1202–1219. doi: 10.1053/jhep.2003.50193 [DOI] [PubMed] [Google Scholar]
- (3).Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- (4).Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020;40(6):1254–1261. doi: 10.1111/liv.14478 [DOI] [PubMed] [Google Scholar]
- (5).Valenti L, Pelusi S. Redefining fatty liver disease classification in 2020. Liver Int. 2020;40(5):1016–1017. doi: 10.1111/liv.14430 [DOI] [PubMed] [Google Scholar]
- (6).Lin S, Huang J, Wang M, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world [published online ahead of print, 2020 Jun 1]. Liver Int. 2020;10.1111/liv.14548. doi: 10.1111/liv.14548 [DOI] [PubMed] [Google Scholar]
- (7).Younossi ZM, Rinella ME, Sanyal A, et al. From NAFLD to MAFLD: Implications of a premature change in terminology [published online ahead of print, 2020 Jun 16]. Hepatology. 2020;10.1002/hep.31420. doi: 10.1002/hep.31420 [DOI] [PubMed] [Google Scholar]
- (8).GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016 [published correction appears in Lancet. 2018 Sep 29;392(10153):1116] [published correction appears in Lancet. 2019 Jun 22;393(10190):e44]. Lancet. 2018;392(10152):1015–1035. doi: 10.1016/S0140-6736(18)31310-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Dongiovanni P, Stender S, Pietrelli A, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283(4):356–370. doi: 10.1111/joim.12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Nasr P, Fredrikson M, Ekstedt M, Kechagias S. The amount of liver fat predicts mortality and development of type 2 diabetes in non-alcoholic fatty liver disease. Liver Int. 2020;40(5):1069–1078. doi: 10.1111/liv.14414 [DOI] [PubMed] [Google Scholar]
- (11).Trépo E, Valenti L. Update on NAFLD genetics: From new variants to the clinic. J Hepatol. 2020;72(6):1196–1209. doi: 10.1016/j.jhep.2020.02.020 [DOI] [PubMed] [Google Scholar]
- (12).Long MT, Massaro JM, Hoffmann U, Benjamin EJ, Naimi TS. Alcohol use is associated with hepatic steatosis among persons with presumed Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2020;18(8):1831–1841.e5. doi: 10.1016/j.cgh.2019.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]