Abstract
Background:
C-type lectin receptors, including Dectin-2, are pattern recognition receptors on monocytes and macrophages that mainly recognize sugars and sugar-like structures present on fungi. Activation of C-type lectin receptors induces downstream CARD9 signalling, leading to the production of cytokines. We hypothesized that under hyperglycaemic conditions, as is the case in diabetes mellitus, glycosylated protein (sugar-like) structures activate C-type lectin receptors, leading to immune cell activation and increased atherosclerosis development.
Methods:
Low-density lipoprotein receptor-deficient mice were lethally irradiated and transplanted with bone marrow from control wild-type, Dectin-2−/− or Card9−/− mice. After 6 weeks of recovery, mice received streptozotocin injections (50 mg/g BW; 5 days) to induce hyperglycaemia. After an additional 2 weeks, mice were fed a Western-type diet (0.1% cholesterol) for 10 weeks.
Results and Conclusion:
Deletion of haematopoietic Dectin-2 reduced the number of circulating Ly6Chi monocytes, increased pro-inflammatory cytokine production, but did not affect atherosclerosis development. Deletion of haematopoietic CARD9 tended to reduce macrophage and collagen content in atherosclerotic lesions, again without influencing the lesion size. Deletion of haematopoietic Dectin-2 did not influence atherosclerosis development under hyperglycaemic conditions, despite some minor effects on inflammation. Deletion of haematopoietic CARD9 induced minor alterations in plaque composition under hyperglycaemic conditions, without affecting lesion size.
Keywords: Atherosclerosis, CARD9, C-type lectin receptors, Dectin-2, hyperglycaemia, inflammation, monocytes/macrophages
Background
Diabetes largely increases the risk for the development of cardiovascular diseases (CVD), the leading cause of death in type 1 and type 2 diabetic patients. In fact, a 1% increase in haemoglobin (Hb) A1c levels is associated with a 31% increase in cardiovascular events.1 Interestingly, atherosclerotic plaques of diabetic patients display a higher macrophage content as compared to non-diabetic patients independently of other risk factors, which strongly correlates with HbA1c levels.2
Monocytes and macrophages are major drivers of atherosclerosis development, the main underlying cause of CVD, by infiltrating into the arterial wall and accumulating oxidized and/or aggregated lipoproteins. This leads to the formation of foam cells and secretion of pro-inflammatory cytokines.3 High glucose levels increase the transcription of pro-inflammatory cytokines in monocytes and macrophages,4 and monocytes from diabetic patients have a more pro-inflammatory phenotype after ex vivo stimulation.5 Altogether, these data support a link between hyperglycaemia and macrophage/macrophage activation.
Myeloid cells, such as monocytes and macrophages, express pattern recognition receptors (PRRs), which evolved to recognize pathogen-associated molecular patterns (PAMPs) including lipids, carbohydrates and proteins. Thereby, PRRs transduce danger signals to cellular responses. Toll-like receptors (TLRs) are the most extensively studied PRRs and have been shown to play an important role in atherogenesis.6 Interestingly, TLRs not only respond to various exogenous ligands derived from pathogens but also to endogenous ligands, such as lipids, involved in atherosclerosis.7 The C-type lectin receptor (CLR) family is another class of PRRs, characterized by a carbohydrate-binding domain which mainly recognizes sugars and sugar-like structures on fungal pathogens.8,9 Well-known members of the CLR family are Dectin-1, Dectin-2 and Mincle, which signal through the PKCδ-CARD9-Bcl-10-MALT1 axis, leading to transcription of nuclear factor-kappa B (NF-κB) and subsequent secretion of pro-inflammatory cytokines. The caspase recruitment domain-containing protein (CARD) 9 is a part of this axis and a master regulator in signal transduction of all CLRs.10,11
Although the ‘classical’ function of CLRs is to recognize fungal carbohydrates, endogenous ligands have also been described for CLRs,12 such as the recognition of glycosylated protein by Dectin-2.13 We hypothesized that increased levels of carbohydrate structures that are formed during hyperglycaemia (e.g. advanced glycation end products – AGEs) may activate CLRs.14 This would transduce hyperglycaemic conditions into macrophage activation, thereby inducing a chronic pro-inflammatory state and increasing the risk for atherosclerosis development.
Therefore, the aim of this study was to elucidate whether deletion of the CLR Dectin-2 or the downstream master regulator of the CLR family CARD9 protects from atherosclerosis development under hyperglycaemic conditions. To this end, low-density lipoprotein receptor-deficient (Ldlr−/−) mice were irradiated and their bone marrow was reconstituted with bone marrow from wild-type (WT), Dectin-2−/− or Card9−/− mice. After a recovery period, mice were injected with streptozotocin (STZ) to induce hyperglycaemia and were fed a Western-type diet (WTD) containing 0.1% cholesterol to induce hyperlipidaemia and atherosclerosis.
Materials and methods
Animals
Homozygous 6-week-old female Ldlr−/− mice (C57Bl/6 J background) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed under standard conditions in conventional cages in a temperature-controlled room with a 12-h light/dark cycle and ad libitum access to food and water. To induce bone marrow aplasia, female Ldlr−/− recipient mice (8 weeks of age) were irradiated to a dose of 8 Gy at a dose rate of 3.8 Gy/min (X-RAD 320ix; Precision X-Ray, Inc., North Branford, CT, USA). The day thereafter irradiated recipient Ldlr−/− mice received an intravenous injection through the tail vein with 1.2 × 106 bone marrow cells isolated from donor control WT, Dectin2−/− or Card9−/− (all C57Bl/6 J background) female mice, mixed with 0.3 × 106 freshly isolated splenic cells from Rag1−/− (also C57Bl/6 J background) female mice. The successful engraftment of Dectin-2- or CARD9-deficient bone marrow cells was verified by measuring gene expression levels in bone marrow cells and peritoneal macrophages at the end of the study (Supplemental Figure 1(A) and (B)). All mice received water containing antibiotics (0.13 mg/kg/day ciprofloxacin, 0.105 mg/kg/day polymyxin B, 0.15 mg/kg/day amphotericin B) from 1 day before until 4 weeks after bone marrow transplantation (BMT). After 6 weeks of recovery on chow diet, hyperglycaemia was induced by injecting mice on 5 consecutive days with STZ (50 mg/kg, i.p.).15 After 2 weeks, hyperglycaemia was determined directly from the tail tip with a glucometer and mice were regarded as hyperglycaemic when blood glucose levels were above 15 mM. Subsequently, mice were fed a WTD, containing 15% (w/w) cocoa butter, 1% (w/w) corn oil and 0.1% (w/w) cholesterol (AB diets, Woerden, The Netherlands) for 10 weeks. Body weight was monitored weekly. Three times a week, blood glucose levels of mice were checked. Mice were treated with insulin [subcutaneous, 1:4 dilution of Lantus (25 U/mL) in saline] three times a week in case blood glucose levels rose above 25 mM (1 U insulin/mouse) and 30 mM (1.5 U insulin/mouse). All experiments in this study were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, the Dutch Law on Animal Experiments and the Federation of European Laboratory Animal Science Associations (FELASA) regulations. The protocol was approved by the Ethics Committee on Animal Experiments of the Leiden University Medical Center.
Plasma lipid analysis
Blood was drawn from the tail vein of 4-h-fasted mice at the indicated time points. After 10 weeks of WTD, unfasted blood samples were collected through orbital exsanguination in ethylenediaminetetraacetic acid (EDTA)-coated tubes. Plasma from all samples was isolated by centrifugation and assayed for total cholesterol (TC) and triglycerides (TGs) using commercially available enzymatic colorimetric kits (Liquicolor; Human GmbH, Wiesbaden, Germany). Assays were performed according to the manufacturer’s protocols.
Extracellular staining and flow cytometry
Cells within 50 µL fresh blood were stained extracellularly with antibodies for CD45, SiglecF, Ly6C, CD4 (BD Biosciences, CA, USA); CD11b, Ly6G, MHCII, CD3, CD8a, Natural Killer (NK) (Biolegend, Koblenz, Germany); and CD19 (eBioscience/Thermo Fisher, Breda, The Netherlands). Staining was analysed by FACSVerse (BD Biosciences) and CXP software (Beckman Coulter, Woerden, The Netherlands). Whole blood was first gated on total CD45+ leukocyte population. Neutrophils were selected as Ly6G+ and from Ly6G− population, and eosinophils were defined as Cd11b+ SiglecF+. Within the Ly6G− SiglecF− population, monocytes were defined as CD11b+ MHCII− and further subdivided into pro-inflammatory (Ly6C+ high), anti-inflammatory (Ly6C+ low) and intermediate (Ly6C+ medium) monocytes. Lymphoid cells were selected on CD3+ for T-cells. Within the CD3+ population, cytotoxic T-cells were gated on CD8+ CD4− and T-helper cells on CD8− CD4+.
Ex vivo stimulations
For ex vivo cell stimulation experiments, cells were extracted from the peritoneum, the bone marrow (i.e. tibia and femur of hind limb bones) or the spleen. After cleaning with 70% ethanol, bones were cut and flushed with sterile phosphate-buffered saline (PBS). Obtained cells were differentiated in Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher, Breda, The Netherlands) containing 1% Penicillin/Streptomycin (Sigma–Aldrich, Zwijndrecht, The Netherlands) and 30% L929 medium for 7 days, and then counted with a particle counter (Beckman Coulter). For stimulation experiments, 100 µL suspension containing 1 × 105 bone-marrow-derived macrophages (BMDMs) was added to 96-well flat bottom plates (Corning, NY, USA). Spleen cells were isolated by crushing whole spleen with a plunger. The single cell suspension was filtered, spun down and counted. A total of 5 × 105 cells were added to 24-well plates (Greiner, Monroe, NC, USA) in DMEM (Thermo Fisher) medium containing 10% heat inactivated foetal bovine serum. Splenocytes were stimulated for 5 days with Candida albicans (1 × 107/mL), Staphylococcus aureus (1 × 107/mL) or Salmonella typhimurium (1 × 107/mL). Peritoneal macrophages were obtained by injecting 10 mL ice-cold PBS into the peritoneal cavity. Total cavity fluid was collected, spun down, and obtained cells were counted. A 100-µL suspension containing 1 × 105 peritoneal macrophages was added to 96-well round-bottom plates (Greiner). Culture medium used was Roswell Park Memorial Institute (RPMI, no glucose) 1640 Dutch modifications (Sigma–Aldrich, The Netherlands), supplemented with 0.3% glutamax, 1% gentamycin (Life Technologies, Nieuwerkerk, The Netherlands), 1% HEPES and 5.5 mM D-glucose (Sigma–Aldrich, The Netherlands). BMDM and peritoneal macrophages were challenged with Escherichia coli (E. coli) lipopolysaccharide (LPS, 10 ng/mL) (serotype O55:B5; Sigma–Aldrich, St. Louis, MO, USA) or Pam3Cys (P3 C, 10 µg/mL). Supernatants of stimulated bone marrow cells and peritoneal macrophages were collected after 24 h and stored at −80°C until assessed.
Cytokine assay
The concentrations of tumour necrosis factor (TNF)-α (R&D Systems, Minneapolis, MN, USA) and mouse interleukin (IL)-6 (Sanquin, Amsterdam, The Netherlands) were measured in the cell culture supernatants using enzyme-linked immunosorbent assays (ELISAs), according to the manufacturers’ instructions.
RNA isolation and qPCRs
Peritoneal macrophages were harvested as described above and stored in liquid nitrogen until mRNA assessment. Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used according to the manufacturer’s protocol to extract mRNA, which was then transcribed into complementary DNA (cDNA) by reverse transcription using iScript cDNA synthesis kit (Bio-Rad Laboratories BV, Veenendaal, The Netherlands). Relative expression was determined using SYBR Green method (Applied Biosystems, Thermo Fisher) on an Applied Bioscience Step-one PLUS qPCR machine (Applied Biosystems; Life technologies) and the values were expressed as fold increases in mRNA levels relative to those of WT mice, with 36b4 as a housekeeping gene. Primers used for the experiments (final concentration 10 µM) are listed in Supplemental Table S1.
Atherosclerosis development
Hearts were collected and fixed in 4% phosphate-buffered formaldehyde, embedded in paraffin and cross-sectioned (5 µm) throughout the aortic root area, starting from the appearance of open aortic valve leaflets. Per mouse, six sections with 50 μm intervals were used for atherosclerosis quantification. Sections were stained with hematoxylin-phloxine-saffron for histological analysis. Macrophage area was determined using rat anti-mouse antibody MAC3 (1:1000; BD Pharmingen, San Diego, CA, USA). Collagen area was determined using Sirius Red staining. T-cells were stained with CD3 antibody (1:50; DakoCytomation, Glostrup, Denmark). The lesion area and composition were quantified using ImageJ Software.
Statistical analysis
Differences between groups were determined using one-way analysis of variance (ANOVA) with the Dunnett’s post hoc test. Differences at probability values less than 0.05 were considered statistically significant. Data are presented as means ± SEM. All statistical analyses were performed with the SPSS 20.0 software package for Windows (SPSS, Chicago, IL USA).
Results
Deletion of haematopoietic Dectin-2 or CARD9 does not influence metabolic parameters
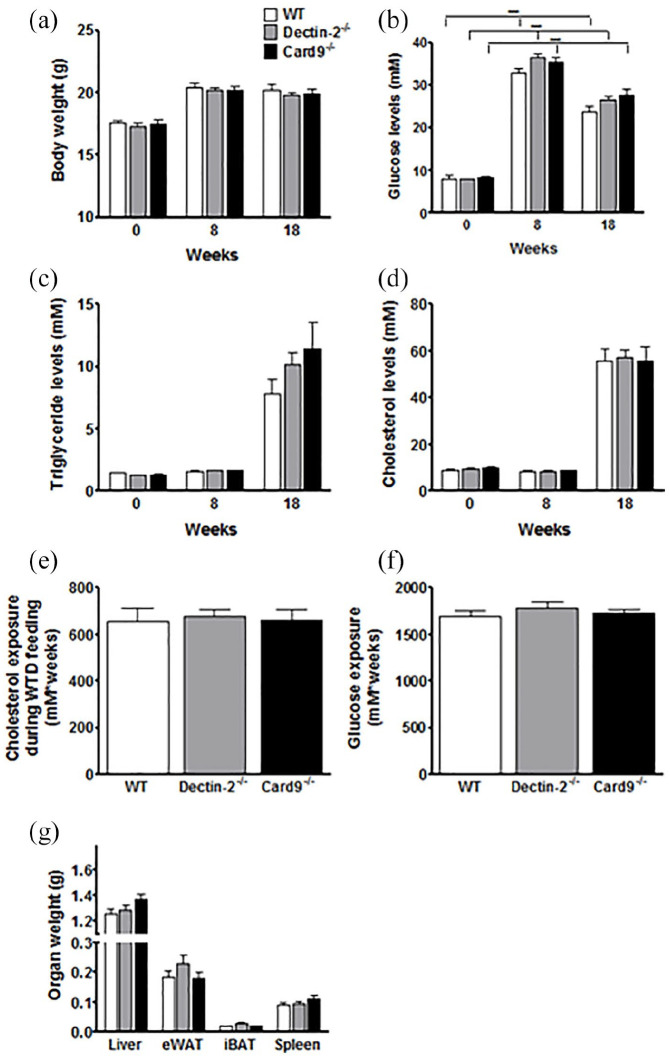
To study the effect of haematopoietic deletions of Dectin-2 or CARD9 on STZ-induced hyperglycaemia and atherosclerotic parameters, bone marrow of donor Dectin-2−/−, Card9−/− or control WT mice was transplanted into Ldlr−/− recipient mice (t = 0). After 6 weeks of recovery, hyperglycaemia was induced using STZ injections. Two weeks thereafter, at t = 8 weeks, mice were switched to WTD to induce atherosclerosis during 10 weeks (Figure 1). We first assessed the effect of deletion of haematopoietic Dectin-2 or CARD9 on metabolic parameters. Body weight was similar for all genotypes during the study (Figure 2(a)). Glucose levels were markedly higher after STZ injection for all genotypes as compared to t = 0, confirming successful induction of hyperglycaemia (Figure 2(b)). Deletion of haematopoietic Dectin-2 or CARD9 did not influence glucose levels compared to WT controls. Plasma TG (Figure 2(c)) and TC (Figure 2(d)) levels were higher after feeding WTD, but similar for all groups. Consequently, the groups had similar TC exposure (Figure 2(e)) and glucose exposure (Figure 2(f)) during the 10 weeks of WTD feeding. Also, haematopoietic deletion of Dectin-2 and CARD9 did not influence the weight of several organs at the end of the study (Figure 2(g)). These data show that deletion of haematopoietic Dectin-2 or CARD9 does not influence body weight or plasma glucose and lipid parameters under hyperglycaemic conditions.
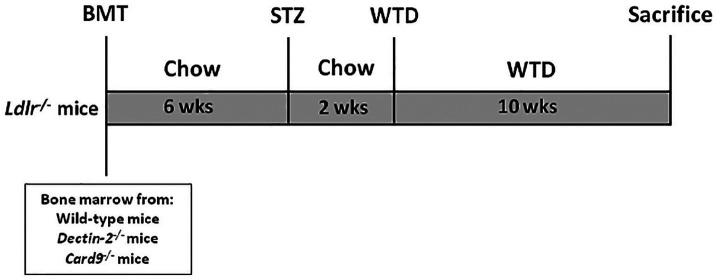
Figure 1.
Study setup.
Ldlr−/− mice were transplanted with bone marrow from control C57BL/6 wild-type, Dectin-2−/− or Card9−/− mice. After 6 weeks of recovery, mice were treated with STZ to induce hyperglycaemia. After 8 weeks of BMT, mice were fed a Western-type diet for 10 weeks and metabolic parameters, inflammatory status and atherosclerosis development were determined.
Figure 2.
Deletion of haematopoietic Dectin-2 or CARD9 does not influence metabolic parameters.
Ldlr−/− mice were transplanted with bone marrow from control (WT), Dectin-2−/− or Card9−/− mice. After 6 weeks of recovery, mice were treated with STZ to induce hyperglycaemia. After 8 weeks of BMT, mice were fed a Western-type diet for 10 weeks. (a) Body weight, plasma levels of (b) glucose, (c) triglycerides and (d) cholesterol were monitored during the study. At the end of the study, (e) total cholesterol and (f) total glucose exposure during WTD feeding was calculated and mice were sacrificed and (g) organs collected and weighed. Data are expressed as means ± SEM; n = 12–17/group. ***p < 0.0001.
Deletion of haematopoietic Dectin-2, but not CARD9, reduces circulating Ly6Chi monocytes
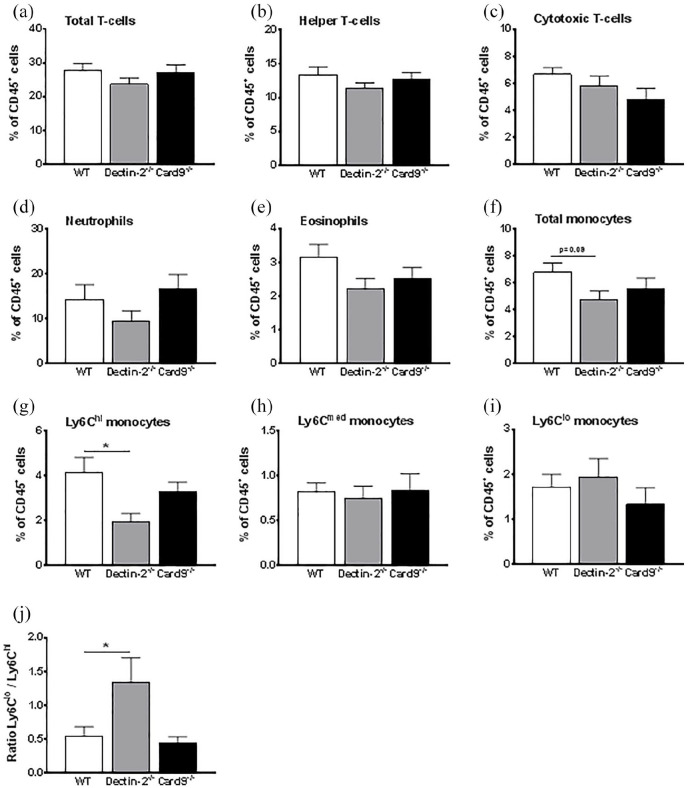
In the following set of experiments, we evaluated whether deletion of haematopoietic Dectin-2 or CARD9 influenced the inflammatory state under hyperglycaemic conditions. To this end, we first performed flow cytometry on blood cells at the end of the study. Haematopoietic deletion of Dectin-2 or CARD9 did not influence circulating levels of total T-cells (Figure 3(a)), cytotoxic T-cells (Figure 3(b)) and helper T-cells (Figure 3(c)) as compared to control mice. Deletion of haematopoietic Dectin-2 or CARD9 also did not influence the circulating neutrophils (Figure 3(d)) and eosinophils (Figure 3(e)). However, total monocytes tended to be lower in mice with deletion of haematopoietic Dectin-2, but not with haematopoietic CARD9-deletion (Figure 3(f)). This was due to a reduction in Ly6Chi monocytes, while Ly6Cmed and Ly6Clo monocytes were similar (Figure 3(g)–(i)). As a consequence, deletion of haematopoietic Dectin-2 increased the ratio between Ly6Clo and Ly6Chi monocytes (Figure 3(j)).
Figure 3.
Deletion of haematopoietic Dectin-2, but not CARD9, reduces circulating monocytes.
At the end of the study, after 10 weeks of WTD feeding, (a–i) circulating immune cells in whole blood were determined by flow cytometry. And the (j) ratio between Ly6Clo/Ly6Chi monocytes was calculated. Data are expressed as means ± SEM; n = 8/group; *p < 0.05.
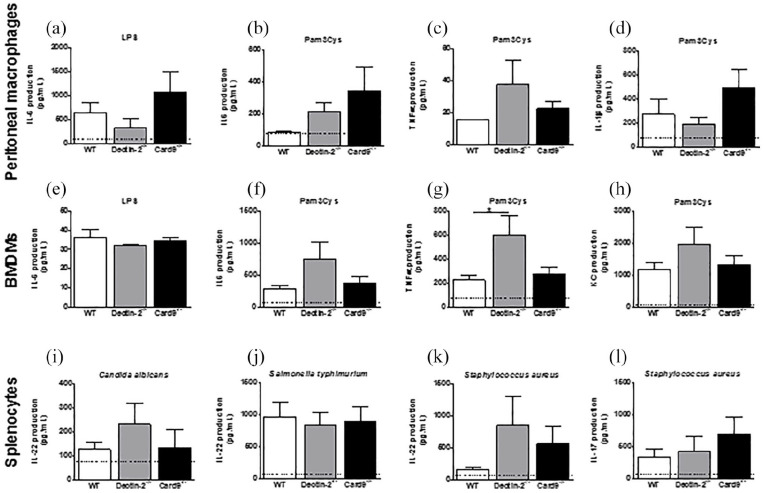
We then assessed whether the reduction in Ly6Chi monocytes in mice with deletion of haematopoietic Dectin-2 also resulted in functional changes. To evaluate the general inflammatory response of these macrophages, isolated peritoneal macrophages and BMDMs were stimulated ex vivo with the TLR ligands LPS or Pam3Cys. LPS-stimulated IL-6 production by peritoneal macrophages and BMDM was not different between groups (Figure 4(a) and (e)). Deletion of haematopoietic Dectin-2 seemed to consistently increase IL-6 and TNFα production in both peritoneal macrophages and BMDMs, resulting in only a significant increase in Pam3Cys-stimulated BMDMs (Figure 4(b)–(d) and (f)–(h)). Deletion of haematopoietic CARD9 does not seem to have influenced the Pam3Cys stimulated IL-6 or TNFα response. However, generally there was a large variation in response, resulting in the absence of statistical differences between the groups (Figure 4(a)–(h)). Thus, deletion of haematopoietic CARD9 under hyperglycaemic conditions does not influence inflammatory response capacity, while deletion of haematopoietic Dectin-2 reduces circulating pro-inflammatory monocytes but increases their pro-inflammatory cytokine production. To investigate whether deletion of haematopoietic deletion of Dectin-2 or CARD9 influenced T-cell activation, IL-22 and IL-17 secretion by splenocytes was determined after stimulation for 5 days with Candida albicans, Staphylococcus aureus or Salmonella typhimurium. The secretion of the two T-cell cytokines IL-22 and IL-17 was not altered among the three genotypes (Figure 4(i)–(l)).
Figure 4.
Deletion of haematopoietic Dectin-2, but not CARD9, increases TNFα production by Pam3Cys stimulated bone-marrow-derived macrophages.
At the end of the study, after 10 weeks of WTD feeding, mice were killed and (a–c) peritoneal macrophages and (d–f) bone-marrow-derived macrophages were isolated and ex vivo stimulated with LPS (10 ng/mL) or Pam3Cys (10 μg/mL) for 24 h. Splenocytes were isolated and ex vivo stimulated with Staphylococcus aureus (1 × 107/mL), Salmonella typhimurium (1 × 107/mL) or Candida aureus (1 × 107/mL) for 5 days. Production of (a, b, e, f) IL-6, (c, g) TNFα, (d) IL-1β, (h) KC, (i–k) IL-22 and (l) Il-17 was determined in medium. Black dotted line indicates detection limit of the assay. Data are expressed as means ± SEM; n = 7–8/group; *p < 0.05.
Deletion of haematopoietic Dectin-2 or CARD9 does not influence expression of inflammatory genes in peritoneal macrophages
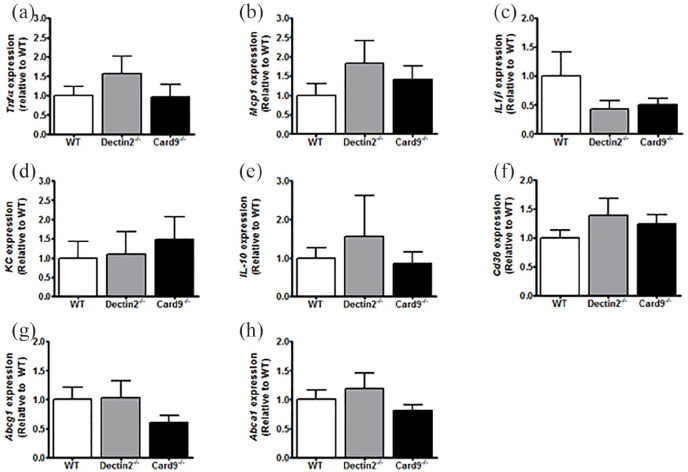
We assessed whether these functional changes were paralleled by changes in the expression of inflammatory genes in peritoneal macrophages. The expression of Tnfα, Mcp1 Il1β, Kc and Il-10 in peritoneal macrophages was similar between all genotypes (Figure 5(a)–(e)).
Figure 5.
Deletion of haematopoietic Dectin-2 or CARD9 does not influence expression of inflammatory genes in peritoneal macrophages.
At the end of the study, after 10 weeks of WTD feeding, mice were killed and peritoneal macrophages were collected. RT-qPCR was used to determine the expression of the inflammatory genes (a) Tnfα, (b) Mcp1, (c) Il1β, (d) Kc and (e) IL-10. Also, expression of genes involved in lipid uptake (f) Cd36, and lipid efflux (g) Abcg1 and (h) Abca1 was measured. Data are expressed as means ± SEM; n = 7–8/group.
Since monocytes and macrophages are not only important for the production of cytokines but also for foam cell formation, we next assessed the gene expression of transporters for cholesterol uptake (Cd36) and efflux (Abcg1, Abca1). Again, the expression of these genes was similar between the groups (Figure 5(d)–(f)). These data show that haematopoietic deletion of Dectin-2 or CARD9 under hyperglycaemic conditions neither influences expression of inflammatory genes nor expression of genes involved in cellular cholesterol transport in peritoneal macrophages.
Deletion of haematopoietic Dectin-2 or CARD9 does not influence atherosclerotic lesion size
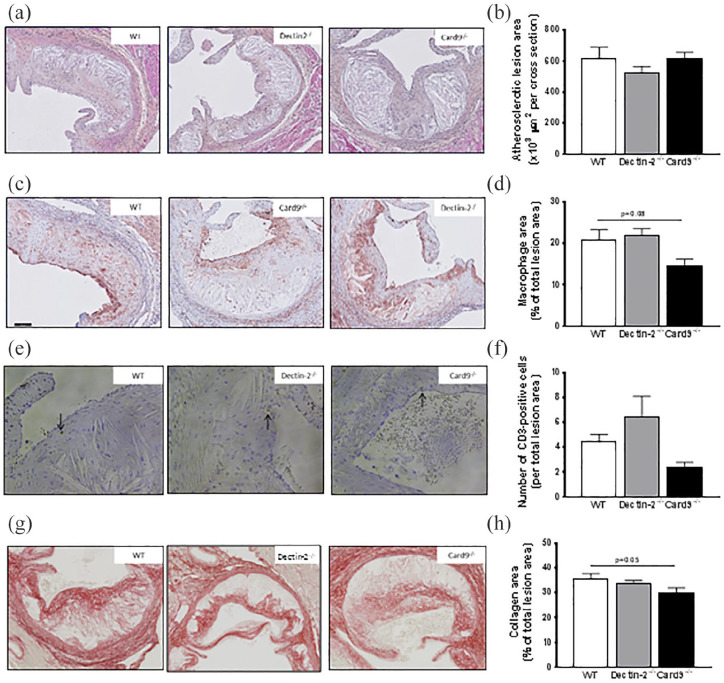
Finally, we assessed whether deletion of haematopoietic Dectin-2 or CARD9 could attenuate atherosclerosis development under hyperglycaemic conditions. Therefore, we determined the atherosclerotic lesion area and composition in the valve area of the aortic root of the heart at the end of the study. Deletion of haematopoietic Dectin-2 or CARD9 did not influence lesion area throughout the aortic root of the heart (Figure 6(a) and (b)). Deletion of haematopoietic Dectin-2 did also not influence the macrophage content (Figure 6(c) and (d)), CD3-positive cell content (Figure 6(e) and (f)) or collagen content (Figure 6(g) and (h)) in the lesions. Slight differences were seen in mice with a deletion of haematopoietic CARD9, in which the macrophage content (Figure 6(c) and (d)), CD3-positive cell content (Figure 6(e) and (f)) and collagen content (Figure 6(g) and (h)) of the atherosclerotic lesions tended to be reduced. Taken together, these findings indicate that although deletion of haematopoietic CARD9 slightly alters lesion composition, neither deletion of Dectin-2 nor CARD9 does influence atherosclerotic lesion size under hyperglycaemic conditions.
Figure 6.
Deletion of haematopoietic CARD9 reduces macrophage content in plaques.
At the end of the study, after 10 weeks of WTD feeding, mice were killed and slides of the valve area of the aortic root were stained with hematoxylin-phloxine-saffron (HPS) and representative pictures are shown. (a) Scale bar represents 100 μm. (b) The average total lesion area of these four cross-sections per mouse was calculated. Representative pictures of (c) MAC3 staining, (e) CD3 staining (20X magnification) and (g) Sirius Red staining are presented. The mean of the (d) MAC3-positive macrophage area, (f) CD3-positive cells and the mean of the Sirius Red positive area were determined. Data are expressed as means ± SEM; n = 12–17/group.
Discussion
Hyperglycaemia associates with pro-inflammatory monocytes that highly contribute to atherosclerosis development. The results of this study show that, in opposite to our hypothesis, haematopoietic deletion of the CLR Dectin-2 does not influence atherosclerosis development, despite moderate changes in the inflammatory phenotype of peripheral monocytes and macrophages. Haematopoietic deletion of the downstream master regulator of the CLR family CARD9 also does not influence atherosclerotic lesion size but moderately impacts the composition of atherosclerotic plaques, without affecting the inflammatory phenotype of peripheral innate immune cells.
Deletion of haematopoietic Dectin-2 reduced the number of circulating Ly6Chi monocytes. These monocytes are regarded pro-inflammatory as they are more prone to become macrophages that turn into foam cells after uptake of oxidized LDL (oxLDL) in the vessel wall. Subsequently, these foam cells secrete both pro-inflammatory cytokines and reactive oxygen species, thereby promoting lesion progression. In contrast, Ly6Clo monocytes are involved in tissue repair and regarded as anti-inflammatory.14 The reduced pro-inflammatory Ly6Chi monocyte count in mice with haematopoietic deletion of Dectin-2 was not paralleled by a reduced pro-inflammatory phenotype of tissue macrophages, as determined in both peritoneal macrophages and BMDMs. We even observed an increased TNFα (and a trend for increased IL-6) secretion from Pam3Cys-stimulated macrophages from haematopoietic Dectin-2-deficient as compared to WT mice. This increased cytokine response seems to be specific for TLR2-dependent Pam3Cys stimulation16 and was not observed after TLR4 stimulation using LPS. One could speculate that Dectin-2-deficiency causes compensatory upregulation of other CLRs, such as Dectin–1. Since Dectin-1 cooperates with TLR2,17 this could possibly explain the TLR2-specific increased cytokine production in Dectin-2-deficient macrophages. Interestingly, the TLR2 response is explicitly activated during hyperglycaemia, since these pro-inflammatory responses in BMDMs from mice with deletion of haematopoietic Dectin-2 were not observed under normoglycaemic conditions.18 High glucose levels enhance expression of TLRs19 and may thus also increase the sensitivity of TLR responses during hyperglycaemic conditions. Most importantly, these subtle changes in the inflammatory phenotype of monocytes and macrophages eventually did not influence atherosclerotic lesion size, or composition, in mice with deletion of haematopoietic Dectin–2.
Deletion of haematopoietic CARD9 neither influenced circulating immune cell count and phenotype nor cytokine production upon stimulation of BMDM or peritoneal macrophages. This was unexpected since total body CARD9 deletion attenuates immune responses under conditions of high-fat diet (HFD)-induced mild hyperglycaemia.20 Similar to Dectin-2, deletion of haematopoietic CARD9 did not influence atherosclerotic lesion size, which is in contrast to the more severe lesions and increased lesion size that had been observed in deletion of haematopoietic CARD9 under normoglycaemic conditions.18 It might be that the deletion of CARD9 has opposite effects in hyperlipidaemic and hyperglycaemic conditions. Although haematopoietic CARD9-deficiency increased plaque size under hyperlipidaemic conditions, it may attenuate plaque formation under hyperglycaemic conditions, as hypothesized in the current study. However, in a model with both hyperlipidaemia and hyperglycaemia, the net effect of haematopoietic CARD9-deficiency will be no difference in plaque formation. Being a main driver of atherosclerosis in the Ldlr−/− mouse model,21 it should also be noted that mice with STZ-induced hyperglycaemia also had markedly increased plasma cholesterol levels as compared to mice under normoglycaemic conditions. Therefore, it might be possible that potential differences in atherosclerotic plaque formation among the three genotypes under hyperglycaemic condition were shed by the high plasma cholesterol levels.
Despite the lack of effect on atherosclerotic lesion size, deletion of haematopoietic CARD9 tended to reduce the macrophage and collagen content within the plaque. The reduced macrophage content in the plaque is in line with a previous study that showed a reduced number of macrophages in the heart and improved myocardial function in total CARD9-deficient mice with diet-induced obesity and insulin resistance.20 Presumably, CARD9 signalling plays a role in macrophage infiltration under hyperglycaemic conditions. It would be interesting to evaluate which receptors are responsible for activation of CARD9 under these conditions. Since the macrophage content was not reduced in lesions from mice with deletion of haematopoietic Dectin-2, it is most likely that signal transduction of other CLRs, such as Dectin-122 or Mincle,23 is involved. So far, it has been shown that Dectin-1 expression is increased on monocytes of type 2 diabetes patients,22 and Mincle is highly expressed in adipose tissue of obese mice and humans.24 Whether Dectin-1 and Mincle are indeed activated by hyperglycaemic conditions, and whether they are involved in hyperglycaemia-induced macrophage activation and atherosclerosis formation, needs further investigation. It is important to mention that besides multiple CLRs, CARD9 can also be activated by other PRRs, such as nucleotide-binding oligomerization domain-containing protein 2 (NOD2). NOD2 is an intracellular PRR that recognizes peptidoglycan, a polymer of sugars and amino acids that is normally found in the cell wall of most bacteria.25 Interestingly, hyperglycaemia increases NOD2 expression in both preclinical models26 and human studies,27 which makes NOD2 a potentially interesting candidate to evaluate in the process of hyperglycaemia-induced macrophage infiltration in atherosclerotic plaques.
Our observations suggest that the role of carbohydrate-binding CLRs in atherosclerosis development during hyperglycaemia is limited at most. This is especially indicated by the absence of any effects of deletion of haematopoietic Dectin-2 on atherosclerosis development, and the relatively small effects of deletion of haematopoietic CARD9. It is, however, possible that CLRs play a role in hyperglycaemia-induced atherosclerosis via other non-haemopoietic derived cell types, such as endothelial cells. This has also been described for the known receptor for AGEs (RAGE). AGE-binding to RAGE is known to influence atherosclerotic development in mice.28,29 However, these studies were conducted in total body RAGE deletions28,29 and atherosclerosis development was particularly associated to AGE-binding to endothelial RAGE.28 Therefore, it is possible that we missed effects particularly in deletion of haematopoietic CARD9 as this modulator is also expressed in endothelial cells.30
Innate immune cells are crucially involved in every step of the atherosclerotic plaque formation.31 However, due to tight connection between innate and adaptive immune cell reactions, we also studied T-cell activation using the IL-22 cytokine, known as marker for T-cell activation and assigned with protective effects against the development of atherosclerosis.32 Although secretion of IL-22 is known to be regulated by CARD9,33 our results revealed no differences in mice with a deletion of haematopoietic CARD9 and Dectin-2 compared to WT animals. Of note, these results do not exclude differential regulation of IL-22 between the different genotypes in the local atherosclerotic plaque tissue.
Our study has limitations. First, it is possible that haematopoietic Dectin-2 and CARD9 are important at different stages of atherosclerosis development than studied here. The involvement of innate immune cells in atherosclerosis development is most critical during early atherosclerosis development when the uptake of oxidized lipids by macrophages precedes foam cells formation.34 Interestingly, oxLDL-induced pro-inflammatory responses have shown to be dependent on CARD9,35 suggesting that CARD9 may influence the initiation of atherosclerosis development. However, in the current study, all mice had developed advanced atherosclerosis with severe lesions due to the high plasma lipid levels and relatively long duration of the study. It can, therefore, not be excluded that deletion of haematopoietic Dectin-2 or, more likely, CARD9 may have anti-atherogenic effects in early atherosclerosis development. Related to this, high plasma cholesterol level, rather than the high glucose level, may have been the strongest driver of atherosclerosis development in our mouse model. The known reduction in lipoprotein clearance upon STZ injection15 caused an increase in plasma cholesterol levels around twofold compared to mice under normoglycaemic condition (Supplemental Figure 1)18, which likely contributes to the advanced atherosclerotic lesions observed. Since Keren et al.36 showed an involvement of the immune system in hyperglycaemia accelerated atherosclerosis using the same model of combined hyperglycaemia and atherosclerosis, we chose for this model to study similar effects on innate immune cells. However, another interesting follow-up study, which would also circumvent the effect of STZ injections on lipid levels, would be to transplant bone marrow cells from STZ-treated Dectin-2 and CARD9-deficient mice into Ldlr−/− donor mouse to study the role of Dectin-2 and CARD9 in conferring persistent effects of hyperglycaemia on immune cells and consequently the development of atherosclerosis. Finally, a limitation of our study is that the inflammatory response in peripheral macrophages and BMDMs may not completely reflect the response of the population of immune cells that resides within the plaque.35 It is thus possible that the macrophages inside the lesions have a different (i.e. anti-inflammatory) phenotype.
Conclusion
Our data do not support an important role for haematopoietic Dectin-2 or CARD9 in atherosclerosis development under hyperglycaemic conditions. Which receptors and signalling pathways are involved in atherosclerosis development under hyperglycaemic conditions, and which underlying molecular pathways are involved, remains a topic for future investigations.
Supplemental Material
Supplemental material, Supplementary_Figure_1 for Deletion of haematopoietic Dectin-2 or CARD9 does not protect from atherosclerosis development under hyperglycaemic conditions by Kathrin Thiem, Geerte Hoeke, Enchen Zhou, Anneke Hijmans, Tom Houben, Margien G Boels, Isabel M Mol, Esther Lutgens, Ronit Shiri-Sverdlov, Johan Bussink, Thirumala D Kanneganti, Mariëtte R Boon, Rinke Stienstra, Cees J Tack, Patrick CN Rensen, Mihai G Netea, Jimmy FP Berbée and Janna A van Diepen in Diabetes & Vascular Disease Research
Supplemental material, Supplementary_Figure_2 for Deletion of haematopoietic Dectin-2 or CARD9 does not protect from atherosclerosis development under hyperglycaemic conditions by Kathrin Thiem, Geerte Hoeke, Enchen Zhou, Anneke Hijmans, Tom Houben, Margien G Boels, Isabel M Mol, Esther Lutgens, Ronit Shiri-Sverdlov, Johan Bussink, Thirumala D Kanneganti, Mariëtte R Boon, Rinke Stienstra, Cees J Tack, Patrick CN Rensen, Mihai G Netea, Jimmy FP Berbée and Janna A van Diepen in Diabetes & Vascular Disease Research
Supplemental material, S_Table_1_revision for Deletion of haematopoietic Dectin-2 or CARD9 does not protect from atherosclerosis development under hyperglycaemic conditions by Kathrin Thiem, Geerte Hoeke, Enchen Zhou, Anneke Hijmans, Tom Houben, Margien G Boels, Isabel M Mol, Esther Lutgens, Ronit Shiri-Sverdlov, Johan Bussink, Thirumala D Kanneganti, Mariëtte R Boon, Rinke Stienstra, Cees J Tack, Patrick CN Rensen, Mihai G Netea, Jimmy FP Berbée and Janna A van Diepen in Diabetes & Vascular Disease Research
Acknowledgments
The authors thank Lianne van der Wee-Pals for her excellent technical assistance.
Footnotes
Author contributions: K.T., G.H., J.F.P.B. and J.A.v.D. conceived the study and designed experiments; K.T., G.H., A.H., T.H., M.G.B., I.M.M., M.R.B., J.B. and J.A.v.D. performed the experiments; K.T., G.H. and E.Z. analysed the data; K.T., G.H., E.L., R.S., C.J.T., M.G.N., P.C.N.R., J.F.P.B. and J.A.v.D. were involved in interpretation of the data; K.T., G.H., J.F.P.B. and J.A.v.D. drafted the article; all other authors revised the manuscript critically, and all authors approved the final version to be published.
Availability of data and material: All data generated or analysed during this study are included in this published article and its supplementary information files.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval and consent to participate: All experiments in this study were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, the Dutch Law on Animal Experiments and the FELASA regulations. The protocol was approved by the Ethics Committee on Animal Experiments of the Leiden University Medical Center.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Dutch Diabetes Research Foundation (#2013.81.1674) and the Netherlands CardioVascular Research Initiative: The Dutch Heart Foundation, Dutch Federation of University Medical Centers, the Netherlands Organisation for Health Research and Development and the Royal Netherlands Academy of Sciences’ for the GENIUS project ‘Generating the best evidence-based pharmaceutical targets for atherosclerosis’ (CVON2011-9). R.S. is supported by a VIDI grant from the Netherlands Organisation for Scientific Research. M.G.N. is supported by an ERC Consolidator Grant (#310372) and a Spinoza Grant of the Netherlands Organisation for Scientific Research. J.A.v.D. is supported by a Veni Grant of the Netherlands Organisation for the Scientific Research (#91616083).
ORCID iD: Kathrin Thiem  https://orcid.org/0000-0001-8144-1294
https://orcid.org/0000-0001-8144-1294
Supplemental material: Supplemental material for this article is available online.
References
- 1. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Risk factors for cardiovascular disease in type 1 diabetes. Diabetes 2016; 65: 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burke AP, Kolodgie FD, Zieske A, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol 2004; 24: 1266–1271. [DOI] [PubMed] [Google Scholar]
- 3. Libby P. Inflammation in atherosclerosis. Nature 2002; 420: 868–874. [DOI] [PubMed] [Google Scholar]
- 4. Shanmugam N, Reddy MA, Guha M, et al. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 2003; 52: 1256–1264. [DOI] [PubMed] [Google Scholar]
- 5. Devaraj S, Glaser N, Griffen S, et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 2006; 55: 774–779. [DOI] [PubMed] [Google Scholar]
- 6. Seneviratne AN, Sivagurunathan B, Monaco C. Toll-like receptors and macrophage activation in atherosclerosis. Clin Chim Acta 2012; 413: 3–14. [DOI] [PubMed] [Google Scholar]
- 7. Lee JY, Sohn KH, Rhee SH, et al. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 2001; 276: 16683–16689. [DOI] [PubMed] [Google Scholar]
- 8. Gow NA, Netea MG, Munro CA, et al. Immune recognition of Candida albicans beta-glucan by Dectin-1. J Infect Dis 2007; 196: 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kerrigan AM, Brown GD. Syk-coupled C-type lectins in immunity. Trends Immunol 2011; 32: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gross O, Gewies A, Finger K, et al. CARD9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006; 442: 651–656. [DOI] [PubMed] [Google Scholar]
- 11. Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 2012; 13: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia-Vallejo JJ, van Kooyk Y. Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev 2009; 230: 22–37. [DOI] [PubMed] [Google Scholar]
- 13. Mori D, Shibata K, Yamasaki S. C-type lectin receptor Dectin-2 binds to an endogenous protein beta-glucuronidase on dendritic cells. PLoS ONE 2017; 12: e0169562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Diepen JA, Thiem K, Stienstra R, et al. Diabetes propels the risk for cardiovascular disease: sweet monocytes becoming aggressive. Cell Mol Life Sci 2016; 73: 4675–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldberg IJ, Hu Y, Noh HL, et al. Decreased lipoprotein clearance is responsible for increased cholesterol in LDL receptor knockout mice with streptozotocin-induced diabetes. Diabetes 2008; 57: 1674–1682. [DOI] [PubMed] [Google Scholar]
- 16. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011; 11: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weinstock A, Pevsner-Fischer M, Porat Z, et al. Cultured mesenchymal stem cells stimulate an immune response by providing immune cells with toll-like receptor 2 ligand. Stem Cell Rev Rep 2015; 11: 826–840. [DOI] [PubMed] [Google Scholar]
- 18. Thiem K, Hoeke G, van den Berg S, et al. Deletion of hematopoietic Dectin-2 or CARD9 does not protect against atherosclerotic plaque formation in hyperlipidemic mice. Sci Rep 2019; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Romero MM, Basile JI, Corra Feo L, et al. Reactive oxygen species production by human dendritic cells involves TLR2 and Dectin-1 and is essential for efficient immune response against mycobacteria. Cell Microbiol 2016; 18: 875–886. [DOI] [PubMed] [Google Scholar]
- 20. Dasu MR, Devaraj S, Zhao L, et al. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 2008; 57: 3090–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishibashi S, Goldstein JL, Brown MS, et al. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest 1994; 93: 1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao L, Qin X, Peterson MR, et al. CARD9 knockout ameliorates myocardial dysfunction associated with high fat diet-induced obesity. J Mol Cell Cardiol 2016; 92: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cortez-Espinosa N, Garcia-Hernandez MH, Reynaga-Hernandez E, et al. Abnormal expression and function of Dectin-1 receptor in type 2 diabetes mellitus patients with poor glycemic control (HbA1c>8%). Metabolism 2012; 61: 1538–1546. [DOI] [PubMed] [Google Scholar]
- 24. Clement M, Basatemur G, Masters L, et al. Necrotic cell sensor Clec4e promotes a proatherogenic macrophage phenotype through activation of the unfolded protein response. Circulation 2016; 134: 1039–1051. [DOI] [PubMed] [Google Scholar]
- 25. Ichioka M, Suganami T, Tsuda N, et al. Increased expression of macrophage-inducible C-type lectin in adipose tissue of obese mice and humans. Diabetes 2011; 60: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schaefer AK, Melnyk JE, Baksh MM, et al. Membrane association dictates ligand specificity for the innate immune receptor NOD2. ACS Chem Biol 2017; 12: 2216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Du P, Fan B, Han H, et al. NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney Int 2013; 84: 265–276. [DOI] [PubMed] [Google Scholar]
- 28. Harja E, Bu DX, Hudson BI, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE–/– mice. J Clin Invest 2008; 118: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 2008; 57: 2461–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freed JK, Greene AS. Proteomic analysis of shear stress-mediated protection from TNF-alpha in endothelial cells. Microcirculation 2010; 17: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore KJ, Tabas I, Macrophages in the pathogenesis of atherosclerosis. Cell 2011; 145: 341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fatkhullina AR, Peshkova IO, Dzutsev A, et al. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity 2018; 49: 943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamas B, Richard M, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 2016; 22: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biessen EAL, Wouters K. Macrophage complexity in human atherosclerosis: opportunities for treatment? Curr Opin Lipidol 2017; 28: 419–426. [DOI] [PubMed] [Google Scholar]
- 35. Rhoads JP, Lukens JR, Wilhelm AJ, et al. Oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcgammaR cooperation and is dependent on CARD9. J Immunol 2017; 198: 2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keren P, George J, Shaish A, et al. Effect of hyperglycemia and hyperlipidemia on atherosclerosis in LDL receptor-deficient mice: establishment of a combined model and association with heat shock protein 65 immunity. Diabetes 2000; 49: 1064–1069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure_1 for Deletion of haematopoietic Dectin-2 or CARD9 does not protect from atherosclerosis development under hyperglycaemic conditions by Kathrin Thiem, Geerte Hoeke, Enchen Zhou, Anneke Hijmans, Tom Houben, Margien G Boels, Isabel M Mol, Esther Lutgens, Ronit Shiri-Sverdlov, Johan Bussink, Thirumala D Kanneganti, Mariëtte R Boon, Rinke Stienstra, Cees J Tack, Patrick CN Rensen, Mihai G Netea, Jimmy FP Berbée and Janna A van Diepen in Diabetes & Vascular Disease Research
Supplemental material, Supplementary_Figure_2 for Deletion of haematopoietic Dectin-2 or CARD9 does not protect from atherosclerosis development under hyperglycaemic conditions by Kathrin Thiem, Geerte Hoeke, Enchen Zhou, Anneke Hijmans, Tom Houben, Margien G Boels, Isabel M Mol, Esther Lutgens, Ronit Shiri-Sverdlov, Johan Bussink, Thirumala D Kanneganti, Mariëtte R Boon, Rinke Stienstra, Cees J Tack, Patrick CN Rensen, Mihai G Netea, Jimmy FP Berbée and Janna A van Diepen in Diabetes & Vascular Disease Research
Supplemental material, S_Table_1_revision for Deletion of haematopoietic Dectin-2 or CARD9 does not protect from atherosclerosis development under hyperglycaemic conditions by Kathrin Thiem, Geerte Hoeke, Enchen Zhou, Anneke Hijmans, Tom Houben, Margien G Boels, Isabel M Mol, Esther Lutgens, Ronit Shiri-Sverdlov, Johan Bussink, Thirumala D Kanneganti, Mariëtte R Boon, Rinke Stienstra, Cees J Tack, Patrick CN Rensen, Mihai G Netea, Jimmy FP Berbée and Janna A van Diepen in Diabetes & Vascular Disease Research